Abstract
Aim
To assess the repeatability of Eger macular stressometer (EMS) measures of photostress recovery and determine their association with other measures of visual function.
Methods
EMS photostress recovery time was measured in 90 patients with bilateral exudative age related macular degeneration (AMD), 19 with bilateral atrophic AMD and 47 with both forms of the condition (mean age 79 (SD 13) years). Measurements were made on two occasions separated by 1 year. Intrasession repeatability was assessed by repeating the measures after a 10 minute recovery period at the first visit. Distance visual acuity was measured with a logMAR chart, near visual acuity with a MNRead chart at 25 cm, contrast sensitivity with a Pelli‐Robson chart, and the presence of central visual disturbance assessed with an Amsler grid. A questionnaire was used to assess self reported difficulties with glare recovery.
Results
The average EMS recovery time was 11.0 (SD 8.9) seconds, decreasing by 1.6 (5.2) seconds on repeated measurement (p<0.05). EMS photostress recovery was not correlated with visual function measures or subjective difficulties with lights (p>0.05). EMS photostress recovery time did not predict those whose vision decreased over the following year compared with those among whom it remained stable.
Conclusions
The EMS test is not a useful tool in determining the severity or progression of AMD.
Keywords: photostress recovery time, Eger macular stressometer, age related macular degeneration, vision
The photostress test involves exposing the eye to intense light for a set duration of time and measuring the time taken for visual acuity to recover to a predetermined level.1 The reduction in visual acuity results from photopigment depletion and the recovery time are dependent on the rate of photopigment regeneration.1 The time course for dark adaptation is delayed for both rods and cones over the entire stimulated retina and not just in the macular region.2
A variety of photostress tests have been advocated and assessed including pen torches, ophthalmoscopes, Maxwellian view optical systems, pupilometers, scanning laser ophthalmoscopes, and visually evoked potentials.3,4,5 Photostress recovery time has been shown to increase in patients with macular conditions such as central serous retinopathy, cystoid macular oedema and age related macular degeneration (AMD).6,7,8,9
There are reports that photostress recovery time is sensitive to the early stages of atrophic AMD6,8,9 and can be used to predict second eye development of choroidal neovascular membranes.10 However, studies examining photostress recovery time in AMD have generally assessed few patients and there has been a lack of standardisation between techniques.6,7,8,9,11,12
Few studies to date have related photostress recovery time to subjective symptoms in AMD. Collins and Brown7 reported a correlation between subjective adaptation difficulties at night and prolonged glare recovery times. However, only two of their 10 participants with AMD reported difficulty adjusting to the dark when walking outside at night. No association was found with either headlight glare when driving at night or adjusting to sudden changes in light level when walking indoors on a sunny day.
Two published studies13,14 have examined the commercially available Eger macular stressometer test (EMS; Gulden Ophthalmics, PA, USA). Bartlett and colleagues found that patients with age related maculopathy (n = 17) and AMD (n = 12) had significantly longer EMS photostress recovery times compared to age matched controls.13 However, Schmitt and co‐workers showed no difference between cataract (n = 30), diabetic retinopathy (n = 16), glaucoma (n = 16), and AMD patients (n = 30).14 Only one patient with exudative AMD was assessed between these two studies combined.
This study aimed to measure photostress recovery time using the EMS test and to assess measurement repeatability in a large cohort including both atrophic and exudative AMD patients. The correlation between subjectively reported glare problems and EMS photostress recovery time and the ability of the EMS to predict those whose visual acuity or contrast sensitivity will deteriorate over the subsequent year were also determined.
Methods
The mean age of the 156 participants recruited from the Queen's Medical Centre, Nottingham was 78.96 (SD 6.64) years (64% female). Patients were considered suitable if they had AMD irrespective of severity or duration, treated or untreated, in one (n = 6; 3.8%) or both eyes. Exclusion criteria were eye disease other than AMD and inability to understand or speak English. Ninety people (57.7%) had exudative AMD, 19 (12.2%) had atrophic AMD and the remaining patients a combination of the two forms of the disease. All gave informed consent to take part in the study, which was approved by the local research ethics committee (Nottingham) and adhered to the tenets of the Declaration of Helsinki.
The EMS consists of a Thyristor photo flash linked to a digital timer and a near acuity chart. Patients, with vision best corrected by their reading prescription, read the smallest logMAR near acuity line of capital letters at 40 cm (illuminated by 900 lux) using binocular vision. The EMS was then moved to 15 cm and the patients binocularly exposed to the flash while looking into its centre (visual angle 14.2° horizontally and 6.8° vertically). The reading chart was immediately repositioned at 40 cm and time until the patient could read print one line (0.1 logMAR) larger than their pre‐exposure visual acuity was measured. The measurement was repeated after a 10 minute recovery period to allow retinal re‐adaptation following bleaching.13,15
Distance and near (25 cm working distance) visual acuity was measured using Bailey‐Lovie logMAR charts. Contrast sensitivity was assessed using Pelli‐Robson charts and the presence of central distortion or scotoma with an Amsler grid. Subjective difficulties with vision in bright sunlight, adapting to bright sunlight, adapting to dim light, being dazzled by bright lights and having to shade their eyes because of excessive light were each rated on a six point scale.
The EMS recovery time and visual function assessment was remeasured after 1 year in 135 participants. Progression of distance visual acuity was taken as a reduction in logMAR of >0.1 units (95% confidence interval of repeatability).16 Progression of contrast sensitivity loss was taken as a reduction exceeding 0.3 Pelli‐Robson log units (95% confidence interval of repeatability).17 All results are presented as means (SD).
Results
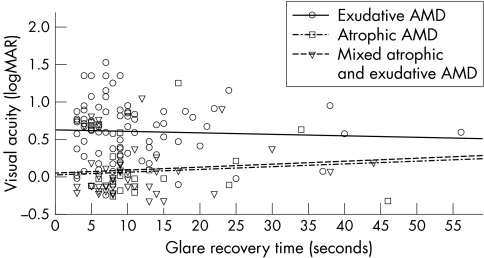
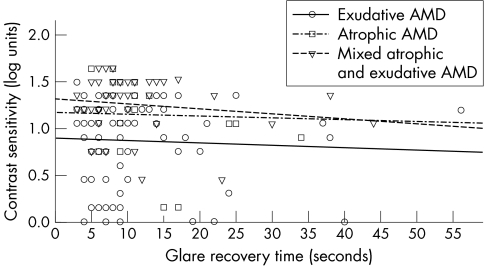
Averaged across the full sample of patients, EMS recovery time was 11.0 (SD 8.9) seconds. The visual function of the three AMD groups is given in table 1. The relation between visual acuity and EMS result is shown in figure 1. EMS recovery time was 1.6 (5.2) seconds shorter on intrasession repeated measure (p<0.05). Initial EMS photostress recovery was not correlated with distance visual acuity in all three AMD groups (p>0.05). This was also the case for contrast sensitivity (fig 2) and near visual acuity. Those patients with a central visual field defect or distortion had a similar EMS photostress recovery time as those with an intact central visual field in exudative (p = 0.56), atrophic (p = 0.38), or mixed AMD (p = 0.10). Subjective difficulties with vision were unrelated to EMS photostress recovery time in all three AMD groups (p>0.05).
Table 1 Visual function of the three age related macular degeneration (AMD) groups.
| Exudative AMD | Atrophic AMD | Mixed AMD | |
|---|---|---|---|
| Distance visual acuity (logMAR) | 0.60 (0.39) | 0.08 (0.39) | 0.07 (0.38) |
| Contrast sensitivity (log units) | 0.87 (0.45) | 1.14 (0.45) | 1.26 (0.29) |
| Near visual acuity (logMAR) | 0.54 (0.43) | 0.31 (0.48) | 0.23 (0.23) |
| Central visual field defect (%) | 65 | 42 | 44 |
| EMS recovery time (seconds) | 10.1 (8.7) | 14.7 (10.6) | 11.3 (8.6) |
Figure 1 Photostress recovery time (seconds) as a function of distance visual acuity in exudative, atrophic, and mixed atrophic and exudative AMD (n = 156).
Figure 2 Photostress recovery time (seconds) as a function of contrast sensitivity in exudative, atrophic, and mixed atrophic and exudative AMD (n = 156).
EMS recovery time and visual function were assessed again after a 1 year period. There was no difference in the initial EMS photostress recovery time among patients with exudative AMD whose distance visual acuity (10.6 (8.7) v 7.9 (3.4) seconds, p = 0.15) or contrast sensitivity (10.8 (9.4) v 11.1 (7.5) seconds, p = 0.92) deteriorated compared with those among whom these two measures remained stable. There was also no difference between the change in EMS photostress recovery time over 1 year in those with exudative AMD whose distance visual acuity (3.3 (10.9) v 2.0 (4.1) seconds, p = 0.28) or contrast sensitivity (4.3 (9.4) v 4.1 (9.1) log units, p = 0.99) deteriorated compared with those in whom these two measures remained stable. Too few patients in the atrophic or mixed AMD groups had a decrease in distance visual acuity or contrast sensitivity (n = 4/3 and n = 11/3, respectively) for a statistical analysis to be performed.
Discussion
Our results provide no evidence that EMS photostress recovery time is affected by AMD, contrary to previous reports using other photostress techniques.6,7,8,9,11,12 Indeed, the average EMS recovery time in our study was similar to that found in previous studies using similar short exposure flash in healthy patients.13,18 The EMS mean recovery time is generally lower than that obtained with other techniques for measuring photostress recovery time,1,6,7,8,9,11,12 which suggests the device does not bleach sufficient photopigment to challenge outer retinal physiology and hence identify people with AMD. The coefficient of repeatability of 10.2 seconds in this study was larger than that found by Bartlett and colleagues13 using the EMS test in a younger population without eye disease, although there was a similar decrease in recovery time with repeated measurement. However, the Bartlett study found significantly longer recovery times in AMD. We are unable to speculate on the differences between these studies, as the central field status of patients was not reported in the Bartlett study. The EMS large coefficient of repeatability limits the sensitivity of the test.
The EMS photostress recovery was not correlated with visual function measures or subjective difficulties with lights and changing light conditions in AMD. There was also no difference in the initial EMS photostress recovery time or change over a year in recovery time among those whose vision decreased over the following year compared with those among whom it remained stable. It would therefore appear that photostress recovery time as assessed by the EMS test is not a useful tool in determining either the severity or the progression of AMD.
The transient state of insensitivity subjectively perceived after photostress is experienced as a scotomatous afterimage.6 The amount of photopigment bleached is dependent on the intensity and duration of the flash.19 In addition, pupil size and eccentricity of viewing will affect the result. Elderly patients tend to have smaller pupils and therefore may need longer exposure to the light source for the photoreceptors to be fully bleached. Furthermore, patients with AMD often adopt eccentric viewing and therefore the effect may be further reduced.1 As such, redesign of the test with a longer flash duration of greater visual angle may be appropriate. Bleaching the photoreceptors with a direct ophthalmoscope for 30 seconds has been suggested to be the most reliable test of photostress as more photopigment is bleached and the effect of pupil size is limited.1 However, the EMS test has a short assessment time and patients relate to the difficulty of adapting after a flash or sudden change in lighting. Hence, despite the negative findings of this study, if redesign and further development of the EMS test could produce associations with the severity and progression of AMD, it may be an attractive test in clinical practice.
Abbreviations
AMD - age related macular degeneration
EMS - Eger macular stressometer
Footnotes
The authors have no commercial interest in the EMS test.
References
- 1.Margrain T H, Thomson D. Sources of variability in the clinical photostress test. Ophthal Physiol Opt 20022261–67. [DOI] [PubMed] [Google Scholar]
- 2.Brown B, Tobin C, Roche N.et al Cone adaptation in age‐related maculopathy. Am J Optom Physiol Opt 198663450–454. [DOI] [PubMed] [Google Scholar]
- 3.Lovasik J V. An electrophysiological investigation of the macular photostress test. Invest Ophthalmol Vis Sci 198324437–441. [PubMed] [Google Scholar]
- 4.Zabriskie N A, Kardon R H. The pupil photostress test. Ophthalmology 19941011122–1130. [DOI] [PubMed] [Google Scholar]
- 5.Ito Y, Horiguchi M, Miyake Y.et al Extrafoveal photostress recovery testing with a scanning laser ophthalmoscope. Jap J Ophthalmol 199741255–259. [DOI] [PubMed] [Google Scholar]
- 6.Glaser J S, Savino P J, Sumers K D.et al The photostress recovery test in the clinical assessment of visual function. Am J Ophthalmol 197783255–260. [DOI] [PubMed] [Google Scholar]
- 7.Collins M, Brown B. Glare recovery and its relation to other clinical findings in age related maculopathy. Clin Vis Sci 19894155–163. [Google Scholar]
- 8.Collins M, Brown B. Glare recovery and age related maculopathy. Clin Vis Sci 19894145–153. [Google Scholar]
- 9.Midena E, Angeli C D, Blarzino M C.et al Macular function impairment in eyes with early age‐related macular degeneration. Invest Ophthalmol Vis Sci 199738469–477. [PubMed] [Google Scholar]
- 10.Sandberg M A, Weiner A, Miller S.et al High‐risk characteristics of fellow eyes of patients with unilateral neovascular age‐related macular degeneration. Ophthalmology 1998105441–447. [DOI] [PubMed] [Google Scholar]
- 11.Forsius H, Eriksson A W, Krause U. The dazzling test in diseases of the retina. Acta Ophthalmol 19634255–63. [DOI] [PubMed] [Google Scholar]
- 12.Wu G, Weiter J J, Santos S.et al The macular photostress test in diabetic retinopathy and age‐related macular degeneration. Arch Ophthalmol 19901081556–1558. [DOI] [PubMed] [Google Scholar]
- 13.Barlett H, Davies L N, Eperjesi F. Reliability, normative data , and the effect of age‐related macular disease on the Eger Macular Stressometer photostress recovery time. Ophthal Physiol Opt 200424594–599. [DOI] [PubMed] [Google Scholar]
- 14.Schmitt N, Grover D, Feldon S. The Eger Macular Stressometer: pilot study. Am J Ophthalmol 2003136314–317. [DOI] [PubMed] [Google Scholar]
- 15.Collins M. The onset of prolonged glare recovery with age. Ophthal Physiol Opt 19899368–371. [PubMed] [Google Scholar]
- 16.Bailey I, Bullimore M, Raasch T, Taylor H. Clinical grading and the effects of scaling. Invest Ophthalmol Vis Sci 199132422–432. [PubMed] [Google Scholar]
- 17.Elliott D B, Sanderson K, Conckey A. The reliability of the Pelli‐Robson contrast sensitivity chart. Ophthal Physiol Opt 19901021–24. [PubMed] [Google Scholar]
- 18.Gomez‐Ulla F, Louro O, Mosquera M. Macular dazzling test on normal subjects. Br J Ophthalmol 198670209–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollins M, Alpern M. Dark adaptation and visual pigment regeneration in human cones. J Gen Physiol 197362430–447. [DOI] [PMC free article] [PubMed] [Google Scholar]