Abstract
Aims
To assess the correlation between optical coherence tomography (OCT) and leakage on fundus fluorescein angiography (FFA) following photodynamic therapy (PDT) with verteporfin for choroidal neovascularisation (CNV).
Methods
Retrospective comparative observational case series of patients who were treated with PDT for CNV from one centre. All patients had 3 monthly FFA and OCT following initial PDT to assess if further treatment was required. A pair of FFA and OCT images from the same visit at a random follow up date were taken from each patient's series and assessed separately by different observers. The presence of pigment epithelial detachment, subretinal fluid, vitreomacular traction, intraretinal fluid, absence of foveal depression, and the retinal thickness on OCT were correlated with presence of leaks on FFA.
Results
A total of 121 eyes of 121 patients were included. The presence of subretinal fluid, gross cystoid macular oedema, sponge‐like retinal thickening and retinal thickness of more than 350 μm on OCT correlated well with leak on FFA (p value <0.01). The likelihood ratios were 3.0, 5.7, 2.7, and 3.6, respectively. The presence of a solitary foveal cyst did not correlate well with leaks on FFA.
Conclusions
The presence of subretinal fluid, intraretinal fluid in the form of gross cystoid macular oedema, or sponge‐like retinal thickening, or a retinal thickness more than 350 μm correlates with leaks on FFA and so suggests the need for repeat PDT.
Keywords: choroidal neovascular membrane, optical coherence tomography, fundus fluorescein angiography, photodynamic therapy
Photodynamic therapy (PDT) with verteporfin for age related macular degeneration (AMD) usually requires a course of treatment. The decision to re‐treat is based on identifying leaks on a fundus fluorescein angiogram (FFA); however, leaks can be hard to distinguish from fluorescein staining, especially after several treatments.1,2 Optical coherence tomography (OCT) represents a fast and non‐invasive examination technique that generates two dimensional sections of the posterior pole in vivo.3,4,5 Changes to the OCT findings following PDT have been presented but which features correlate most closely with the presence of leaks on an FFA have not been fully assessed.6,7,8,9,10
We aimed to compare the appearance of the OCT with the presence or absence of leaks on FFA during the course of PDT follow up to see which OCT features best correlated with the presence of leaks and so give guidance to help re‐treatment decisions.
Materials and methods
The data were collected retrospectively from a pool of patients who had all received initial PDT with verteporfin for a classic or predominantly classic subfoveal CNV secondary to AMD between July 2001 and October 2004, to allow at least 3 months of follow up. The greatest linear dimension (GLD) of the total lesion had to be <5400 μm. PDT was performed as standard in the TAP study.1
From the series of 3 monthly follow up visits, one visit was randomly chosen for each patient from our collected database. The OCT and FFA from the same visit were analysed. Where bilateral treatment had been performed, one eye was randomly chosen for analysis. Random visits were chosen to reduce possible bias from knowledge of previous visits. In this study we did not assess the clinical findings or changes in vision as the features of the FFA and OCT were to be recorded objectively, without knowledge of the subjective changes. The investigators were masked to the treatment course, number of treatments, and whether treatment was given or not at that visit.
Stereo FFA was performed by an accredited ophthalmic photographer using the standard protocol of the Digital Angiography Reading Centre (DARC), Wisconsin, with a Zeiss Fundus Camera (FF450IR). The image capture software was Digital Healthcare (version 4.11.0.15). The presence of leaks was assessed on the FFA independently by AVM. In cases where leak was uncertain, SJT also assessed the images and if doubt remained, the FFA was recorded as not leaking. This meant that OCT features truly associated with leaks are more likely to be found.
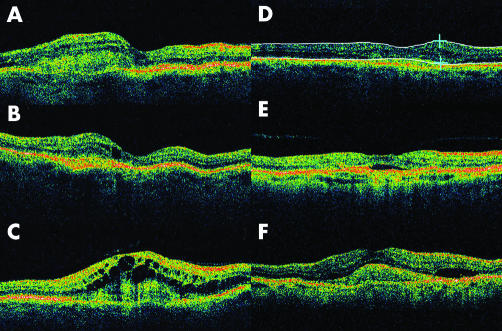
The same accredited ophthalmic photographer performed OCT, using the OCT3 (Zeiss, Jena, Germany) for each patient. Six radial 6 mm scans were centred on the best possible fixation the patient could manage. Each OCT image was evaluated independently by SSS. Proportional analysis for each OCT image was performed. The presence of a pigment epithelial detachment (PED), subretinal fluid (SRF), intraretinal fluid, vitreomacular traction (VMT), and a recognisable foveal depression were documented. Intraretinal fluid was subdivided into a solitary foveal cyst, sponge‐like retinal appearance with the presence of small intraretinal cysts, or gross cystoid macular oedema (CMO) (fig 1). The single retinal thickness analysis protocol on OCT3 was used to measure the retinal thickness of the treated eyes. The retinal thickness of the fellow eyes with inactive or no CNV was also measured. The largest measure of retinal thickness was recorded after analysing all six OCT cuts. Areas of SRF or CMO were included in the retinal thickness measurement but we excluded the outer high reflectivity band of the outer border, which would include the CNV complex if apparent (fig 1). The position of the lines generated by the software of the OCT3 was assessed and if this misread the neuroretinal thickness, the measurement was performed by manual positioning of the callipers. If the fellow eye had no evidence of CNV this was used as a control for analysis of retinal thickness.
Figure 1 Illustration of OCT findings. The subclassification of intraretinal fluid: (A) sponge‐like retinal appearance with the presence of intraretinal cysts; (B) a single foveal cyst; (C) gross cystoid macular oedema; (D) callipers used to measure retinal thickness, in this case 350 μm; (E) subretinal fluid (SRF) and vitreomacular traction; (F) choroidal neovascularisation complex, SRF, and intraretinal thickening.
Data were collected and analysed using Microsoft Excel and Graph Pad Instat 3 and Prism 4. Sensitivity, specificity, positive predictive value, negative predictive value, with the 95% confidence interval (CI), the likelihood ratio, and two sided p value using the Fisher's exact test were calculated. Ethics approval has been given for the collection of data on these patients. All patients had given full informed consent for their investigation and treatment.
Results
The records of 136 consecutive patients (136 eyes) treated with PDT between July 2001 and October 2004 were analysed; 17 eyes were excluded because of the poor quality of the OCT or FFA. Therefore, the images of 121 eyes were compared. Of these, 66 (54.5%) were female. Mean age was 73.9 years (range 30–94 years). There were 62 (51.2%) left eyes. There were 32 fellow eyes with no evidence of CNV. Follow up visits ranged from 3–24 months after initial PDT treatment, with 43 eyes at 3 months (35.5%), 29 at 6 months (24%), 21 at 9 months (17.4%), 12 at 12 months (9.9%), and 16 being between 15 months and 24 months (13.2%). On analysing the FFAs, 70 (57.85%) eyes showed a leak originating from a CNV complex after PDT.
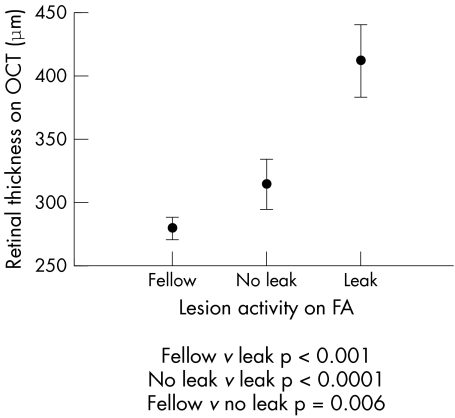
The parameter on OCT with the highest positive predictive value to leakage on FFA was the presence of gross CMO (0.89). Other factors with a high positive predictive value were the presence of SRF (0.80), a sponge‐like retinal appearance (0.79), and a retinal thickness >350 μm (0.83). The parameter on OCT with the highest sensitivity for leakage on FFA was a retinal thickness >300 μm (0.87) and presence of intraretinal fluid (0.83). The highest specificity was found for gross CMO (0.96), followed by subretinal fluid (0.84), sponge‐like retinal thickening (0.835), and retinal thickening >350 μm (0.835) (tables 1 and 2, and fig 2).
Table 1 Prevalence on optical coherence tomography of pigment epithelial detachment, subretinal fluid, intraretinal fluid (with a differentiation between gross cystoid macular oedema, sponge‐like retinal thickening and odd foveal cyst), absence of foveal depression, and retinal thickness more than 350 μm in the eyes with “leak” versus “no leak” on fundus fluorescein angiography (FFA).
| Leak on FFA | No leak on FFA | p Values | |
|---|---|---|---|
| Pigment epithelial detachment | 4/70 (5.7%) | 0/51 (0%) | |
| Subretinal fluid | 33/70 (47.1%) | 8/51 (15.7%) | 0.0004 |
| Intraretinal fluid | 58/70 (82.9%) | 24/51 (47.1%) | <0.0001 |
| Gross cystoid macular oedema | 16/70 (22.9%) | 1/51 (2%) | <0.0001 |
| Sponge‐like retinal thickening | 33/70 (47.1%) | 10/51 (19.6%) | 0.0009 |
| Solitary foveal cyst | 9/70 (12.9%) | 13/51 (25.5%) | 0.0945 |
| Absence of foveal depression | 38 /70 (54.3%) | 18/51 (35.3%) | 0.044 |
| Retinal thickness >350 μm | 44/70 (62.9%) | 9/51 (17.6%) | <0.0001 |
Table 2 Sensitivity, specificity, positive predictive value, negative predictive value (with 95% confidence intervals, CI), likelihood ratio and p value for each structural parameter on optical coherence tomography when compared to leak on fundus fluorescein angiography.
| Sensitivity (CI) | Specificity (CI) | Positive predictive value (CI) | Negative predictive value CI) | Likelihood ratio | p Value | |
|---|---|---|---|---|---|---|
| Subretinal fluid | 0.4714 (0.35 to 0.59) | 0.8431 (0.71 to 0.93) | 0.8049 (0.65 to 0.91) | 0.5375 (0.42 to 0.65) | 3.005 | 0.0004 |
| Intraretinal fluid | 0.8286 (0.72 to 0.91) | 0.5294 (0.38 to 0.67) | 0.7073 (0.6 to 0.8) | 0.6923 (0.52 to 0.83) | 1.761 | <0.0001 |
| Gross cystoid macular oedema | 0.2254 (0.13 to 0.34) | 0.9608 (0.87 to 0.99) | 0.8889 (0.65 to 0.99) | 0.4712 (0.37 to 0.57) | 5.746 | 0.0041 |
| Sponge‐like retinal thickening | 0.4714 (0.35 to 0.59) | 0.8235 (0.69 to 0.92) | 0.7857 (0.63 to 0.9) | 0.5316 (0.42 to 0.65) | 2.671 | 0.0009 |
| Solitary foveal cyst | 0.1268 (0.06 to 0.23) | 0.7451 (0.6 to 0.86) | 0.4091 (0.21 to 0.64) | 0.3800 (0.29 to 0.48) | 0.4973 | 0.0945 |
| Retinal thickness >300 μm | 0.8714 (0.77 to 0.94) | 0.5098 (0.37 to 0.65) | 0.7093 (0.6 to 0.8) | 0.7429 (0.57 to 0.87) | 1.778 | <0.0001 |
| Retinal thickness >350 μm | 0.6286 (0.50 to 0.74) | 0.8235 (0.69 to 0.92) | 0.8302 (0.70 to 0.92) | 0.6176 (0.49 to 0.73) | 3.562 | <0.0001 |
| No foveal depression | 0.5429 (0.42 to 0.66) | 0.6471 (0.5 to 0.78) | 0.6786 (0.54 to 0.8) | 0.5077 (0.38 to 0.63) | 1.538 | 0.044 |
Figure 2 The 95% confidence interval (CI) range of maximal retinal thickness on optical coherence tomography (OCT) of “leak” and “no leak” lesions on fundus fluorescein angiography (FA) in study eyes compared with fellow eyes.
The absence of a foveal depression did correlate statistically with leak on FFA (p = 0.044), but with a positive predictive value of only 0.68, a sensitivity of 0.54, and a specificity of 0.65.
There were nine eyes (7.4%) with vitreomacular traction on OCT. Three of these were thought to have leaks on FFA.
Discussion
Several studies have shown a correlation between leakage on FFA from an active CNV and increased retinal thickness on OCT.6,8 Costa et al showed a positive correlation between an increase in retinal thickness on OCT caused by fluid accumulation and leakage on FFA during the first 120 minutes after PDT for CNV in nine AMD patients.6 Subsequent cessation of fluorescein leak from CNV 1 week after PDT was accompanied by a reduction in maximum retinal thickness on OCT in all patients. Three months after treatment, fluorescein leakage within the CNV was noted in eight of nine patients and, accordingly, intraretinal or subretinal fluid accumulation on OCT was noted in these with an increase in the foveal thickness.
A limitation of OCT is that with six radial scans, it is still possible to miss a remnant leak area of the CNV complex. However, in our study we aimed to analyse all six radial scans so as not to miss important structural pathology. Fixation is a major problem when obtaining OCT scans of the macular area. The advantage of obtaining and analysing six scans improves the chances of passing through the fovea.
When analysing OCT scans in patients with subfoveal CNV secondary to AMD following PDT, Sahni et al showed high reproducibility between observers for measuring the neuroretinal foveal thickness, intraretinal fluid, subretinal fluid, and bilaminar foveal thickness.9 However, the reproducibility of the outer high reflectivity band measurements was low with the authors admitting that the difficulty in identifying the true outer margin. In our study, the retinal thickness included the neurosensory retina with CMO or SRF if present, excluding the outer high reflectivity band of the outer border, which we agree is indeterminable in the presence of RPE atrophy.
In a recent paper by Salinas‐Alamán et al, 62 eyes of 53 patients with active CNV were followed before and after PDT with FFA and OCT 2000, with a follow up time of 12 months for 42 eyes, and 6 months for 20 eyes.10 With FFA as the standard, OCT achieved a sensitivity of 0.96 in determining CNV activity after PDT. However, OCT obtained a moderate specificity of 0.59. The likelihood ratio for a positive test was 2.3, and for a negative test was 0.08. A diagnostic efficiency of 82.95% was calculated for the presence of intraretinal or subretinal fluid in OCT to detect post‐treatment CNV activity.
In our study, we subdivided intraretinal fluid on OCT into three groups: gross CMO, sponge‐like retinal appearance, and a solitary foveal cyst to try to increase diagnostic specificity. We found that gross CMO, SRF, and retinal thickness >350 μm had the highest positive predictive value, but that a solitary intraretinal foveal cyst on OCT does not always correlate with activity of CNV on FFA and may represent longstanding structural change rather than leak. No OCT finding was 100% diagnostic of leak so cannot replace FFA (table 2). However, we think that this study has provided us with a ranking of the most important parameters to consider on OCT in deciding whether to re‐treat.
Abbreviations
CMO - cystoid macular oedema
CNV - choroidal neovascularisation
FFA - fundus fluorescein angiography
GLD - greatest linear dimension
OCT - optical coherence tomography
PDT - photodynamic therapy
PED - pigment epithelial detachment
SRF - subretinal fluid
VMT - vitreomacular traction
Footnotes
None of the authors has a financial or proprietary interest in any material or method mentioned.
Ethics approval has been given for the collection of data on these patients.
References
- 1.Treatment of Age‐related Macular Degeneration with Photodynamic Therapy (TAP) Study Group Photodynamic therapy of subfoveal choroidal neovascularisation in age‐related macular degeneration with verteporfin. One year results of 2 randomized clinical trials—TAP Report 1. Arch Ophthalmol 19991171329–1345. [PubMed] [Google Scholar]
- 2.Coscas G, Coscas F, Soubrane G. [Monitoring the patient after treatment: angiographic aspects of recurrence and indications for retreatment]. J Fr Ophtalmol 20042781–92. [DOI] [PubMed] [Google Scholar]
- 3.Chauhan D S, Marshall J. The interpretation of optical coherence tomography images of the retina. Invest Ophthalmol Vis Sci 1999402332–2342. [PubMed] [Google Scholar]
- 4.Hee M R, Izatt J A, Swanson E A.et al Optical coherence tomography of the human retina. Arch Ophthalmol 1995113325–332. [DOI] [PubMed] [Google Scholar]
- 5.Sandhu S S, Talks S J. The correlation of optical coherence tomography, with or without additional colour fundus photography, with stereo fundus fluorescein angiography in diagnosing choroidal neovascular membranes. Br J Ophthalmol 200589967–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costa R A, Farah M E, Cardillo J A.et al Immediate indocyanine green angiography and optical coherence tomography evaluation after photodynamic therapy for subfoveal choroidal neovascularization. Retina 200323159–165. [DOI] [PubMed] [Google Scholar]
- 7.Rogers A H, Martidis A, Greenberg P B.et al Optical coherence tomography findings following photodynamic therapy of choroidal neovascularization. Am J Ophthalmol 2002134566–576. [DOI] [PubMed] [Google Scholar]
- 8.Montero J A, Ruiz‐Moreno J M, Tavolato M. Follow‐up of age‐related macular degeneration patients treated by photodynamic therapy with optical coherence tomography 3. Graefes Arch Clin Exp Ophthalmol 2003241797–802. [DOI] [PubMed] [Google Scholar]
- 9.Sahni J, Stanga P, Wong D.et al Optical coherence tomography in photodynamic therapy for subfoveal choroidal neovascularisation secondary to age related macular degeneration: a cross sectional study. Br J Ophthalmol 200589316–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salinas‐Alamán A, García‐Layana A, Maldonado M J.et al Using optical coherence tomography to monitor photodynamic therapy in age related macular degeneration. Am J Ophthalmol 200514023–28. [DOI] [PubMed] [Google Scholar]