Abstract
Aim
To determine the acute and chronic vascular effects of endoscopic cyclophotocoagulation (ECP) versus trans‐scleral cyclophotocoagulation (TCP) in a rabbit model.
Methods
20 rabbits underwent ECP in one eye and another 20 rabbits had unilateral TCP. Five treated eyes from each group underwent endoscopic fluorescein angiography (EFA) of the treated ciliary processes at each of the following time points: immediate, 1 day, 1 week, and 1 month. Five untreated rabbits were used as controls. The NIH Image software program was used to trace ciliary processes in order to determine their mean intensity, as a measure of their perfusion. Histopathology was also performed on eyes from each time point.
Results
Immediately and 1 day after laser, both TCP and ECP eyes demonstrated severely reduced or non‐existent blood flow in the areas of treatment. TCP treated processes essentially remained non‐perfused at the 1 week and 1 month time points. ECP treated processes showed some reperfusion at 1 week and greater reperfusion by 1 month. Histopathology confirmed the overall greater vascular occlusion seen with TCP.
Conclusions
Chronic poor perfusion of the ciliary body after TCP may account, in part, for its efficacy, as well as the significant complications including hypotony and phthisis. Late reperfusion of this region after ECP may provide some insight into the differences in efficacy and complication rates compared to TCP.
Keywords: endoscopic cyclophotocoagulation, trans‐scleral cyclophotocoagulation, glaucoma laser surgery, ciliary process histology, rabbit model
Endoscopic cyclophotocoagulation (ECP) is the laser treatment of ciliary processes within the eye using direct endoscopic visualisation. It has been used for the treatment of refractory glaucomas1,2,3,4,5,6 and as an adjunct procedure at the time of cataract extraction.2,4 The purpose of this study was to determine the effect of ECP using a diode laser on the vascular perfusion of the ciliary body, compared to diode laser trans‐scleral cyclophotocoagulation (TCP). It has been suggested that ECP is a more selective procedure that is less destructive to the ciliary processes as well as to adjacent tissues.1,2,3
Contact TCP with the diode laser has become an increasingly popular technique for treating refractory glaucoma.7,8,9,10 This is due, in part, to the compact design of the laser unit and the relative ease of use of the procedure. It has been shown in a prospective, randomised comparative trial that diode has equivalent efficacy and safety profiles as neodymium:yttrium‐aluminium‐garnet (Nd:YAG) laser.10 However, TCP by both types of laser has significant potential side effects.7,8,9,10,11,12,13,14,15,16,17,18
Clinical series examining the use of ECP suggest that this approach may have a lower incidence of serious complications, although there are no currently published reports comparing the two procedures in a prospective fashion. In the largest published series on diode ECP,3 success in controlling intraocular pressure (IOP) (defined as <22 mm Hg) was 90% at last follow up. There were no cases of phthisis or chronic hypotony among the 68 eyes treated with ECP, and the rate of severe vision loss (defined as loss of ⩾2 Snellen lines of acuity) was 6%. However, case series involving only paediatric eyes have reported cases of retinal detachment, hypotony, and choroidal effusion.5
Differences in rates and spectrums of complications between the two laser treatments may be further predicted by a better understanding of their histological and vascular effects. The potentially lower rates of devastating complications with ECP may be related to a lesser degree of vascular destruction within the ciliary processes and adjacent structures. We have developed an animal model for performing endoscopic fluorescein angiography (EFA) to study the in vivo integrity of the vascular supply to the ciliary processes. Ciliary perfusion has not been previously studied in an animal model to compare the effects of TCP versus ECP on blood flow. In this study, we compared the effects of TCP and ECP on vascular perfusion immediately, 1 day, 1 week, and 1 month after the procedure. In addition, we evaluated the histological effects at these time points.
Methods
A total of 45 Dutch belted pigmented rabbits were used in this study. This project was approved by the committee on animal research (CAR) at the University of California, San Francisco (UCSF). All experiments were conducted according to the Declaration of Helsinki and conformed to the ARVO statement for the use of animals in ophthalmic and vision research. Five rabbits served as controls for EFA and histopathology. Twenty rabbits underwent ECP in one eye and the remaining 20 rabbits received unilateral diode TCP. Approximately 100 degrees of ciliary processes were treated by ECP or TCP. Five rabbits in each treatment group were assessed by EFA and enucleated at the following time points: immediate, 1 day, 1 week, and 1 month.
Anaesthesia, antisepsis, IOP measurement, and postoperative care
Preoperatively, rabbits were sedated and anaesthetised with ketamine 35–50 mg/kg and xylazine 5–10 mg/kg intramuscularly. Topical oxybuprocaine (proparacaine) 0.5% eye drops were also applied to the operated eye. ECP treated eyes were also prepared with povidone‐iodine solution (0.5%) and covered with sterile drapes. Postoperatively, topical neomycin/polymyxin ointment and atropine 1% eye drops were applied.
IOP was checked by pneumotonometry (Mentor Medical, Inc, NJ, USA) within 30 minutes of anaesthesia administration at pre‐cyclophotocoagulation and at the time points when the rabbits were sacrificed.
Diode TCP
The diode laser (Iris Oculight SLx (Iris Medical, Inc, Mountain View, CA, USA)), fitted with the G‐probe, was used for TCP. The duration was set at 2.0 seconds. Laser power was titrated by increasing in 20 mW increments until a “pop” was noted and then reduced by 10–20 mW to avoid excessive tissue destruction. The range of power was 500–750 mW.19 The laser tip of the G‐probe was placed at the limbus since the pigmented rabbit's ciliary processes are located more anteriorly than in the human.20,21,22 Seven to eight shots were applied over the 100 degrees of limbus.
ECP
After creation of a bevelled incision into the anterior chamber with a 2.5 mm keratome, viscoelastic (Healon 5, AMO, Inc, Irvine, CA, USA) was injected to deepen the anterior chamber and posterior sulcus for visualisation and treatment. The 18 gauge endoscopic probe was inserted and treatment was carried out over 100 degrees.
The endoscopic diode laser unit (Endo Optiks 2, Little Silver, NJ, USA) incorporates a diode laser that emits pulsed continuous wave energy at 810 nm, a 175 W xenon light source, a helium‐neon laser aiming beam, and video camera imaging that can be recorded. Laser application was continuous, at 200–500 mW, to achieve whitening and shrinkage of the ciliary processes. Care was taken to avoid overtreatment, which would result in bubble formation. After completion of the ECP, viscoelastic was removed from the anterior chamber through Simcoe irrigation and aspiration. The wound was closed with one or two single 10‐0 nylon sutures, and the anterior chamber was refilled with balanced salt solution. There were no cases of flat or shallow anterior chamber noted during follow up examinations.
EFA
The ECP laser unit was fitted with a blue glass filter at the light source (excitation stimulus for fluorescein) and a yellow filter at the camera source (emission band for fluorescein). Five rabbits in each laser treatment group were examined for ciliary body perfusion at each study time point. Intraocular EFA was performed by inserting the endoscopic probe into the eye and recording the fluorescence from the ciliary processes, 5–10 seconds after fluorescein injection. Fluorescein at a concentration of 10% was injected into the ear vein of the rabbit. Video recording from the ECP laser unit was onto digital tape using a Sony digital video camcorder (model DCR TRV20, one megapixel).
The fluorescein angiography for the control and treatment eyes was interpreted in terms of the area and degree of perfusion. A single frame from each eye at approximately 10 seconds after fluorescein injection was selected for assessment. Grading was by a single masked observer and by computerised pixel intensity calculation, using the NIH‐Image software to trace around each ciliary process. The program can determine the average pixel intensity over the area within each tracing. Background autofluorescence was accounted for by subtracting the value obtained from transposition of the above tracing to an image of the same area that was taken before fluorescein injection.
In ECP eyes, the endoscopic probe was directed towards the ciliary processes within the central portion of the 100 degree area of treatment. In TCP eyes, the probe was directed towards processes in the area of direct TCP treatment, which could be visualised externally as a darkening of the scleral limbus. The probe was positioned such that the three to four ciliary processes were within the central portion of the 100 degrees of treatment. The number of ciliary processes in a Dutch belted rabbit is approximately 21 for 100 degrees of the circumference.23 Three to four processes were chosen within the viewing area since the typical distance from the processes used during treatment allows for visualisation of three to four processes in the field. If a larger field of view is used, the ciliary processes become too small to adequately delineate their borders.
Histopathology
At the end of the follow up period for each rabbit, euthanasia was performed via an intracardiac injection of sodium pentobarbital (200 mg/kg), and the treated eye was enucleated and placed within 2% formaldehyde. Eyes were dehydrated in successively increasing concentrations of alcohol and embedded within paraffin for sectioning. Sections through treated areas were stained with haematoxylin and eosin, and examined for change.
Results
The mean IOPs at baseline and each assessment time point are summarised in table 1. The mean reduction at day 1 was statistically significant for both TCP and ECP. Although the pressures at week 1 and month 1 suggested a general trend towards reduction, IOPs in treated eyes were not significantly different when compared to baseline with both types of lasers.
Table 1 Intraocular pressures (IOP) prelaser and post‐laser treatment among eyes treated by endoscopic cyclophotocoagulation (ECP) and trans‐scleral cyclophotocoagulation (TCP).
| Laser procedure | IOP pre‐laser (mm Hg) | IOP post‐laser (mm Hg) | IOP reduction (mm Hg) | p Value | |
|---|---|---|---|---|---|
| ECP | |||||
| 1 day | 24.7 (3.9) | 12.0 (3.5) | 12.7 | 0.007 | |
| 1 week | 24.9 (3.0) | 20.4 (9.5) | 4.5 | 0.607 | |
| 1 month | 25.9 (3.4) | 21.6 (4.7) | 4.3 | 0.124 | |
| TCP | |||||
| 1 day | 26.3 (2.3) | 13.6 (3.2) | 12.7 | 0.004 | |
| 1 week | 28.2 (2.8) | 25.0 (4.0) | 3.2 | 0.373 | |
| 1 month | 26.4 (2.7) | 23.1 (4.2) | 3.3 | 0.887 |
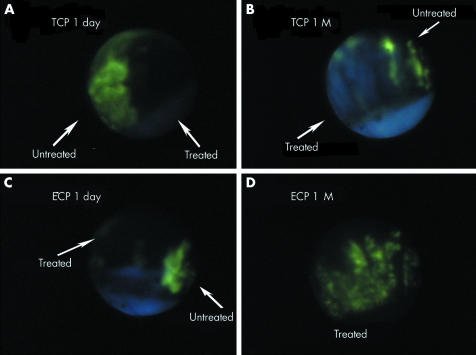
Normal, untreated ciliary processes demonstrated fluorescence within 10 seconds of fluorescein injection (fig 1). Both TCP and ECP treatment resulted in severely reduced or nonexistent blood flow at the immediate and 1 day time points (fig 2A and C). The ciliary tissues of TCP eyes exhibited areas of central hypofluorescence with surrounding zones of hyperfluorescence, corresponding to focal points of laser and adjacent “skip areas” between treatment spots. The areas of hypofluorescence were shaped irregularly over the iris root, ciliary processes, and the pars plana, demonstrating an extensive target area from external treatment. In comparison, ECP eyes showed continuous areas of dense hypofluorescence over the ciliary tissues (fig 2C). At 1 week, both TCP and ECP eyes had similar angiographic findings as the immediate and 1 day eyes. At 1 month, TCP eyes showed persistent sites of marked hypofluorescence, which corresponded to sites of laser treatment (fig 2B). By contrast, ECP eyes demonstrated partial reperfusion of the ciliary processes that was uniform but of lower intensity when compared to adjacent untreated tissue (fig 2D).
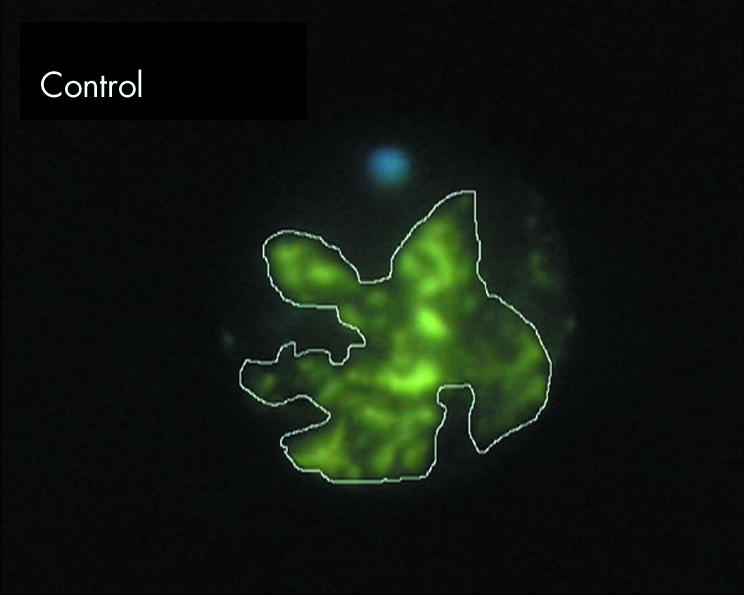
Figure 1 Endoscopic fluorescein angiography of untreated ciliary processes, 10 seconds after intravenous injection of 10% fluorescein. NIH Image software was used to trace around ciliary processes (seen as white line tracing) to determine the mean intensity of pixels within the tracings. In table 2, the baseline autofluorescence values were subtracted.
Figure 2 Endoscopic fluorescein angiography of the ciliary processes in eyes treated by cyclophotocoagulation. (A) Trans‐scleral cyclophotocoagulation (TCP) treated eye, 1 day. Junction between treated and untreated processes, with normal fluorescence in the untreated area and almost complete non‐perfusion in the treated area. (B) TCP treated eye, 1 month (M). Junction showing white, non‐perfused processes adjacent to “skip” area of non‐treatment. (C) Endoscopic cyclophotocoagulation (ECP) treated eye, 1 day. Junction of treated area and untreated shows non‐perfusion in treated portion of ciliary processes. (D) ECP treated eye, 1 month (M). Treated processes demonstrate partial reperfusion.
The average intensity of the fluorescence over the area analysed on EFA was determined using NIH Image software. A sample tracing of ciliary processes is shown in figure 1. The mean pixel intensity was reduced to less than half of controls in both TCP and ECP groups at the acute and 1 day time points (table 2). At 1 month, the treatment spots in TCP eyes remained severely underperfused with a similarly depressed intensity of fluorescence (36% of control levels), whereas the ECP eyes showed some degree of reperfusion, with the intensity increased to 80% of control levels. It should be noted that scarred, white ciliary processes exhibited a baseline autofluorescence, which accounted for a substantial amount of intensity in tissues that had very minimal subjective fluorescein perfusion. This autofluorescence is similar to that observed clinically from optic nerve drusen, in which the white autofluorescence includes the wavelengths which pass through the yellow detection filter for fluorescein. It was controlled for by subtracting the autofluorescence values from the measured values after fluorescein injection, as described in the Methods section.
Table 2 Fluorescence intensity of ciliary process with endoscopic fluorescein angiography, determined using NIH Image software.
| Mean | SD | |
|---|---|---|
| Control | 56.00 | 1.61 |
| ECP | ||
| Acute | 20.63 | 12.35 |
| 1 day | 12.58 | 2.26 |
| 1 week | 16.44 | 5.46 |
| 1 month | 44.98 | 9.42 |
| TCP | ||
| Acute | 24.16 | 15.51 |
| 1 day | 24.81 | 7.64 |
| 1 week | 22.83 | 8.47 |
| 1 month | 20.33 | 7.62 |
A sample tracing of ciliary processes is shown in figure 1. As noted in text, background autofluorescence were subtracted.
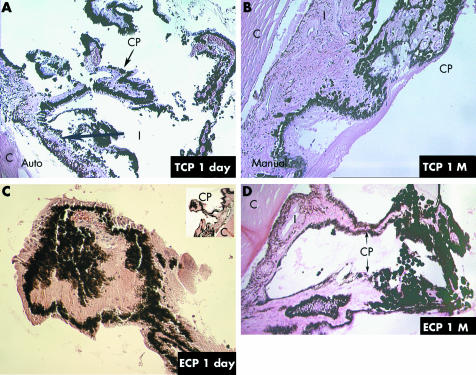
Histopathology of the ECP treated eyes showed substantial shrinkage, coagulation necrosis, and disorganisation of the architecture of the ciliary processes at 1 day, with loss of epithelium and shrinkage and avascularity of the stroma (fig 4C). For comparison, a histological section from an untreated eye is provided in figure 3. By 1 month after ECP, deeper ciliary vessels appeared patent, consistent with the physiological reperfusion seen with EFA (fig 4D). At 1 day post‐laser, TCP treated eyes demonstrated severe ciliary tissue damage, closure of many of the large and small vessels, and vascular stasis within the remaining vessels (fig 4A). In addition, adjacent anatomical structures such as the pars plana demonstrated damage and disorganisation, especially in histological sections through areas in which the laser probe was not well centred over the ciliary tissue (not shown). Examination of the gross anatomy of the ciliary processes at day 1 also supported the anatomical variability of the coagulation necrosis from TCP, whereas the whitening of the processes after ECP appeared more consistent across the area of treatment. By 1 month, processes showed sclerosis of the stromal tissue and absence of normal epithelial architecture with a continuing exudative response (fig 4B).
Figure 4 (A) Histology of trans‐scleral cyclophotocoagulation (TCP) treated eye, 1 day. Severe disruption of individual processes can be observed. Pigment and cellular dispersion is evident (40× magnification). (B) Histology of TCP treated eye, 1 month (M). The ciliary processes demonstrate atrophy and collapse. Normal epithelial layering is disrupted and an exudative membrane covers much of the pars plana and pars plicata (40× magnification). (C) Histology of endoscopic cyclophotocoagulation (ECP) treated eye, 1 day. Ciliary processes appear shrunken, with disruption of the stroma and epithelial layers (100× magnification with lower magnification shown in upper right corner). (D) Histology of ECP treated eye, 1 month (M). Ciliary processes are disorganised, disrupted, and scarred, with pigment concentration to the right. Relatively normal process with patent vessels is seen to the left (40× magnification).
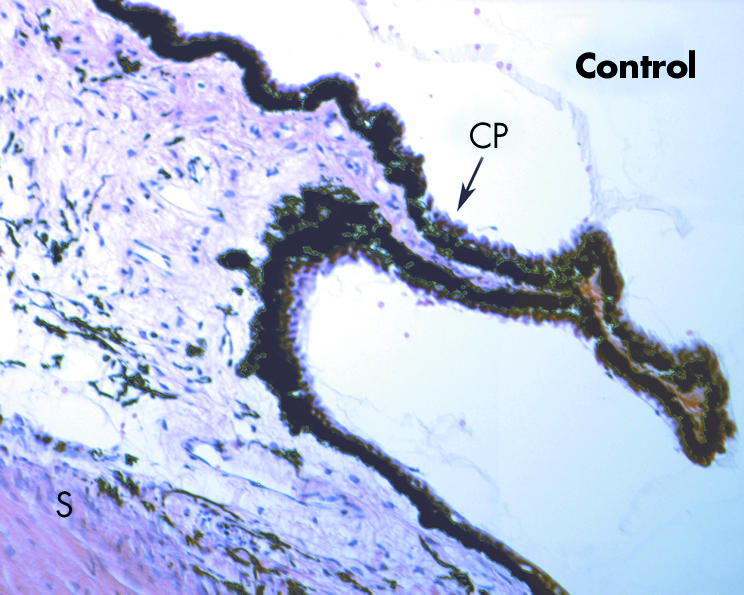
Figure 3 Histology of the ciliary region from a normal, untreated eye. Small capillary‐like vessels can be observed in the stroma of the ciliary process, and larger vessels are present in the deeper stroma (100× magnification).
Discussion
This is the first report to document the vascular perfusion of the ciliary processes after trans‐scleral and endoscopic cyclophotocoagulation in an animal model. A previous evaluation of perfusion in human patients has been performed,24 although treatment parameters were not standardised in a prospective manner. In the current rabbit model, cyclophotocoagulation by both approaches produced substantial early non‐perfusion of the pars plicata. However, perfusion significantly increased by 1 month after ECP but remained low with TCP at the same time point.
The chronic non‐perfusion after TCP may have relevance to the clinical efficacy of this procedure as well as the associated risks for hypotony and phthisis. Numerous clinical studies have documented the effectiveness of TCP in the treatment of refractory glaucomas.7,8,9,10,11,12,13,14 These effects can be long term although sometimes require re‐treatment.7,8,9,10,11,12,13,14 The ability to achieve near complete or complete shutdown of aqueous production may lead to greater overall efficacy, as well as an increased risk for hypotony and/or phthisis.
Another contributing mechanism by which cyclodestruction may achieve reduction in aqueous flow is by injury to the ciliary epithelium. In our study, histological sections through treated ciliary processes showed coagulation necrosis of the pigmented and non‐pigmented epithelia after both ECP and TCP. Previous studies have also shown significant damage to the epithelium with both trans‐scleral and endophotocoagulation,25,26 as well as the ability for regeneration after cyclophotocoagulation.27 It may be that both types of cyclophotocoagulation lead to reduced aqueous secretion by the epithelium, but TCP provides further aqueous suppression by chronically inhibiting vascular perfusion. In addition, this greater capacity to shut down aqueous production may contribute to an increased potential for hypotony.
With regard to hypotony and phthisis, other mechanisms may contribute to chronically low pressures and inadequate ocular nutrition. For example, cyclitic membrane formation may lead to late ciliary body detachment and further aqueous suppression. In our study, proteinaceous and fibrinous accumulation was observed surrounding the ciliary processes of TCP eyes, suggesting possible later formation of a cyclitic membrane. Clinically, hypotony may occur after both TCP7,8,9,10,11,12,13,14 and ECP,5 though clinical series suggest possibly higher rates with TCP,1,2,3,4,5,6,7,8,9,10,11,12,13,14 particularly in adults.
In the present study, the overall IOP reductions at the week 1 and month 1 time points were not statistically significant with either ECP or TCP. The most likely explanation is that only 100 degrees of ciliary processes were treated, in order to observe the vascular and histopathological effects. In the clinical situation, usually 180 degrees or more are treated. Moreover, ECP is often performed for up to 360 degrees.3
One of the major limitations of our study is the difference in ocular anatomy between humans and rabbits. In the pigmented Dutch belted rabbit eye, the ciliary processes extend into the posterior portion of the peripheral iris. As noted in the Methods section, treatment with TCP was at the limbal area, resulting in laser energy affecting the iris root and peripheral iris. Although the area of treatment is shifted anteriorly, there should not be a substantial difference in the major finding that TCP results in chronic reduction of vascular perfusion. Another anatomical difference is that the rabbit sclera is thinner than the human sclera, potentially biasing the treatment effect to appear greater in the rabbit (that is, overtreatment) compared with the human eye. However, overtreatment was avoided by titrating the energy applied to a level below the “pop”—an indicator of overtreatment.
In summary, we have found in a rabbit model that TCP is associated with persistent poor perfusion of the ciliary processes and ECP results in early reduction of perfusion with later reperfusion. In our study, we avoided overtreatment by using considerably lower treatment levels than in humans, to allow for the thin sclera. Finally, these results may serve as a basis for further investigation, perhaps in a primate model, and be of use to those who are exploring improved techniques for cyclodestruction, such as photodynamic therapy of ciliary processes.28
Acknowledgements
The authors thank the following individuals for their assistance with the research: Robin Troyer, Hiliary Frigillana, and Jeanne Koelling.
Abbreviations
ECP - endoscopic cyclophotocoagulation
EFA - endoscopic fluorescein angiography
IOP - intraocular pressure
TCP - trans‐scleral cyclophotocoagulation
Footnotes
Grant support: That Man May See, Inc, San Francisco, CA; Research to Prevent Blindness, Los Angeles, CA, USA.
Competing interests: SL has received honoraria for speakerships for Medtronics, Inc.
The authors do not have a financial/proprietary interest in any of the devices, equipment, instruments, or drugs discussed in this article.
The results of this study have been presented at the Association for Research in Vision and Ophthalmology (ARVO) meeting in Fort Lauderdale, FL, USA, May, 2002.
References
- 1.Uram M. Ophthalmic laser microendoscope ciliary process ablation in the management of neovascular glaucoma. Ophthalmology 1992991823–1828. [DOI] [PubMed] [Google Scholar]
- 2.Uram M. Combined phacoemulsification, endoscopic ciliary process photocoagulation, and intraocular lens implantation in glaucoma management. Ophthalmic Surg 199526346–352. [PubMed] [Google Scholar]
- 3.Chen J, Cohn R A, Lin S C.et al Endoscopic photocoagulation of the ciliary body for treatment of refractory glaucomas. Am J Ophthalmol 1997124787–796. [DOI] [PubMed] [Google Scholar]
- 4.Gayton J L, Van De Karr M, Sanders V. Combined cataract and glaucoma surgery: trabeculectomy versus endoscopic laser cycloablation. J Cataract Refract Surg 1999251214–1219. [DOI] [PubMed] [Google Scholar]
- 5.Neely D E, Plager D A. Endocyclophotocoagulation for management of difficult pediatric glaucomas. J AAPOS 20015221–229. [DOI] [PubMed] [Google Scholar]
- 6.Lin S C, Chen J, Hwang D G.et al Endoscopic cyclophotocoagulation for the treatment of glaucoma in keratoplasty patients [abstract]. Invest Ophthalmol Vis Sci 1998392157B14 [Google Scholar]
- 7.Kosoko O, Gaasterland D E, Pollack I P.et al Long‐term outcome of initial ciliary ablation with contact diode laser transscleral cyclophotocoagulation for severe glaucoma. The Diode Laser Ciliary Ablation Study Group. Ophthalmology 19961031294–1302. [DOI] [PubMed] [Google Scholar]
- 8.Bloom P A, Tsai J C, Sharma K.et al “Cyclodiode”: transscleral diode laser cyclophotocoagulation in the treatment of advanced refractory glaucoma. Ophthalmology 19971041508–1520. [DOI] [PubMed] [Google Scholar]
- 9.Spencer A F, Vernon S A. “Cyclodiode”: results of a standard protocol. Br J Ophthalmol 199983311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Youn J, Cox T A, Herndon L W.et al A clinical comparison of transscleral cyclophotocoagulation with neodymium:YAG and semiconductor diode lasers. Am J Ophthalmol 1998126640–647. [DOI] [PubMed] [Google Scholar]
- 11.Wright M M, Grajewski A I, Feuer W J. Nd:YAG cyclophotocoagulation: outcome of treatment for uncontrolled glaucoma. Ophthalmic Surg 199122279–283. [PubMed] [Google Scholar]
- 12.Brancato R, Giovanni L, Trabucchi G.et al Contact transscleral cyclophotocoagulation with Nd:YAG laser in uncontrolled glaucoma. Ophthalmic Surg 198920547–551. [PubMed] [Google Scholar]
- 13.Schuman J S, Puliafito C A, Allingham R R.et al Contact transscleral continuous wave neodymium:YAG laser cyclophotocoagulation. Ophthalmology 199097571–580. [DOI] [PubMed] [Google Scholar]
- 14.Schuman J S, Bellows A R, Shingleton B J.et al Contact transscleral Nd:YAG laser cyclophotocoagulation: midterm results. Ophthalmology. 1992;99: 1089–94; discussion, 1095 [DOI] [PubMed]
- 15.Edward D P, Brown S V L, Higginbothom E.et al Sympathetic ophthalmia following neodymium:YAG cyclotherapy. Ophthalmic Surg 198920544–546. [PubMed] [Google Scholar]
- 16.Lam S. Tessler HH, Lam BL, et al. High incidence of sympathetic ophthalmia after contact and noncontact neodymium: YAG cyclotherapy, Ophthalmology 1992991818–1822. [DOI] [PubMed] [Google Scholar]
- 17.Bechrakis N E, Müller‐Stolzenurg N W, Helbig et al Sympathetic ophthalmia following laser cyclophotocoagulation. Arch Ophthalmol 19941180–84. [DOI] [PubMed] [Google Scholar]
- 18.Azuara‐Blanco A, Dua H S. Malignant glaucoma after diode laser cyclophotocoagulation. Am J Ophthalmol 1999127467–469. [DOI] [PubMed] [Google Scholar]
- 19.Ueda N, Obana A, Miki T. Treatment parameters for the efficacy of transscleral cyclophotocoagulation in rabbits using a diode laser. Jpn J Ophthalmol 200044205–213. [DOI] [PubMed] [Google Scholar]
- 20.Schuman J S, Jacobson J J, Puliafito C A.et al Experimental use of semiconductor diode laser in contact transscleral cyclophotocoagulation in rabbits. Arch Ophthalmol 19901081152–1157. [DOI] [PubMed] [Google Scholar]
- 21.Schlote T, Beck J, Rohrbach J M.et al Alteration of the vascular supply in the rabbit ciliary body by transscleral diode laser cyclophotocoagulation. Graefes Arch Clin Exp Ophthalmol 200123953–58. [DOI] [PubMed] [Google Scholar]
- 22.Brancato R, Leoni G, Travucchi G.et al Histopathology of continous wave neodymium: yttrium aluminum garnet and diode laser contact transscleral lesions in rabbit ciliary body. A comparative study. Invest Ophthalmol Vis Sci 19913286–92. [PubMed] [Google Scholar]
- 23.Prince J H, Diesem C D, Eglitis I.et alAnatomy and histology of the eye and orbit in domestic animals. Springfield, IL: AU, publisher, 196080
- 24.Uram M. Endoscopic fluorescein angiography of the ciliary body in glaucoma management. Ophthalmic Surg Lasers 199627174–178. [PubMed] [Google Scholar]
- 25.Haller J A. Transvitreal endocyclophotocoagulation. Trans Am Ophthal Soc 199694589–676. [PMC free article] [PubMed] [Google Scholar]
- 26.Noecker R J, Kelly T, Patterson E.et al Diode laser contact transscleral cyclophotocoagulation: getting the most from the G‐probe. Ophthalmic Surg Lasers Imaging 200435124–130. [PubMed] [Google Scholar]
- 27.McKelvie P A, Walland M J. Pathology of cyclodiode laser: a series of nine enucleated eyes. Br J Ophthalmol 200286381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill R A, Crean D H, Doiron D R.et al Photodynamic therapy of the ciliary body with tin ethyl etiopurpurin and tin octaethyl benzochlorin in pigmented rabbits. Ophthalmic Surg Lasers 199728948–953. [PubMed] [Google Scholar]