Abstract
Aims
To search for the induction of the expression of antimicrobial peptides in corneal fibroblasts treated with bacterial components.
Methods
RT‐PCR was performed to search for mRNAs expression of antimicrobial peptides and toll‐like receptors (TLRs) in murine primary cultures of corneal fibroblast (PCCF) treated with lipopolysaccharide (LPS) from Escherichia coli, peptidoglycan from Staphylococcus aureus, and cytosine‐phosphorous‐guanine oligonucleotide (CpG‐ODN). Cellular activation was blocked with anti‐TRL antibodies.
Results
LPS did not induce expression of antimicrobial peptide in corneal fibroblasts. Cathelin related antimicrobial peptide (CRAMP) and α‐defensin 3 were overexpressed in a time and dose dependent manner in corneal fibroblasts treated with peptidoglycan and with CpG‐ODN, respectively. CRAMP expression was blocked when PCCF were treated with anti‐TLR‐2 antibodies. α‐Defensin 3 was not expressed in NIH murine corneal fibroblasts (which do not express the TLR‐9 molecule) treated with CpG‐ODN.
Conclusion
Results suggest that corneal fibroblasts, which are the second cellular barrier of the cornea, can play an important part in the innate immunity of the eye via TLR stimulation.
Keywords: CRAMP, α‐defensin 3, corneal fibroblast, toll‐like receptors, antimicrobial peptides
Every organ and tissue can be threatened by the invasion of environmental pathogens. The eye straddles these threats as the ocular surface has direct contact with environmental pathogens, whereas the internal compartments of the eye are vulnerable to blood borne pathogens. Pathogens have developed special skills to invade tissues and organs but the immune system has created a wide variety of responses to eliminate the micro‐organisms.1
The ocular immune privilege provides the eye with a limited range of immune effector mechanisms against pathogens that represent a minimal risk to vision integrity. This innate immunity plays an important part in protecting the eye against pathogens, mediated by antimicrobial peptides that have been reported on the ocular surface.2
The antimicrobial peptides are proteins that participate in the primary immune response of plants, insects, and mammals. There are two groups of antimicrobial peptides called defensins and cathelicidins. Three forms of defensin, α, β, and θ, are recognised. The α‐defensins are also known as human neutrophil peptides (HNP) 1–3, because they have been localised in neutrophils and Paneth cells of the intestine, whereas β‐defensins are expressed by many epithelia.3,4 Six human β‐defensins (hBD‐1–6) have been identified.5,6,7,8 hBD‐1 is constitutively expressed, whereas hBD‐2 and hBD‐3 are inducible by cytokines and bacterial products. hBD‐4 appears to have a more limited distribution than hBD‐1, hBD‐2, or hBD‐3; in addition its expression can be upregulated by bacterial infection but not by inflammatory factors that upregulate hBD‐2 and hBD‐3.7 The most recently identified family members are hBD‐5 and hBD‐6, which have been localised to the epididymis.8
On the other hand, cathelicidins have been observed to be present as a unique gene in mammals. This gene has different names depending on its location: in humans, it has been called LL‐37; in mouse, cathelin related antimicrobial peptide (CRAMP); in pig, PR39.9 The common denominator of this protein family is a conserved cathelin, whereas the C‐terminal domain is represented by highly variant antibacterial peptides.10 The gene is expressed in bone marrow and testis, granulocytes and in human wounds.11,12,13
The molecular structures that contribute to the makeup of micro‐organisms have been described as pathogen associated molecular patterns (PAMPs) and include lipopolysaccharide (LPS) and peptidoglycans (PGNs). These have been reported to induce expression of antimicrobial peptides in cells of the immunological system and in cells of several epithelial tissues.14,15 Toll‐like receptors (TLRs) are involved in the recognition of the PAMPs.16 TLRs are transmembrane receptors with an extracellular domain, involved in the recognition of the PAMP ligand, and an intracellular domain called TLR/IL‐1 receptor (TIR), essential for the signal transduction that drives the activation of the nuclear factor kappa B (NF‐kB)17,18 to induce expression of endogenous signals, such as inflammatory cytokines and antimicrobial peptides.17 TLR4 is required for recognition of the LPS contained in Gram negative bacteria.19 TLR2 form heterodimeric complexes with TLR6 or TLR1 for the recognition of PGN, lipotechoic acid, and soluble tuberculosis factor.20 TLR9 is activated by bacterial DNA.21
The expression of antimicrobial peptides in different tissues of the eye, such as corneal epithelial cells and ciliary body cells has been documented.2,22 However, in corneal fibroblasts, which are the second cellular barrier of the cornea, the expression of antimicrobial peptides by bacterial stimulus has not been explored. In a previous study, we found that most TLRs are expressed in the primary culture of corneal fibroblasts (PCCF) and that LPS is able to induce overexpression of all TLRs in these cells.23 In this work, we studied in PCCF the antimicrobial peptide expression in response to LPS, PGN, and cytosine‐phosphorous‐guanine oligonucleotide (CpG‐ODN).
Methods
Animals
Five Balb/c and five NIH mice were used. All the mice were healthy, 4–6 weeks old, and did not present any ophthalmological alterations. The experimental protocols were performed in accordance with the ENCB‐IPN statements for the use of animals in research protocols.
PCCF and cellular stimulation assay
The corneal fibroblasts were obtained according to the methods of Berryhill et al.24 Briefly, Balb/c and NIH mice corneas were digested with 0.6U of dispase and 80U of collagenase solution (Invitrogen, Carlsbad, CA, USA) at 37°C for 1 hour, followed by 15 minutes of digestion with trypsin‐EDTA (Invitrogen, Carlsbad, CA, USA) at 37°C. After being washed with DMEM, the cells were resuspended in DMEM (150 000 cells/ml) containing 5% of FBS (Gibco, Rockwille, MD, USA), gentamicin, and fungizone. The corneal fibroblasts were cultured overnight in 25 cm2 flasks, the medium with non‐attached cells was discarded and the incubation was continued in a new medium which was changed every 3 days, until 90% confluence was achieved.
The Balb/c PCCF were treated with 10 μg/ml of LPS from Escherichia coli 0111:B4 (Sigma chemical, St Louis, MO, USA), 10 μg/ml of PGN from Staphylococcus aureus (Sigma Fluka, St Louis, MO, USA), 3 ng/ml of CpG‐ODN (TCC ATG ACG TTC CTG ACG TT; Invitrogen, Carlsbad CA, USA), or with 3 ng/ml of irrelevant oligonucleotide without CpG sequence (TCC AGG ACT TCT CTT CAG GTT; Invitrogen) according to the stimulus, then dissolved in fresh MEM medium for different times: 0, 3, 6, 12, 24, 48, and 72 hours (time‐response assay). For the dose‐response assay the PCCF were treated with different concentrations of LPS, PGN, and CpG‐ODN and incubated at the optimal time found in the time‐response assay. The total RNA of treated and non‐treated cells was obtained with Trizol reagent (Invitrogen) for analysis, through RT‐PCR, mRNA expression of: α‐defensin‐3 (AD‐3), α‐defensin‐4 (AD‐4), α‐defensin‐5 (AD‐5), β‐defensin‐1 (BD‐1), β‐defensin‐2 (BD‐2), β‐defensin‐3 (BD‐3), cathelin related antimicrobial peptide (CRAMP), TLR‐1, TLR‐2, TLR‐4, TLR‐6, and TLR‐9.
Blocking of cellular stimulation
For the blocking of cellular stimulation assay, the Balb/c PCCF were incubated with 2.5 µg, 5 µg, 10 µg, and 15 µg of anti‐TLR2 antibody (Santa Cruz Biotechnology, Inc Santa Cruz, CA, USA) for 1 hour before treatment with PGN. The cultures were incubated with the corresponding PAMP during the optimal time found in the time‐response assay. On the other hand, the NIH‐PCCF, which do not express TLR‐9,23 were treated with 3 ng/ml CpG‐ODN at different times.
RNA isolation and RT‐PCR analysis
Total RNA extraction was performed with Trizol reagent. Total RNA was treated with free RNAse‐DNAse I and RNA was re‐extracted. For the reverse transcriptase (RT) reaction, total RNA (3 μg) with 0.5 µg oligo‐(dT)15‐18 (Invitrogen) was denatured at 70°C for 10 minutes. Then, 1X single strand buffer, 0.5 mM DTT, 500 µM of each dNTP, and 200U of MMLV reverse transcriptase (Invitrogen) were added. The RT reactions were performed at 42°C for 1 hour. The polymerase chain reaction (PCR) reactions were performed with 1 µl of cDNA, 1X buffer, 1 mM MgCl2, 200 µM of each dNTP, and 0.2 µM of each antimicrobial peptide, TLRs, and β actin specific primers (table 1). Optimal PCR conditions were 30 cycles of 30 seconds at 92°C, 30 seconds at 60°C, and 30 seconds at 72°C.
Table 1 Primer sequence.
| Primer sequence | Amplified segment (pb) | |||
|---|---|---|---|---|
| β actin | Sense: 5′‐TGGAATCCTGTGGCATCCATGAAAC‐3′ | 324 | ||
| Anti‐sense: 5′‐TAAAACGCAGCTCAGTAACAGTCCG‐3′ | ||||
| TLR1 | Sense: 5′‐GGACTTCCACATGTCTCCACTATCC‐3′ | 596 | ||
| Anti‐sense: 5′‐TCCATGCTTGTTCTTCTCTGTGG‐3′ | ||||
| TLR2 | Sense: 5′‐GTGGTACCTGAGAATGATGTGGG‐3′ | 541 | ||
| Anti‐sense: 5′‐GTTAAGGAAGTCAGGAACTGGGTG‐3′ | ||||
| TLR4 | Sense: 5′‐CTGGGTGAGAAATGAGCTGG‐3′ | 249 | ||
| Anti‐sense: 5′‐GATACAATTCCACCTGCTGCC‐3′ | ||||
| TLR6 | Sense: 5′‐TTAACTGACCTTCCTGGGTGTGG‐3′ | 308 | ||
| Anti‐sense: 5′‐GCAGAACAGTATCACAGGACAGTGG‐3′ | ||||
| TLR9 | Sense: 5′‐GACTTACTGTTGGAGGTGCAGACC‐3′ | 313 | ||
| Anti‐sense: 5′‐GAACACCACGAAGGCATCATAGG‐3′ | ||||
| CRAMP | Sense 5′‐ ATGCAGTTCCAGAGGGACGTCC ‐3′ | 315 | ||
| Anti‐sense 5′‐ CTGCTCCGGCTGAGGTACAAG ‐3′ | ||||
| AD‐3 | Sense 5′‐ TCCTGCTGGCCTTCCAGGTCC ‐3′ | 236 | ||
| Anti‐sense 5′‐ TCAGCGACAGCAGAGTGT‐3′ | ||||
| AD‐4 | Sense 5′ TCCTGCTGGCCTTCCAGGTCC‐ ‐3′ | 231 | ||
| Anti‐sense 5′‐ TCAGCGGCGGGGGCAGCA ‐3′ | ||||
| AD‐5 | Sense 5′‐ TCCTGCTGGCCTTCCAGGTCC‐3′ | 258 | ||
| Anti‐sense 3′‐ TCAGCTACAGCAGAATACGAAAG ‐3′ | ||||
| BD‐1 | Sense 5′‐ CCCTGGCTGCCACCACTATGA ‐3′ | 239 | ||
| Anti‐sense 5′‐ CGCTCGTCCTTTATGTCCATTC ‐3′ | ||||
| BD‐2 | Sense 5′‐ ATGAGGACTCTCTGCTCTCTGCTG‐3′ | 243 | ||
| Anti‐sense 5′‐ TTTCATGACTTGCAACAGGGGT ‐3′ | ||||
| BD‐3 | Sense 5′‐ GCTTCAGTCATGAGGATCCATTACC‐3′ | 252 | ||
| Anti‐sense 5′‐ CCATCTTCATGGAGGAGCAAATTC‐3′ |
Results
PCCF treated with LPS
When the corneal fibroblasts were stimulated with LPS from E coli, we observed only the expression of the antimicrobial peptides BD‐1 (fig 1) and BD‐3 (data not shown) at all the times assayed. However, an overexpression of TLR‐4 at 6 hours and of tumour necrosis factor α (TNFα) at 12 hours was observed (fig 1).

Figure 1 RT‐PCR of antimicrobial peptides and TLRs of Balb/c PCCF stimulated with LPS (10 μg/ml) of Escherichia coli. Antimicrobial peptides BD‐2, BD‐3, AD‐3, AD‐4, AD‐5, and CRAMP were also assayed but they were not expressed. The experiment was done in duplicate.
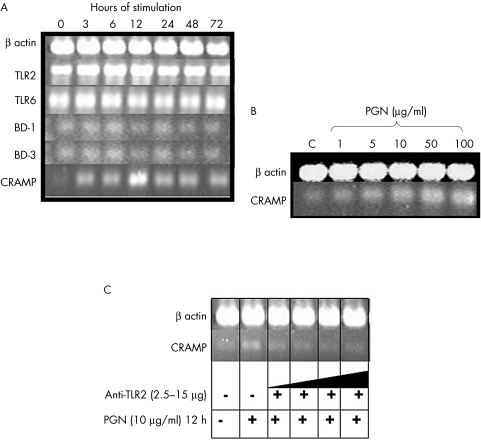
Overexpression of CRAMP in PCCF treated with PGN
When the corneal fibroblasts were stimulated with PGN from S aureus, we observed an overexpression of antimicrobial peptide CRAMP, which started at 3 hours and reached its highest expression at 12 hours after the stimulus (fig 2A). Expression of BD‐1 and BD‐3 was similar at all the times assayed. TLR‐2 was also overexpressed at 6 hours and 12 hours and the expression of TLR‐6 was similar at all the times (fig 2A). To corroborate this overexpression of CRAMP by PGN, we stimulated the cells with different concentrations of PGN at 12 hours and observed that the overexpression of CRAMP increased when the amount of PGN was increased (fig 2B). On the other hand, we observed that the anti‐TLR‐2 antibody blocked CRAMP expression when different amounts of this antibody were incubated before stimulating the cells with PGN (fig 2C). No expression of BD‐2, AD‐3, AD‐4, or AD‐5 were found.
Figure 2 RT‐PCR of antimicrobial peptides and TLRs of Balb/c PCCF stimulated with PNG of Staphylococcus aureus. (A) Time‐response assay with 10 µg/ml of PGN. TLR‐1 as well as the antimicrobial peptides BD‐2, AD‐3, AD‐4, and AD‐5 were also assayed but they were not expressed. (B) Dose‐response assay of CRAMP expression done at 12 hours of stimulation. (C) Blocking CRAMP expression assay done with 2.5 µg, 5 µg, 10 µg, and 15 µg of anti‐TLR2 antibody before stimulating with PGN (10 μg/ml) at 12 hours.
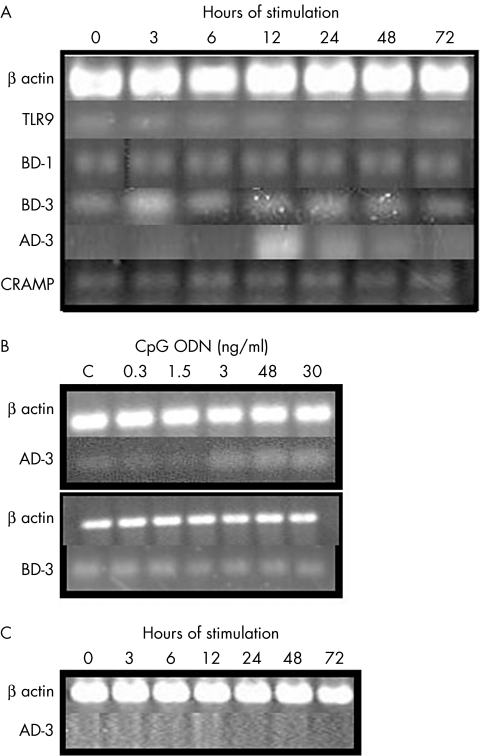
Expression of AD‐3 in Balb/c PCCF treated with CpG‐ODN
CpG‐ODN was also used to stimulate corneal fibroblasts in this study. We observed that CpG‐ODN induced expression of AD‐3 in the Balb/c corneal fibroblasts at 12 hours after the stimulus (fig 3A). The oligonucleotide without CpG sequence, used as control, did not induce AD‐3 expression (data not shown). Expression of TLR9 in the stimulated cells was similar at all the times. When we analysed the dose‐dependent response to AD‐3 expression in these cells, we observed that the expression of this antimicrobial peptide was augmented with the increase in the amount of CpG‐ODN (fig 3B). We also observed an overexpression of BD‐3 at 3 hours of stimulation, but the overexpression of BD‐3 did not increase in the dose‐response assay (fig 3B). To study the participation of TLR‐9 in the expression of AD‐3 induced by CpG‐ODN, we used PCCF from NIH mice (which do not express TLR‐923) because the anti‐TLR‐9 antibody is not available commercially. In figure 3C, we can observe that AD‐3 was not expressed in NIH corneal fibroblasts stimulated with CpG‐ODN at different times. CRAMP and BD‐1 were expressed at all the stimulation times (fig 3A), whereas the other antimicrobial peptides studied were not expressed in these cells.
Figure 3 RT‐PCR of antimicrobial peptides and TLRs of PCCF stimulated with CpG‐ODN. Time‐response assay with 3 ng/ml of CpG‐ODN in Balb/c (A) and NIH (C) PCCF. In Balb/c mice, the antimicrobial peptides BD‐2, AD‐4, and AD‐5 were also assayed but they were not expressed. (B) Dose‐response assay to AD‐3 and BD‐3 expression, done at 12 hours and 3 hours of stimulation, respectively.
Discussion
The expression of antimicrobial peptides at epithelial surfaces, such as eye, skin, lung, intestine, salivary system, and in some oral epithelia is thought to provide protection against infection.22,25,26,27,28
In mammals, several gene families encode peptides with antibacterial activity, such as the defensins and cathelicidins. The TLRs are molecules that also participate in innate immunity and can induce the expression of different effector molecules, such as cytokines, chemokines, and antimicrobial peptides.17
In a previous work, we reported that all TLRs were expressed in the whole healthy eye of Balb/c mice and that all the TLRs were overexpressed in the corneal fibroblasts treated with LPS from E coli.23 Similarly, it has been reported that the corneal epithelium has functional TLR‐2, TLR‐4, and TLR‐9 by stimulation with Pam(3)Cys, LPS, and CpG‐ODN, respectively.29
When an ocular infection or trauma occurs, the keratocytes and fibroblasts are the cells that has an important role in the repair of ocular damage. Therefore, in this work, we studied the expression of antimicrobial peptides in corneal fibroblasts stimulated with three bacterial components; LPS, PGN, and CpG‐ODN. Gottsch et al reported that the mRNA and the protein of defensins have not been detected in normal human corneal stroma, but they are readily detectable in the corneal stroma in cases of rejected transplants and post‐infectious keratitis.30 The LPS did not induce the overexpression of antimicrobial peptides in corneal fibroblasts, however BD‐1 was constitutively expressed. It has been reported that BD‐1 mRNA is constitutively expressed in the cornea.31,32 However, an overexpression of TLR4 and TNFα by LPS after 12 hours and 6 hours of treatment was observed, respectively, in these cells. It has been reported that TNFα and IL‐1α induce defensin mRNA in keratocytes after 18 hours of treatment.30
On the other hand in the human corneal epithelial cells, which are the first defence barrier of the cornea, both TLR‐2 and TLR‐4 have been detected intracellularly, but not at the cell surface, suggesting the possibility that these cells fail to elicit innate immune responses that contribute to an immunosilent environment at the ocular mucosal epithelium.33 However, Song et al reported that human corneal epithelial cells express CD14 and TLR‐4, and that these molecules are overexpressed after induction with Pseudomonas aeruginosa LPS.34 Furthermore, TLR‐2 expression has been upregulated in conjunctival epithelial cells by IFN‐γ and Staphylococcus aureus‐CWE.35 Real time PCR analysis revealed gene expression for TLRs 1‐7, 9, and 10 in human RPE cells and TLRs 2, 3, and 4 were increased by treatment with poly I:C or interferon γ.36
In this work, we also observed a time and dose dependent expression of the murine cathelicidin CRAMP in the corneal fibroblasts stimulated with PGN from S aureus. Furthermore, this expression occurred through the TLR‐2 pathway because TLR‐2 overexpression was observed at 6 hours after stimulation and the anti‐TLR‐2 antibody blocked CRAMP expression.
CRAMP is the homologous protein of human cathelicidin/LL‐37 and this is the sole identified murine cathelicidin. CRAMP has been shown to have both antimicrobial and angiogenic activities.37 CRAMP, like human cathelicidin/LL‐37, also exhibits a direct effect on the migration and function of leucocytes and is chemotactic for human monocytes, neutrophils, macrophages, and mouse peripheral blood leucocytes through a receptor for the formyl peptide receptor‐like‐2 (FPRL2).38 Thus, CRAMP functions as both a chemoattractant for phagocytic leucocytes and an enhancer of adaptive immune response. CRAMP can cause a rapid permeabilisation of the inner membrane of Escherichia coli39 and can impair the replication of an intracellular bacterial pathogen in macrophages.40 It has been suggested that members of this gene family can participate in host defence through their antimicrobial effects and activate mesenchymal cells during wound repair.
There are only two reports about the cathelicidin expression in the eye. One indicates that LL37/cathelicidin was detected frequently in samples of ocular surface epithelia41 and the other pointed out that mRNA for LL‐37 was absent in the nasolacrimal duct.42 In other tissues, such as adult testis, spleen, stomach, and intestine CRAMP expression has been detected by RT‐PCR.43
In this work, we also found that AD‐3 can be induced through the TLR pathway in corneal fibroblasts. The antimicrobial peptide AD‐3 was induced in a time and dose dependent fashion via TLR‐9 in Balb/c corneal fibroblasts treated with CpG‐ODN. There are few reports about the expression of α‐defensin in the eye. Human AD‐1, AD‐2, and AD‐3 (HNP‐1, HNP‐2, HNP‐3) are upregulated in the tear after surgery.44 Messenger RNAs of AD‐5 and AD‐6 are absent in the healthy nasolacrimal duct epithelium, in conjunctiva, cornea, and lacrimal gland,42 whereas AD‐1, AD‐2, and AD‐3 were detected in normal tears, lacrimal gland, and inflamed conjunctiva.2
The expression of antimicrobial peptides by the TLR‐PAMPs pathway has been demonstrated in other tissues different from the eye. Human primary uterine epithelial cells stimulated with the TLR‐3 agonist poly(I:C) expressed BD‐1 and BD‐2.45 LPS, PGN, and bacterial lipopeptide stimulated BD‐2 in a TLR‐4 and TLR‐2 dependent manner in intestinal epithelial cells, airway epithelial cells, and human tracheobronchial epithelial cells.14,19,26 We are reporting the expression of antimicrobial peptides by a TLR dependent pathway in corneal fibroblasts.
So far, antimicrobial peptides have mainly been reported in corneal epithelial cells, but this work was focused on the observation of the pattern of antimicrobial peptide expression in corneal fibroblasts stimulated with bacterial components. These cells, which are the second cellular barrier of the cornea, expressed few antimicrobial peptides, but CRAMP and AD‐3 increased their level of expression by PGN and CpG‐ODN, respectively, via TLR, and in a time and dose dependent manner. Our results suggest that corneal fibroblasts can also participate in the innate immune response of the eye.
Acknowledgements
This work was supported by CONACYT (grant 47424) and by the Instituto Politécnico Nacional (CGPI20050056). Sandra Rodríguez‐Martínez received scholarships from CONACYT and PIFI‐IPN for her graduate studies in CQB. Mario E Cancino‐Díaz is fellow of COFAA‐IPN, EDI‐IPN and SNI‐CONACYT. Juan C Cancino‐Díaz is fellow of EDI‐IPN and SNI‐CONACYT.
Abbreviations
AD‐3 - α‐defensin 3
BD‐1 - β‐defensin‐1
CpG‐ODN - cytosine‐phosphorous‐guanine oligonucleotide
CRAMP - cathelin related antimicrobial peptide
HNP - human neutrophil peptides
LPS - lipopolysaccharide
NF‐kB - nuclear factor kappa B
PAMPs - pathogen associated molecular patterns
PCCF - primary cultures of corneal fibroblast
PCR - polymerase chain reaction
PGNs - peptidoglycans
RT - reverse transcriptase
TIR - TLR/IL‐1 receptor
TLRs - toll‐like receptors
TNF - tumour necrosis factor
References
- 1.Streilein J W. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nature Rev Immunol 20033879–889. [DOI] [PubMed] [Google Scholar]
- 2.Haynes R J, Tighe P J, Dua S. Antimicrobial defensin peptides of the human ocular surface. Br J Ophthalmol 199983737–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganz T, Lehrer R I. Defensins. Pharmacol Ther 199566191–205. [DOI] [PubMed] [Google Scholar]
- 4.Schroder J M. Epithelial peptide antibiotics. Biochem Pharmacol 199957121–134. [DOI] [PubMed] [Google Scholar]
- 5.Bensch K W, Raida M, Magert H J.et al hBD‐1: a novel β‐defensin from human plasma. FEBS Lett 1995398331–335. [DOI] [PubMed] [Google Scholar]
- 6.Harder J, Bartels J, Christophers E.et al Isolation and characterization of human β‐defensin‐3, a novel human inducible peptide antibiotic. J Biol Chem 20002765707–5713. [DOI] [PubMed] [Google Scholar]
- 7.Garcia J R C, Krause A, Schulz S.et al Human beta defensin 4: a novel inducible peptide with a specific salt‐sensitive spectrum of antimicrobial activity. FASEB J 2001151819–1821. [PubMed] [Google Scholar]
- 8.Yamaguchi Y, Nagase T, Makita R.et al Identification of multiple novel epididymis‐specific β‐defensin isoforms in humans and mice. J Immunol 20021692516–2523. [DOI] [PubMed] [Google Scholar]
- 9.Yu K, Park K, Kang S W.et al Solution structure of a cathelicidin‐derived antimicrobial peptide, CRAMP as determined by NMR spectroscopy. J Pept Res 2002601–9. [DOI] [PubMed] [Google Scholar]
- 10.Zanetti M, Gennaro R, Romeo D. Cathelicidins: a novel protein family with a common proregion and a variable C‐terminal antimicrobial domain. FEBS lett 19953741–5. [DOI] [PubMed] [Google Scholar]
- 11.Gudmundsson G H, Agerberth B, Odeberg J.et al The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL‐37 in granulocytes. Eur J Biochem 1996238325–332. [DOI] [PubMed] [Google Scholar]
- 12.Cowland J B, Johnsen A H, Borregard N. hCAP‐18, a cathelin/pro‐bactenecin‐like protein of human neutrophil specific granules. FEBS lett 1995368173–176. [DOI] [PubMed] [Google Scholar]
- 13.Frohm M, Gunne H, Bergman A C.et al Biochemical and antibacterial analysis of human wound and blister fluid. Eur J Biochem 199623786–92. [DOI] [PubMed] [Google Scholar]
- 14.Vora P, Youdim A, Thomas L S.et al Beta‐defensin‐2 expression is regulated by TLR signaling in intestinal epithelial cells. J Immunol 20041735398–5405. [DOI] [PubMed] [Google Scholar]
- 15.Biragyn A, Ruffini P A, Leifer C A.et al Toll‐like receptor 4‐dependent activation of dendritic cells by beta‐defensin 2. Science 20022981025–1029. [DOI] [PubMed] [Google Scholar]
- 16.Rock F L, Hardman G, Timans J C.et al A family of human receptors structurally related to Drosophila toll. Proc Natl Acad Sci USA 199895588–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medzhitov R. Toll like receptors and innate immunity. Nature Rev Immunol 20011135–145. [DOI] [PubMed] [Google Scholar]
- 18.Medzhitov R, Preston‐Hurlburt P, Kopp E.et al MyD88 is an adaptor protein in the hToll/IL‐1 signaling. Science 19972791612–1615. [Google Scholar]
- 19.Becker M N, Diamond G, Verghese M W.et al CD14‐dependent lipopolysaccharide‐induced beta‐defensin‐2 expression in human tracheobronchial epithelium. J Biol Chem 200027529731–29736. [DOI] [PubMed] [Google Scholar]
- 20.Hajjar A M, O'Mahony D S, Ozinsky A.et al Cutting edge: functional interactions between toll‐like receptor (TLR) 2 and TLR1 or TLR6 in response to phenol‐soluble modulin. J Immunol 200116615–19. [DOI] [PubMed] [Google Scholar]
- 21.Hemmi H, Takeuchi O, Kawai T.et al A toll‐like receptor recognizes bacterial DNA. Nature 2000408740–745. [DOI] [PubMed] [Google Scholar]
- 22.Haynes R J, McElveen J E, Dua H S.et al Expression of human beta‐defensins in intraocular tissues. Invest Ophthalmol Vis Sci 2000413026–3031. [PubMed] [Google Scholar]
- 23.Rodriguez‐Martinez S, Cancino‐Diaz M E, Jimenez‐Zamudio L.et al TLRs and NODs mRNA expression pattern in healthy mouse eye. Br J Ophthalmol 200589904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berryhill B L, Kader R, Kane B.et al Partial restoration of the keratocyte phenotype to bovine keratocytes made fibroblastic by serum. Invest Ophthalmol Vis Sci 2002433416–3421. [PubMed] [Google Scholar]
- 25.Sayama K, Komatsuzawa H, Yamasaki K.et al New mechanisms of skin innate immunity: ASK1‐mediated keratinocyte differentiation regulates the expression of beta‐defensins, LL37, and TLR2. Eur J Immunol 2005351886–1895. [DOI] [PubMed] [Google Scholar]
- 26.Hertz C J, Wu Q, Porter E M.et al Activation of toll‐like receptor 2 on human tracheobronchial epithelial cells induces the antimicrobial peptide human beta defensin‐2. J Immunol 20031716820–6826. [DOI] [PubMed] [Google Scholar]
- 27.Vora P, Youdim A, Thomas L S.et al Beta‐defensin‐2 expression is regulated by TLR signaling in intestinal epithelial cells. J Immunol 20041735398–5405. [DOI] [PubMed] [Google Scholar]
- 28.Murakami M, Ohtake T, Dorschner R A.et al Cathelicidin antimicrobial peptides are expressed in salivary glands and saliva. J Dent Res 200281845–850. [DOI] [PubMed] [Google Scholar]
- 29.Johnson A C, Heinzel F P, Diaconu E.et al Activation of toll‐like receptor (TLR)2, TLR4, and TLR9 in the mammalian cornea induces MyD88‐dependent corneal inflammation. Invest Ophthalmol Vis Sci 200546589–595. [DOI] [PubMed] [Google Scholar]
- 30.Gottsch J D, Li Q, Ashraf M F.et al Defensin gene expression in the cornea. Curr Eye Res 1998171082–1086. [DOI] [PubMed] [Google Scholar]
- 31.Ikeda A, Sakimoto T, Shoji J.et al Expression of alpha‐ and beta‐defensins in human ocular surface tissue. Jpn J Ophthalmol 20054973–78. [DOI] [PubMed] [Google Scholar]
- 32.Shin J S, Kim C W, Kwon Y S.et al Human beta‐defensin 2 is induced by interleukin‐1beta in the corneal epithelial cells. Exp Mol Med 200436204–210. [DOI] [PubMed] [Google Scholar]
- 33.Ueta M, Nochi T, Jang M H.et al Intracellularly expressed TLR2s and TLR4s contribution to an immunosilent environment at the ocular mucosal epithelium. J Immunol 20041733337–3347. [DOI] [PubMed] [Google Scholar]
- 34.Song P I, Abraham T A, Park Y.et al The expression of functional LPS receptor proteins CD14 and toll‐like receptor 4 in human corneal cells. Invest Ophthalmol Vis Sci 2001422867–2877. [PubMed] [Google Scholar]
- 35.Cook E B, Stahl J L, Esnault S.et al Toll‐like receptor 2 expression on human conjunctival epithelial cells: a pathway for Staphylococcus aureus involvement in chronic ocular proinflammatory responses. Ann Allergy Asthma Immunol 200594486–497. [DOI] [PubMed] [Google Scholar]
- 36.Kumar M V, Nagineni C N, Chin M S.et al Innate immunity in the retina: toll‐like receptor (TLR) signaling in human retinal pigment epithelial cells. J Neuroimmunol 20041537–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koczulla R, von Degenfeld G, Kupatt C.et al An angiogenic role for the human peptide antibiotic LL‐37/hCAP‐18. J Clin Invest 20031111665–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurosaka K, Chen Q, Yarovinsky F.et al Mouse cathelin‐related antimicrobial peptide chemoattracts leukocytes using formyl peptide receptor‐like 1/mouse formyl peptide receptor‐like 2 as the receptor and acts as an immune adjuvant. J Immunol 20051746257–6265. [DOI] [PubMed] [Google Scholar]
- 39.Kang S W, Lee D G, Yang S T.et al CRAMP analog having potent antibiotic activity without hemolytic activity. Protein Pept Lett 20029275–282. [DOI] [PubMed] [Google Scholar]
- 40.Rosenberger C M, Gallo R L, Finlay B B. Interplay between antibacterial effectors: a macrophage antimicrobial peptide impairs intracellular Salmonella replication. Proc Natl Acad Sci USA 20041012422–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McIntosh R S, Cade J E, Al‐Abed M.et al The spectrum of antimicrobial peptide expression at the ocular surface. Invest Ophthalmol Vis Sci 2005461379–1385. [DOI] [PubMed] [Google Scholar]
- 42.Paulsen F P, Pufe T, Schaudig U.et al Detection of natural peptide antibiotics in human nasolacrimal ducts. Invest Ophthalmol Vis Sci 2001422157–2163. [PubMed] [Google Scholar]
- 43.Gallo R L, Kim K J, Bernfield M.et al Identification of CRAMP, a cathelin‐related antimicrobial peptide expressed in the embryonic and adult mouse. J Biol Chem 199727213088–13093. [DOI] [PubMed] [Google Scholar]
- 44.Zhou L, Huang L Q, Beuerman R W.et al Proteomic analysis of human tears: defensin expression after ocular surface surgery. J Proteome Res 20043410–416. [DOI] [PubMed] [Google Scholar]
- 45.Schaefer T M, Fahey J V, Wright J A.et al Innate immunity in the human female reproductive tract: antiviral response of uterine epithelial cells to the TLR3 agonist poly(I:C). J Immunol 2005174992–1002. [DOI] [PubMed] [Google Scholar]