Abstract
Aim
To determine the association of spherical equivalent (SE) with low uncorrected visual acuity (VA) along with a proposed definition for myopia using logMAR VA >0.3 as the criteria.
Methods
1334 Chinese schoolchildren (mean age 7.8; range 7–9 years) were enrolled in the study after those who had hyperopia ⩾+2.00 dioptres (D) and astigmatism > = −2.00D were excluded. Uncorrected logMAR VA was measured for both eyes. Cycloplegia autorefraction was achieved by the instillation of three drops of 1% cyclopentolate 5 minutes apart. The average of five successful consecutive refraction and keratometry readings were obtained with calibrated Canon RK5 autokeratorefractometers by well trained optometry students, at least 30 minutes after the instillation of the third drop of cyclopentolate. SE cut‐off points (−0.25D, −0.5D, −0.75D, −1.0D) were evaluated.
Results
Using different SE cut‐off points, the myopia prevalence rates of this sample of schoolchildren varied from 45.8% (SE at least −0.25 D) to 30.7% (SE at least −1.0 D). The cut‐off point of ⩾−0.75 D had a sensitivity and specificity of 91.8% (95% CI, 89.2 to 94.4) and 93.7% (95% CI, 92.1 to 95.3), respectively, to predict low vision defined as uncorrected logMAR VA > 0.3 (either eye). The next best cut‐off point of −0.5D had a higher sensitivity (93.3%), but lower specificity (87.9%).
Conclusions
The cut‐off points of −0.75D and −0.5D in SE refraction are appropriate for the prediction of uncorrected logMAR VA worse than 0.3, which is the criterion for the US common state adult driver licensing standard.
Keywords: myopia, children, Singapore
Myopia definitions vary widely in different studies. In 1952, Hirsch reported a 24% myopia prevalence rate in a non‐cycloplegic retinoscopy survey of 9552 American schoolchildren based on a definition of at least −0.12 dioptres (D), while the rate dropped to 5.4% when the definition of ⩽−1.0D was adopted.1 In Taiwan, Lin et al reported that 12% of 6 year old schoolchildren and 84% of aged 16–18 years old had myopia using spherical equivalents (SE) ⩽−0.25D.2 Notably, SE ⩽−0.5D was adopted by the multicentre Refractive Error Study in Children (RESC) surveys conducted worldwide in children 5–15 years of age with different ethnic origins and environmental settings and the Singapore Cohort Study of Risk Factors for Myopia (SCORM).3,4,5,6,7,8,9,10,11 In the United States, both the Orinda Longitudinal Study of Myopia (OLSM) and the Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error Study (CLEERE) used SE ⩽−0.75D.12,13 On the other hand, SE ⩽−1.0D was employed by Lithander in Oman.14
Studies are difficult to compare because there is no common consensus about the best definition of myopia. We thus aimed to evaluate the ability of different SE (−0.25D, −0.5D, −0.75D, −1.0D) to predict low uncorrected visual acuity (VA) in Singapore children.
Methods
The recruitment of subjects in the SCORM and the examination procedures have been described previously.11 All children aged 7–9 years from three schools in Singapore were recruited in 1999 and 2001. Children with serious medical disorders, such as leukaemia, or chronic eye disorders, such as congenital cataract and any other condition associated with a decrease in visual acuity other than uncorrected refractive error were excluded. A total of 1467 Chinese children (mean age 7.8 (SD 0.02) years) participated in the baseline examination. Informed consent was obtained from the parents. The tenets of the Declaration of Helsinki were observed, and approval was granted by the Singapore Eye Research Institute ethics committee.
Eye examinations
Uncorrected distance logMAR VA were measured in both eyes. In brief, cycloplegia was induced by the instillation of three drops of 1% cyclopentolate 5 minutes apart. At least 30 minutes after the last drop, five consecutive refraction and keratometry readings were obtained with one of two calibrated autokeratorefractometers (model RK5; Canon, Inc Ltd, Tochigiken, Japan).
Definitions and statistical analyses
SE is defined as sphere plus half negative cylinder power. Different cut‐offs of SE of at least −0.25D, −0.5D, −0.75D, and −1D, respectively, were compared. The age specific prevalence rates in this sample and 95% confidence intervals (CI) of myopia using different criteria are presented. The sensitivities and specificities of different refractive error cut‐offs to predict low visual acuity were evaluated in children without astigmatism (⩾−2D) or hyperopia (⩾2D). We chose a criterion of logMAR uncorrected VA >0.3 for low vision according to US driving requirements.15 Thereafter, 1334 Chinese children (692 boys and 642 girls) remained. To estimate the probability that the cut‐off point will correctly predict diagnosis of low vision, the adjusted positive predictive values (PPV) and negative predictive values (NPV) were also calculated by the following equation suggested by Altman and Bland.16 A receiver operating characteristic (ROC) curve to predict low visual acuity (logMAR > 0.3) in either eye was plotted. The relative influences of sphere and cylinder on logMAR VA worse than 0.3 were also evaluated in multiple logistic regression models with visual impairment as the dependent variable. Statistical analyses were conducted with SAS, version 8.02; SAS, Cary, NC, USA.
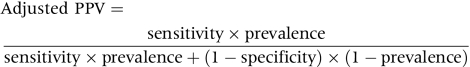 |
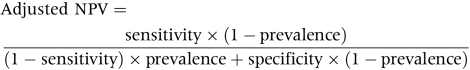 |
Results
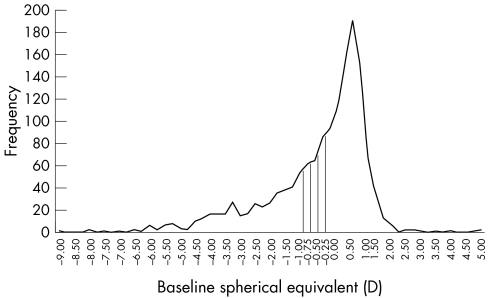
The distribution of refractive error in our study was skewed towards the more myopic values (fig 1). Age specific prevalence rates of myopia in 1467 Singapore Chinese children according to different criteria of SE ⩽−0.25D, ⩽−0.5D, ⩽−0.75D, and ⩽−1.0D were 45.8%, 40.2%, 35.2%, and 30.7%, respectively (table 1).
Figure 1 Cumulative distribution of baseline refractive errors (n = 1467).
Table 1 Age specific prevalence rates of myopia (either eye) by different spherical equivalent (SE) cut‐off points of all Chinese children in the SCORM study (n = 1467).
| Age (years) | No | Prevalence rates of myopia (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SE ⩽−0.25D | No | SE ⩽−0.5D | No | SE ⩽−0.75D | No | SE ⩽−1.00D | No | ||
| SCORM | 1467 | 45.8% (43.2% to 48.4%) | 672 | 40.2% (37.6% to 42.7%) | 589 | 35.2% (32.7% to 37.7%) | 516 | 30.7% (28.4% to 33.2%) | 451 |
| 7 | 645 | 38.8% (35.0% to 42.6%) | 250 | 32.4% (28.8% to 36.2%) | 209 | 26.8% (23.4% to 30.4%) | 173 | 21.7% (18.6% to 25.1%) | 140 |
| 8 | 479 | 43.8% (39.3% to 48.4%) | 210 | 38.2% (33.8% to 42.7%) | 183 | 33.6% (29.4% to 38.0%) | 161 | 29.9% (25.8% to 34.2%) | 143 |
| 9 | 343 | 61.8% (56.4% to 67.0%) | 212 | 57.4% (52.0% to 62.7%) | 197 | 53.1% (47.6% to 58.4%) | 182 | 49.0% (43.6% to 54.4%) | 168 |
Mean uncorrected logMAR VA was 0.31 (SD 0.36). Mean uncorrected logMAR VA of right and left eye were significantly correlated (0.30 (0.36) versus 0.30 (0.35), Pearson correlation coefficient was 0.93, R2 = 0.86, p<0.001). The intraclass correlation coefficient between the right and left eye's refraction was 0.94 (95% CI 0.92 to 0.95; p<0.001).
Girls' uncorrected logMAR VA was better than boys (0.29 (0.34) versus 0.32 (0.37), p = 0.05). Mean uncorrected logMAR VA for children aged 7, 8, and 9 years were 0.25 (0.30), 0.29 (0.35), and 0.44 (0.43), respectively (p<0.001, one way ANOVA).
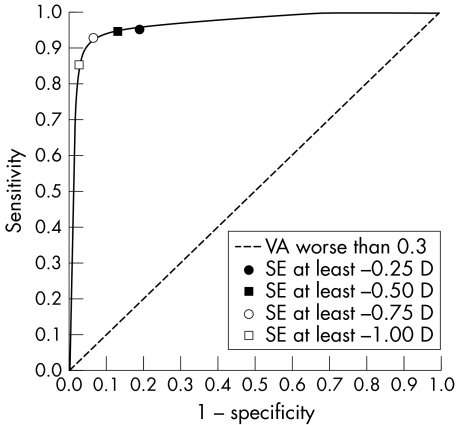
The sensitivities, specificities, PPV, and NPV are presented in table 2. Children with other possible causes of visual impairment, including hyperopia (either eye ⩾+2.0D) or astigmatism (either eye astigmatism ⩾−2.0D) were excluded from this analysis. For the prediction of uncorrected logMAR VA >0.3 in either eye, the cut off of SE ⩽−0.75D gave a sensitivity of 91.8% (95% CI, 91.7 to 96.2), and specificity of 93.7% (95% CI, 92.1 to 95.3). The cut‐off of SE ⩽−0.5D, however, gave a lower specificity of 87.9% (95% CI, 85.7 to 90.0). An analysis of the ROC curve showed that the highest area under the curve values was at a SE cut‐off between −0.5D and −0.75D (data not shown) (fig 2).
Table 2 Sensitivities, specificities, positive predictive values (PPV), negative predictive values (NPV), and 95% CI of different spherical equivalent cut‐offs for the prediction of uncorrected logMAR visual acuity impairment in Singapore schoolchildren (n = 1334).
| Criteria | Impaired vision (either eye uncorrected logMAR visual acuity > 0.3) | |||
|---|---|---|---|---|
| SCORM (7–9 years; n = 1334) | ||||
| Sensitivity (%) | Specificity (%) | Adjusted PPV (%) | Adjusted NPV (%) | |
| SE ⩽−0.25D | 94.9 (92.8 to 97.0) | 80.1 (77.5 to 82.7) | 80.1 (76.9 to 83.4) | 94.9 (93.3 to 96.4) |
| SE ⩽−0.50D | 93.9 (91.7 to 96.2) | 87.9 (85.7 to 90.0) | 83.8 (80.7 to 87.0) | 95.6 (94.2 to 97.0) |
| SE ⩽−0.75D | 91.8 (89.2 to 94.4) | 93.7 (92.1 to 95.3) | 88.8 (85.9 to 91.7) | 95.5 (94.1 to 96.9) |
| SE &les−1.00D | 86.7 (83.5 to 89.9) | 97.8 (96.8 to 98.7) | 94.6 (92.3 to 96.8) | 94.3 (92.8 to 95.8) |
Figure 2 Receiver operating characteristic (ROC) curve. Sensitivity was plotted against 1 − specificity for each mean spherical equivalent cut‐off value (−0.25D, −0.5D, −0.75D, −1.0D) used to define the onset of myopia in logMAR visual acuity (VA) worse than 0.3 groups analysis. Selected data points were labelled with SE cut‐off values. (n = 1334).
For the prediction of uncorrected logMAR VA >0.2 in either eye, the cut‐off of SE ⩽−0.75D gave a sensitivity of 79.9% (95% CI, 76.5 to 83.3), specificity of 96.3% (95% CI, 95.0 to 97.6), PPV of 92.1% (95% CI, 89.6 to 94.6), and a NPV of 89.8% (95% CI, 87.8 to 91.8). The cut‐off of SE ⩽−0.5D gave a sensitivity of 84.8% (95% CI, 81.8 to 87.9), lower specificity of 91.8% (95% CI, 89.9 to 93.7), lower PPV of 87.4% (95% CI, 84.6 to 90.3), and NPV of 90.0% (95% CI, 88.0 to 92.1).
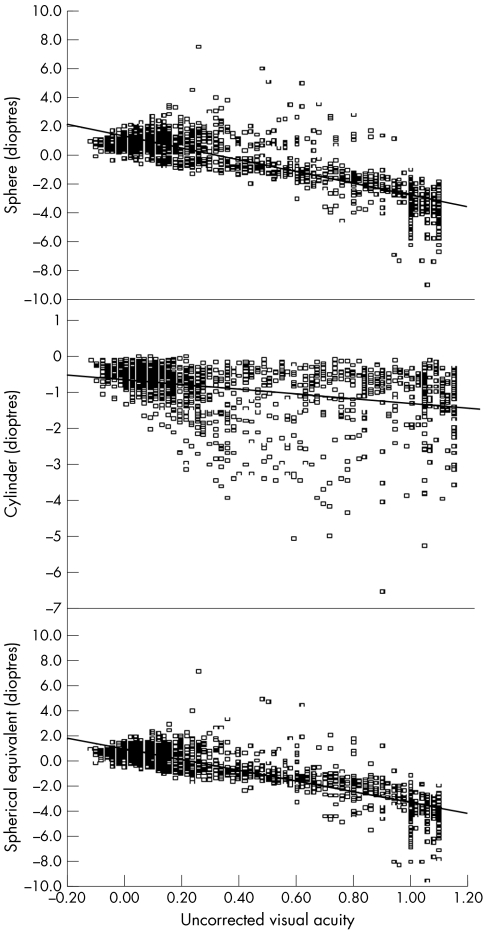
In all 1467 children, the causes of uncorrected logMAR VA >0.3 in the worse eye included myopia only (at least −0.75D) (73.0%), hyperopia only (at least +2.0D) (1.3%), astigmatism only (at least −2.0D) (6.5%), myopia combined with astigmatism (11.2%), and hyperopia combined with astigmatism (0.4%). The effects of spherical and cylindrical error were evaluated in multiple logistic regression models. For every additional dioptre increase in absolute sphere, the odds ratio of poor logMAR VA was 18.9 (95% CI 13.1, 27.3), adjusted for age, sex, school, and absolute cylinder (p<0.001). For every additional dioptre increase in absolute cylinder, the odds ratio of poor logMAR VA was 7.6 (95% CI 4.3, 13.4), adjusted for age, sex, school, and absolute sphere (p<0.001). Scatter plots of uncorrected logMAR VA with SE, sphere and cylinder are shown in figure 3. The Pearson correlation coefficients were −0.84 (95% CI −0.85 to −0.82) for SE and uncorrected logMAR VA, −0.80 (95% CI −0.82 to −0.78) for sphere versus uncorrected logMAR VA, and −0.33 (95% CI −0.37 to −0.28) for cylinder versus uncorrected logMAR VA.
Figure 3 Scatter plots of refractive error and sphere and cylinder (worse eye) and uncorrected logMAR visual acuity (worse eye) (n = 1467).
Discussion
In our study, the criterion of SE of at least −0.75D had the best combination of sensitivities and specificities for the prediction of logMAR VA worse than 0.3, followed by the cut‐off of −0.5D. The influence of sphere on visual impairment was greater compared with cylinder.
Previously used definitions of myopia have depended upon arbitrary criteria, as there is no common international consensus about the accepted definitions for myopia17 (table 3). The Visual Impairment Project in Australia (1999) has reported that a change in cut‐off point from −0.5D to −0.75D would reduce the reported myopia prevalence rates by 22% in 3271 urban and 1473 rural residents who were examined.18 One report from the OLSM had shown that the criterion for myopia of at least −0.5D on non‐cycloplegic autorefraction compared to cycloplegic autorefraction of at least −0.75D more than doubled the estimates of myopia prevalence (44.5% compared to 19.8%).19 We noted that the overall prevalence rate of myopia in our study dropped by about 15% (from 45.8% to 30.7%, table 1) with the change in definition from −0.25D to −1.0D. Other considerations in the comparisons of myopia rates are the uniformity of refractive error measurement techniques and whether cycloplegia was used.
Table 3 Recent studies of myopia prevalence among children.
| Author (year/country) | Study name | Study population (N) | Definition of myopia | Test method | Prevalence rate (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Lin2 (1999/Taiwan) | NA | Aged 6–18 years schoolchildren (n = 11 178) | ⩽−0.25D | cycloplegic autorefraction | 12% at age 6 years, increasing to 56% at age 12 years and 84% in teenagers aged 16–18 years | ||||
| Murthy7 (2002/India) | Refractive Error Study in Children (RESC) | Aged 5–15 years in urban New Delhi (n = 6447) | ⩽−0.5D | cycloplegic autorefraction | 7.4% of all children | ||||
| Zadnik5 (1998/USA); | Orinda Longitudinal Study of Myopia (OLSM) | Aged 6–14 years (n = 994) | ⩽−0.75D | cycloplegic autorefraction | ⩽ 5% before age of 9 years, 21.3% at the age of 14 years | ||||
| Kleinstein6 (2003/USA) | Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error Study (CLEERE), multicentre study | Aged 5–17 years children (534 African‐American, 491 Asian, 463 Hispanic, and 1035 white) (n = 2523) | ⩽−0.75D | cycloplegic autorefraction | 9.2% of all children; Asians (18.5%), Hispanics (13.2%), African Americans (6.5%) Caucasians (4.4%). | ||||
| Lithander16 (1999/Oman) | NA | Aged 6–12 years schoolchildren(n = 6292) | ⩽−1.0D | Cycloplegic retinoscopy | 0.56% in 6 years old, 5.16% in 12 years old |
To our knowledge, no study has attempted to define the optimum cut‐off point of SE for the definition of myopia. We suggest that the relevant level of myopia to use as a cut‐off in prevalence surveys would be that at which a level of functional impairment is seen, rather than an arbitrarily chosen number. The cut‐off of −0.75D provided satisfactory sensitivity or specificity profiles in uncorrected logMAR VA worse than 0.3 prediction analysis, with few myopic children falsely diagnosed as emmetropic (false negatives) and few non‐myopic children falsely diagnosed as myopic (false positives). The more liberal cut‐off of −0.5D is associated with lower specificity and some children without myopia may be falsely labelled as myopic (false positives), thus there may be unnecessary anxiety and extra visits to eye care professionals. The cut‐off point at −0.75D would be a preferred stringent threshold with the best balance of PPV and NPV compared with other cut‐offs. The ROC curve analysis of the most appropriate cut‐off suggests that the interval between −0.5D and −0.75D perform better. However clinically, refractive error is measured in units of 0.25D and therefore the bracketing values of −0.5D and −0.75D were evaluated and −0.75D has the sensitivity >90% and the specificity >90%, respectively. The cut‐off of −0.25D had the lowest specificity and highest false positive rate, and the use of −0.25D could be criticised on those grounds as a number of possibly emmetropic individuals could be miscategorised. The cut‐off point of −1.0D had the lowest sensitivity and highest false negative rates.
A cut‐off value of 0.3 logMAR was chosen because this criterion, the US common state adult driver licensing standard, is a commonly used definition of low vision. We thus employed this uniform definition of low vision in children as well. However, other clinically meaningful cut‐offs such as logMAR VA worse than 0.2 have also been presented. An additional criterion in defining myopia is the possible effects of measurement error. The use of −0.75D or −0.5D should be less affected by this concern as the ROC curve analysis of the most appropriate cut‐off between −0.5D and −0.75D performs better.
In our study, the uncorrected VA of girls was better than boys, partly because the SE of boys is significantly worse than that of girls (right eye −0.60 (1.73)D versus −0.33 (1.41)D; left eye: −0.57 (1.74)D versus −0.33 (1.42)D) and girls could possibly be more compliant when reading logMAR VA chart measurements.
Our primary analysis aimed to evaluate the effects of myopia on decreased logMAR VA by excluding children with astigmatism and hyperopia. In additional analysis that included children with astigmatism, both myopic sphere and cylinder significantly reduced logMAR VA, although sphere had a stronger association with logMAR VA compared with cylinder. Thus, we have to bear in mind that decreased vision in myopic children may be caused by both spherical and cylindrical errors.
Although logMAR VA is a major clinical parameter associated with myopia, myopic individuals may have other deficits like compromised day to day visual performance, visual field defects, and astigmatism.20 Another possible approach would be to assess directly the effects of refractive error on day to day performance via a functional questionnaire based approach such as the VF14.21 It might be expected to yield slightly different results though as most subjects with significant refractive errors would habitually wear refractive correction. The effects of different given SE cut‐offs on visual field defects found on automated perimetry could also be further assessed.20
Of greater interest is the determination of the best cut‐off for high myopia. Criteria for high myopia that have been used in previous studies include −5.0D, −6.0D, −10.0D, and −12.0D and there is no universal definition for high myopia.22,23 It is thought that at this level, the risks of secondary complications, such as retinal detachment and glaucoma may increase.23,24,25 There may also be further deteriorations in visual field, central VA, increased risks of irregular astigmatism, keratoconus, and peripheral visual field defects. The risks of various ocular morbidities associated with different levels of severity of myopia will be evaluated further in the SCORM study.
Clearly, the strengths of this study include a large sample size drawn from a school based population, uniformity of assessments, and the objectivity of table mounted autorefraction. The cycloplegia excluded pseudomyopia and accommodative spasm. Subjects with other possible causes of low vision such as hyperopia (either eye at least +2.0D) and astigmatism (either eye astigmatism at least −2.0D) were excluded. Although the children with diagnosed amblyopia, strabismus, and major eye defects were excluded from this study detailed screening for other more subtle ocular conditions potentially affecting logMAR VA, such as retinal disorders, was not performed. However, the prevalence of such conditions is extremely low in this age group and so would not be expected to influence the results presented here and in the Sydney Myopia Study.26 This sample, however, only included children from one ethnic group, the criterion for low vision acuity based on a US adult driver licensing standard was chosen while other standards could have been selected, and data about corneal topography and irregular astigmatism contributing to low logMAR VA were not available.
In summary, SE ⩽−0.75D and SE ⩽−0.5D are appropriate for the prediction of uncorrected logMAR VA worse than 0.3 in schoolchildren. Further investigations of associations of optimal cut‐offs for myopia with other clinical parameters such as visual field defects are needed.
Abbreviations
CLEERE - Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error Study
NPV - negative predictive values
OLSM - Orinda Longitudinal Study of Myopia
PPV - positive predictive values
RESC - Refractive Error Study in Children
ROC - receiver operating characteristic
SCORM - Singapore Cohort Study of Risk Factors for Myopia
SE - spherical equivalent
VA - visual acuity
References
- 1.Hirsch M J. The changes in refraction between the ages of 5 and 14, theoretical and practical considerations. Am J Optom Arch Am Acad Optom 195229445–459. [DOI] [PubMed] [Google Scholar]
- 2.Lin L L, Shih Y F, Tsai C B.et al Epidemiology study of refraction among schoolchildren in Taiwan in 1995. Optom Vis Sci 199976275–281. [DOI] [PubMed] [Google Scholar]
- 3.Murthy G V, Gupta S K, Ellwein L B.et al Refractive error in children in an urban population in New Delhi. Invest Ophthalmol Vis Sci 200243623–631. [PubMed] [Google Scholar]
- 4.Dandona R, Dandona L, Srinivas M.et al Refractive error in children in a rural population in India. Invest Ophthalmol Vis Sci 200243615–622. [PubMed] [Google Scholar]
- 5.Pokharel G P, Negrel A D, Munoz S R.et al Refractive Error Study in Children: results from Mechi Zone, Nepal. Am J Ophthalmol 2000129436–444. [DOI] [PubMed] [Google Scholar]
- 6.Naidoo K S, Raghunandan A, Mashige K P.et al Refractive error and visual impairment in African children in South Africa. Invest Ophthalmol Vis Sci 2003443764–3770. [DOI] [PubMed] [Google Scholar]
- 7.Maul E, Barroso S, Munoz S R.et al Refractive Error Study in Children: results from La Florida, Chile. Am J Ophthalmol 2000129445–454. [DOI] [PubMed] [Google Scholar]
- 8.Zhao J, Pan X, Sui R.et al Refractive Error Study in Children: results from Shunyi District, China. Am J Ophthalmol 2000129427–435. [DOI] [PubMed] [Google Scholar]
- 9.He M, Zeng J, Liu Y.et al Refractive error and visual impairment in urban children in southern china. Invest Ophthalmol Vis Sci 2004453793–799. [DOI] [PubMed] [Google Scholar]
- 10.Goh P P, Abqariyah Y, Pokharel G P.et al Refractive error and visual impairment in school‐age children in Gombak District, Malaysia. Ophthalmology 2005112678–685. [DOI] [PubMed] [Google Scholar]
- 11.Chua W H, Saw S M, Wu H M.et al Refractive errors in schoolchildren: the Singapore Myopia Cohort Study. Proceedings of the VIII International Conference on Myopia, 7–9 July 2000, Boston, United States. Conference on Myopia 2000
- 12.Mutti D O, Zadnik K, Fusaro R E.et al Optical and structure development of the crystalline lens in childhood. Invest Ophthalmol Vis Sci 199839120–133. [PubMed] [Google Scholar]
- 13.Kleinstein R N, Jones L A, Hullett S.et al Refractive error and ethnicity in children. Arch Ophthalmol 20031211141–1147. [DOI] [PubMed] [Google Scholar]
- 14.Lithander J. Prevalence of myopia in school children in the Sultanate of Oman: a nation‐wide study of 6292 randomly selected children. Acta Ophthalmol Scand 199977306–309. [DOI] [PubMed] [Google Scholar]
- 15.Hyman L, Wu S Y, Connell A M.et al Prevalence and causes of visual impairment in the Barbados Eye Study. Ophthalmology 20011081751–1756. [DOI] [PubMed] [Google Scholar]
- 16.Altman D G, Bland J M. Diagnosis tests. 2: Predictive values, BMJ 1994309102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park D J, Congdon N G. Evidence for an “epidemic” of myopia. Ann Acad Med Singapore 20043321–26. [PubMed] [Google Scholar]
- 18.Wensor M, McCarty C A, Taylor H R. Prevalence and risk factors of myopia in Victoria, Australia. Arch Ophthalmol 1999117658–663. [DOI] [PubMed] [Google Scholar]
- 19.Zadnik K, Mutti D O. Let's define myopia: a need for consensus? Proceedings of the VIII International Conference on Myopia, 7–9 July 2000, Boston, United States. Conference on Myopia 2000
- 20.Aung T, Foster P J, Seah S K.et al Automated static perimetry: the influence of myopia and its method of correction. Ophthalmology 2001108290–295. [DOI] [PubMed] [Google Scholar]
- 21.Saw S M, Gazzard G, Au‐Eong K G.et al Utility values and myopia in teenage school students. Br J Ophthalmol 200387341–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Percival S P. Redefinition of high myopia: the relationship of axial length measurement to myopic pathology and its relevance to cataract surgery. Dev Ophthalmol 19871442–46. [DOI] [PubMed] [Google Scholar]
- 23.Younan C, Mitchell P, Cumming R G.et al Myopia and incident cataract and cataract surgery: the Blue Mountains Eye Study. Invest Ophthalmol Vis Sci 2002433625–3632. [PubMed] [Google Scholar]
- 24.Tong L, Saw S M, Chua W H.et al Optic disk and retinal characteristics in myopic children. Am J Ophthalmol 2004138160–162. [DOI] [PubMed] [Google Scholar]
- 25.Saw S M, Gazzard G, Chan E S Y.et al Myopia and associated pathologic complications. Ophthal Physiol Opt. (in press)
- 26.Robaei D, Rose K, Ojaimi E.et al VA and the causes of visual loss in a population‐based sample of 6‐year‐old Australian children. Ophthalmology 20051121275–1282. [DOI] [PubMed] [Google Scholar]