Abstract
Aims
To assess optic disc characteristics in premature infants with and without ischaemic brain injury and to evaluate the role of optic disc morphology in dating the injury.
Methods
RetCam fundal images, cranial ultrasounds and magnetic resonance imaging (MRI) of 109 premature infants were analysed. The study cohort was divided into subgroups depending on the presence or absence of periventricular leucomalacia (PVL) and intraventricular haemorrhage (IVH). The control group consisted of infants with normal neuroimaging at term and 2 years of age. Using the image analysis software of the RetCam, optic disc diameter (ODD), optic disc area (ODA), and optic cup area (OCA) were measured at 33–34 weeks gestational age. As serial cranial ultrasonography had been performed, it was possible to date the brain injury in those infants with periventricular white matter (PVWM) damage.
Results
Although there was a trend towards reducing ODD, ODA, and OCA with increasing severity of IVH, only the IVH 4 group differed significantly from the controls for these parameters (p = 0.002, p = 0.02, and p = 0.04, respectively). 44.4% of infants with grade 4 IVH had small discs. Only one patient had a large cup in a normal sized disc; this patient had IVH 4. In patients with PVWM damage, the median time of insult was 27 weeks in those with small discs and 28 weeks in those with normal discs. This difference was not significant (p = 0.23).
Conclusions
Premature infants with IVH 4 have an increased incidence of optic nerve hypoplasia. We found no association between disc morphology and timing of brain injury.
Keywords: optic disc morphology, ischaemic neonatal brain injury, RetCam image analysis, infants
Ischaemic brain injury resulting in periventricular white matter (PVWM) damage is a major cause of visual impairment in premature infants where it is a more common cause of visual morbidity than retinopathy of prematurity. PVWM damage may arise as a result of periventricular leucomalacia (PVL) or periventricular haemorrhage. PVL is caused by an ischaemic process in the watershed zone that exists in the PVWM in the immature brain.1 Intraventricular haemorrhage (IVH) occurs in 30–40% of all infants of less than 32 weeks gestational age.2 It arises because of ischaemia of the subependymal germinal matrix tissue of the developing brain with subsequent bleeding from the fragile vasculature into the ventricles.3 Periventricular haemorrhage (PVH) refers to the most severe form of IVH (grade 4 IVH) where there is associated parenchymal damage. As survival rates for premature infants in modern neonatal intensive care units continue to increase, the prevalence of ischaemic neonatal brain injury will also increase.
Several authors have reported the association between IVH and optic nerve hypoplasia (ONH).4,5,6,7 However, the diagnosis of ONH in these studies was based on subjective analysis of the optic disc. Bilateral, mild ONH can prove difficult to diagnose from clinical appearance alone. Furthermore, hypoplasia may occur in optic discs of normal size with large optic cups because of a reduced number of axons.8,9 Pallor may be an additional feature of ONH. The simultaneous occurrence of ONH and optic atrophy is being recognised with increasing frequency, further adding to the confusion over the definition of ONH.8,10
Hellstrom et al analysed fundal photographs of premature children and reported a possible role for optic disc morphology in dating ischaemic brain insults.11,12,13
This study attempts to assess the optic disc in a cohort of premature babies to further elucidate the association between ONH and ischaemic neonatal brain injury and to evaluate the role of optic disc morphology in determining the timing of brain injury.
Methods
We identified all babies screened for ROP (screening criteria of <31 weeks gestational age and/or ⩽1500 g birth weight) since the introduction of the RetCam 120 (Massie Laboratories, Dublin, CA, USA) in the neonatal unit of the National Maternity Hospital, Dublin, in November 1999. These were then cross referenced with a record book of premature babies screened for IVH (screening criteria of <34 weeks gestational age and/or ⩽1500 g birth weight) in the same unit. Portable cranial ultrasonography was routinely performed in the neonatal unit within the first 48 hours of life and subsequently on days 3, 7, 14, and pre‐discharge. Additional scans were performed as indicated by the neurological status of the neonate. Images were obtained through the anterior fontanelle in both right and left sagittal and coronal planes.
RetCam image analysis
All images were taken with the wide angle 130° head for the RetCam and were analysed by a single observer (EML) who was masked to the results of the cranial ultrasonography. The best images for each eye, at a corrected age of 33–34 weeks, were selected for each baby. Eyes with unfocused or poorly centred disc images were excluded. Using the image analysis software of the RetCam 120, the optic disc area (ODA) and optic cup area (OCA) were measured by carefully delineating their outlines with a cursor. The areas were then calculated by the computer. Some of the eyes had no physiological cupping. In these cases, the OCA was assigned a value of 0. The optic disc diameter (ODD) was assessed by marking the limits of the horizontal diameter with the cursor. The RetCam software automatically incorporates a conversion factor of 0.03 mm/pixel to yield real distance values. Optic disc rim area (ORA) was recorded as the difference between ODA and OCA.
Cerebral image analysis
The cranial ultrasound scan (CUSS) examinations were performed and interpreted by a single consultant paediatric radiologist (VD) who was unaware of the optic disc morphology. IVH was graded according to the method of Papile et al: grade 1 is haemorrhage confined to the subependymal germinal matrix, grade 2 is an intraventricular bleed, grade 3 is IVH with ventricular dilation, and grade 4 is IVH associated with parenchymal haemorrhage.14 Grade 4 IVH is also known as periventricular haemorrhage (PVH).
Since November 2001, magnetic resonance imaging (MRI) has been performed on all premature babies at term, and subsequently at 2 years of age, as PVL may not be evident on initial neuroimaging.15 Therefore, for the purposes of this study, we defined our control population as premature babies with normal cranial ultrasonography and normal MRIs at term and at 2 years of age.
In cases where PVWM damage was noted on CUSS, the ultrasound images and any available MRIs were carefully reviewed in order to distinguish between PVH and PVL.16 Intraventricular haemorrhage may be present in both of these conditions and is not helpful in their differentiation. While PVL typically occurs posteriorly, adjacent to the trigone of the lateral ventricles, and PVH typically occurs anteriorly, just dorsal and lateral to the external angle of the lateral ventricle, either lesion may be more extensive and involve the PVWM from frontal to parieto‐occipital regions.3 Other features which may help in distinguishing these conditions are outlined in table 1.17
Table 1 Classification of periventricular white matter damage.
| Characteristic | Periventricular haemorrhage | Periventricular leucomalacia |
|---|---|---|
| Infarct type | Venous | Arterial |
| Periventricular location | Anterior | Posterior |
| Haemorrhagic lesion | Always | Rarely |
| Laterality | Unilateral > bilateral | Bilateral > unilateral |
| Symmetry | Asymmetric | Symmetric |
| Porencephalic cyst | Associated feature | Not associated |
| Ventriculomegaly | Regular dilation of the ventricles | Irregularity of the ventricular outline |
Timing of parenchymal injury
PVWM damage usually occurs between 24–34 weeks gestation.13 As serial CUSS were performed on the premature babies in this study, it was possible to estimate the timing of the parenchymal damage in the PVH and PVL groups in most instances. In other words, if the initial CUSS was normal at 24 hours but parenchymal damage was evident at 72 hours, then the insult was estimated to have occurred within the first postnatal week. In two cases with PVL, the insult could not be timed as the parenchymal damage was only detected on the 2 year MRI scan and was not evident on the initial CUSS. Although prenatal cases of PVH and PVL are known to occur occasionally, the injury occurred postnatally in all of our study patients.
Baseline characteristics
Gestational age and birth weight were recorded for each patient. Maternal notes were reviewed to exclude any cases with a history of maternal alcohol abuse or infection during pregnancy. The severity of retinopathy of prematurity (ROP), if present, was also noted by reviewing all available RetCam images.
Statistical analysis
Analysis was performed using Matlab 6.5 (Statistics Toolbox 4.0). Where data were available for both eyes of an individual, the mean of the measurements of the two eyes was calculated for each optic disc parameter. In cases where only one eye had images of optimal quality, the disc parameters for this eye were included in the analysis. This approach was also adopted by Hellstrom et al in their analysis of optic discs.
As the data were not normally distributed, differences in baseline characteristics and optic disc parameters between the various subgroups were analysed using non‐parametric methods such as Kruskal‐Wallis ANOVA, Wilcoxon rank sum test, and Fisher's exact test as appropriate. A p value of less than 0.05 was considered significant.
The data on the disc parameters are displayed graphically using box plots (a five measure summary of the variables: median, upper and lower quartiles, minimum and maximum values).
Results
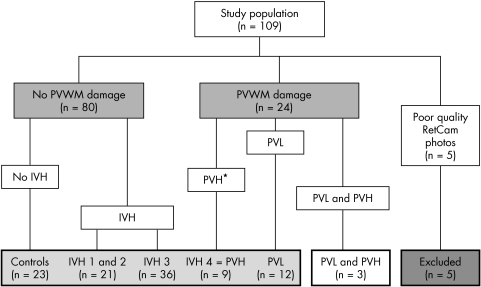
The study population comprised 109 infants with RetCam fundal images and CUSS images. Five patients were excluded because of unfocused or poorly centred fundal images; of these, two had normal neuroimaging, two had IVH 3, and one had PVL. Based on the neuroimaging findings, we categorised our remaining patients into two broad groups; those with and without PVWM damage (fig 1). From table 2 it is evident that babies with PVWM damage had significantly smaller optic discs than babies without PVWM damage (p = 0.03, Wilcoxon rank sum).
Figure 1 Breakdown of the study population. PVWM, periventricular white matter; IVH, intraventricular haemorrhage; PVH, periventricular haemorrhage; PVL, periventricular leucomalacia. *Periventricular haemorrhage is equivalent to IVH grade 4.
Table 2 Optic disc parameters in premature infants with and without periventricular white matter (PVWM) damage.
| Variable (median values) | No PVWM damage (n = 80) | PVWM damage (n = 24) | p Value |
|---|---|---|---|
| Gestational age (weeks) | 28.0 | 27.0 | 0.20 |
| Body weight (g) | 1050.0 | 1008.0 | 0.68 |
| Optic disc diameter (mm) | 1.05 | 0.95 | 0.002 |
| Optic disc area (mm2) | 1.13 | 1.06 | 0.03 |
| Optic cup area (mm2) | 0.09 | 0.08 | 0.07 |
| Optic rim area (mm2) | 1.03 | 0.95 | 0.02 |
The data were further categorised into subgroups in order to elucidate the influence of IVH (grades 1–4) and PVL on optic disc morphology (fig 1). Patients with IVH 1 and IVH 2 were combined into one group as they did not differ significantly in their baseline characteristics and ocular outcomes and there were only four patients with IVH 2. Three infants who had evidence of both periventricular haemorrhage and periventricular leucomalacia on neuroimaging were excluded from further analysis. The baseline characteristics of these subgroups are summarised in table 3.
Table 3 Baseline characteristics of the subgroups.
| Variable | No PVWM damage (n = 80) | PVWM damage (n = 21) | |||
|---|---|---|---|---|---|
| Controls (n = 23) | IVH 1 and 2 (n = 21) | IVH 3 (n = 36) | IVH 4 (n = 9) | PVL (n = 12) | |
| Gestational age (weeks) (median) | 28.0 | 28.0 | 27.5 | 27.0 | 27.5 |
| Body weight (g) (median) | 1100.0 | 980.0 | 1075.0 | 1060.0 | 1008.0 |
| Sex | No (%) | No (%) | No (%) | No (%) | No (%) |
| Male | 14 (61) | 12 (57) | 21 (58) | 2 (22) | 4 (33) |
| Female | 9 (39) | 9 (43) | 15 (42) | 7 (78) | 8 (67) |
| ROP | No (%) | No (%) | No (%) | No (%) | No (%) |
| No ROP | 19 (82) | 13 (62) | 28 (78) | 6 (67) | 5 (42) |
| Subthreshold ROP | 2 (9) | 3 (14) | 3 (8) | 2 (22) | 3 (25) |
| Threshold ROP | 2 (9) | 5 (24) | 5 (14) | 1 (11) | 4 (33) |
IVH, intraventricular haemorrhage; PVL, periventricular leucomalacia; ROP, retinopathy of prematurity.
The gestational age for the study population as a whole ranged from 24 weeks to 33 weeks and the birth weight from 540 g to 2400 g. There was no statistically significant difference in median birth weight between the groups (p = 0.51, Kruskal‐Wallis ANOVA) and the only statistically significant difference in terms of median gestational age was between the control and IVH 4 groups (p = 0.03, Wilcoxon rank sum). Neither was there a significant difference in sex distribution between the groups (p>0.06, Fisher's exact test).
In all, 28.7% of the IVH patients compared to 17.4% of the controls had ROP; however, this was not statistically significant (p = 0.22, Fisher's exact test). Only the PVL group had significantly more patients with ROP than the control group (p = 0.02, Fisher's exact test).
Disc parameters
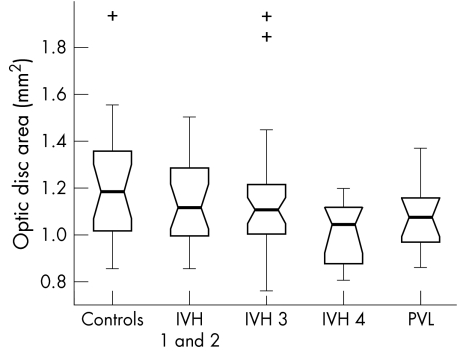
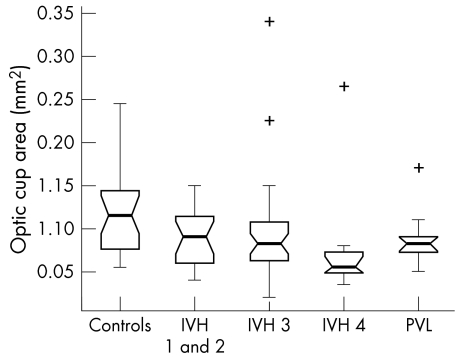
Kruskal‐Wallis analysis of the data identified a significant variation in ODD (p = 0.01) and OCA (p = 0.02) between the study subgroups. No significant difference in ODA (p = 0.15) or ORA (p = 0.20) was detected.
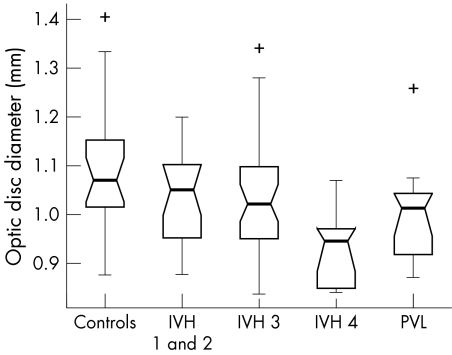
The box plots in figures 2–5 summarise the disc parameter findings for the various subgroups. The IVH 4 group differed significantly from the controls for all four of the disc parameters assessed (Wilcoxon rank sum); median ODD (0.95 mm v 1.07 mm respectively, p = 0.002,), median ODA (1.05 mm2v 1.19 mm2, p = 0.02), median OCA (0.06 mm2v 0.12 mm2, p = 0.007), and median ORA (0.93 mm2v 1.09 mm2, p = 0.04).
Figure 2 Optic disc diameter in the study population (range 0.81–1.41 mm).
Figure 3 Optic disc area in the study population (range 0.66–1.94 mm2).
Figure 4 Optic cup area in the study population (range 0.02–0.34 mm2).
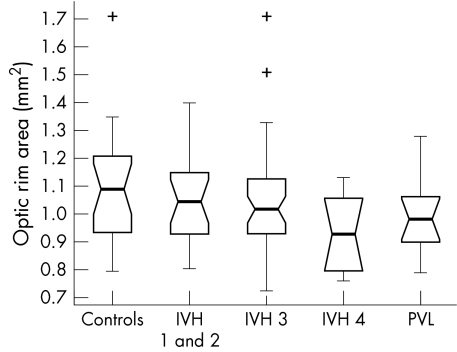
Figure 5 Optic rim area in the study population (range 0.64–1.71 mm2).
The PVL group differed significantly from the controls only in terms of median ODD (1.01 mm v 1.07 mm respectively, p = 0.02). There was no significant difference in median ODA (1.08 mm2v 1.19 mm2, p = 0.08), median OCA (0.08 mm2v 0.12 mm2, p = 0.06) or median ORA (0.98 mm2v 1.09 mm2, p = 0.14).
Neither the IVH 1 and 2 nor the IVH 3 groups differed significantly from controls for any of the optic disc parameters measured (p>0.06, Wilcoxon rank sum).
The box plot data suggest trends of decreasing ODD, ODA, OCA, and ORA with increasing IVH severity. The significance of these trends was assessed by way of a χ2 test for linear trend analysis of the proportion of discs with values less than the median value for the control group. This analysis confirmed that the observed trends were significant for all four disc parameters: ODD (p = 0.002), ODA (p = 0.028), OCA (p = 0.005) and ORA (p = 0.028).
5th and 95th percentiles
Small and large optic discs were defined as those falling outside the 5th and 95th percentiles for ODD and ODA in the control group. Small and large optic cup areas and optic rim areas were similarly defined. From table 4, we can see that the IVH 4 group had significantly more hypoplastic discs than the control group (Fisher's exact test, p = 0.03 for ODD, ODA, and ORA and p = 0.003 for OCA).
Table 4 Number of patients with disc parameters outside the 5th and 95th percentiles of the control group.
| Variable | Controls (n = 23) | IVH 1 and 2 (n = 21) | IVH 3 (n = 36) | IVH 4 (n = 9) | PVL (n = 12) |
|---|---|---|---|---|---|
| No (%) | No (%) | No (%) | No (%) | No (%) | |
| Small ODD (<0.91 mm) | 2 (8.7) | 2 ( 9.5) | 5 (13.9) | 4 (44.4) | 3 (25.0) |
| Large ODD (>1.32 mm) | 2 (8.7) | 0 ( 0.0) | 1 ( 2.8) | 0 ( 0.0) | 0 ( 0.0) |
| Small ODA (<0.94 mm2) | 2 (8.7) | 3 (14.3) | 5 (13.9) | 4 (44.4) | 1 ( 8.3) |
| Large ODA (>1.55 mm2) | 2 (8.7) | 0 ( 0.0) | 2 ( 5.6) | 0 ( 0.0) | 0 ( 0.0) |
| Small OCA (<0.06 mm2) | 1 (4.3) | 4 (19.0) | 6 (16.7) | 5 (55.6) | 1 ( 8.3) |
| Large OCA (>0.24 mm2) | 2 (8.7) | 0 ( 0.0) | 1 ( 2.8) | 1 (0.64) | 0 ( 0.0) |
| Small ORA (<0.87 mm2) | 2 (8.7) | 4 (19.0) | 5 (13.9) | 4 (44.4) | 3 (25.0) |
| Large ORA (>1.35 mm2) | 2 (8.7) | 1 ( 4.8) | 2 ( 5.6) | 0 ( 0.0) | 0 ( 0.0) |
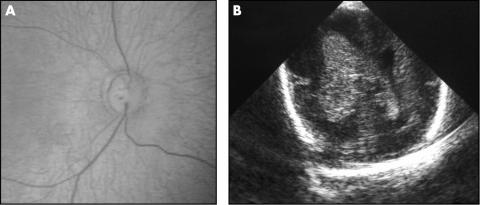
Only one patient had a large optic cup in a normal sized disc (fig 6). The other patients with large optic cups had a correspondingly large optic disc size and therefore did not fit Jacobsons et al criteria for atypical ONH.8
Figure 6 Fundal image (A) and cranial ultrasound (B) of the patient with a large optic cup in a normal sized disc in the presence of a right grade 4/left grade 3 IVH.
Timing of parenchymal injury
The estimated timing of the parenchymal damage in the PVH (IVH 4) and PVL groups is given in table 5. There was no significant difference in the median time of insult between the two groups (27 weeks v 28 weeks respectively, p = 0.14, Wilcoxon rank sum). The time of insult ranged from 25–30 weeks (median 27 weeks) for the five patients with periventricular white matter damage and small optic disc areas and ranged from 26–31 weeks (median 28 weeks) for the remaining patients with PVWM damage but normal optic disc areas (table 5). This was not statistically significant (p = 0.23, Wilcoxon rank sum). Evaluation of Spearman's rank correlation between timing of insult and the optic disc parameters did not yield any significant results (ODD: p = 0.60, ODA: p = 0.55, OCA: p = 0.29, ORA: p = 0.76). Neither was severity of injury nor location of injury correlated with optic disc morphology in patients with PVWM damage.
Table 5 Patients with periventricular white matter (PVWM) damage.
| Patient | GA | ODA (mm2) | PVWM damage | Associated IVH grade | Associated porencephaly | Time of insult | Diagnosis | ||
|---|---|---|---|---|---|---|---|---|---|
| Severity | Localisation† | Symmetry | |||||||
| 1 | 29 | 1.19 | Severe | (R) ant‐mid | No | (R) G4, (L) G3 | Yes | Day 3 | PVH |
| 2 | 26 | 1.09 | Severe | (R) ant‐mid | No | (R) G4, (L) G3 | No | Day 2 | PVH |
| 3 | 25 | 0.93 | Severe | (R) ant‐mid‐post, (L) ant‐mid‐post | No | (R) G4, (L) G4 | Yes | Day 2 | PVH |
| 4 | 25 | 0.90 | Severe | (R) ant‐mid | No | (R) G4, (L) G3 | Yes | Day 2 | PVH |
| 5* | 28 | 1.07 | Mod | (R) ant‐mid | No | (R) G4, (L) G3 | No | Day 3 | PVH |
| 6 | 27 | 0.81 | Mod | (L) ant‐mid | No | (R) G3, (L) G4 | No | Day 2 | PVH |
| 7 | 28 | 1.20 | Severe | (R) ant‐mid‐post, (L) ant‐mid‐post | No | (R) G4, (L) G4 | No | Day 2 | PVH |
| 8 | 27 | 0.83 | Severe | (L) ant‐mid | No | (R) G3, (L) G4 | No | Day 3 | PVH |
| 9 | 26 | 1.05 | Severe | (R) ant‐mid, (L) ant‐mid‐post | No | (R) G4, (L) G4 | Yes | Day 3 | PVH |
| 10 | 27 | 1.07 | Severe | (R) post, (L) mid‐post | No | (R) G3, (L) G3 | No | Day 2 | PVL |
| 11 | 29 | 1.09 | Mod | (R) mid‐post, (L) mid‐post | Yes | (R) G1, (L) G1 | No | Day 7 | PVL |
| 12 | 25 | 1.37 | Mod | (R) mid‐post | No | (R) G3, (L) G2 | No | Day 4 | PVL |
| 13 | 27 | 0.95 | Mod | (L) post | No | (R) G3, (L) G3 | No | Day 2 | PVL |
| 14 | 27 | 1.09 | Severe | (L) mid‐post | No | (L) G3 | No | Day 12 | PVL |
| 15 | 32 | 1.25 | Mod | (R) post, (L) post | Yes | (R) G1 | No | NA | PVL |
| 16 | 30 | 0.86 | Severe | (R) ant‐mid‐post, (L) ant‐mid‐post | Yes | None | No | Day 2 | PVL |
| 17 | 30 | 0.99 | Mod | (R) mid‐post | No | (L) G1 | No | Day 2 | PVL |
| 18 | 28 | 1.19 | Severe | (R) ant‐mid‐post, (L) ant‐mid‐post | Yes | None | No | Day 14 | PVL |
| 19 | 26 | 1.13 | Mod | (R) mid‐post, (L) mid‐post | No | (R) G2, (L) G2 | No | Day 2 | PVL |
| 20 | 25 | 1.02 | Severe | (R) mid‐post, (L) mid‐post | Yes | (R) G3, (L) G3 | No | Day 12 | PVL |
| 21 | 28 | 0.96 | Mild | (R) post, (L) post | Yes | None | No | NA | PVL |
GA, gestational age; ODA, optic disc area; IVH, intraventricular haemorrhage; PVH, periventricular haemorrhage; PVL, periventricular leucomalacia; NA, not able to accurately time the ischaemic insult.
*Only patient with normal sized disc but a large optic cup.
†Ant, mid, and post refer to the frontal, parietal, and occipital regions of the periventricular white matter, respectively.
The ODA values in bold type indicate the small optic discs.
Discussion
The incidence of IVH has been shown to increase progressively with decreasing gestational age18,19; 61% of our study population, with gestational age ranging from 24–33 weeks, had IVH. Only the IVH 4 group differed significantly from the controls in terms of median gestational age, suggesting that low gestational age also predisposes to a more severe grade of IVH.
Although there was a trend towards a higher incidence of ROP in the IVH patients, this was not found to be statistically significant. King and Cronin, Amato et al, and Phillips et al also found no statistical association between ROP and IVH.6,20,21 However, other authors have reported an association.22,23,24 Of note, in these latter studies, CUSS alone was used to diagnose IVH; therefore cases of PVL may have been included in these cohorts. Interestingly, our PVL group did have significantly more patients with ROP than the control group (p = 0.02, Wilcoxon rank sum). Perhaps, the increased incidence of ROP in the PVL group reflects a more severe hypoxia in these patients, which also impacts on the immature retinal vasculature.
Within our study population, only those babies with grade 4 IVH had significantly more hypoplastic discs than the control group. As the optic radiations pass adjacent to the trigone of the lateral ventricles, PVWM damage can result in ganglion cell axonal loss by retrograde trans‐synaptic degeneration across the geniculate body.25 Jacobson et al have postulated that “early” prenatal damage to the white matter, before the supporting tissues around the optic nerve are fully developed, results in smaller optic disc size.13 The median time of injury for the four patients with the small discs in our IVH 4 (PVH) group was 26 weeks.
Jacobson et al also reported that abnormal disc morphology in a child with PVL or PVH could be used to time the brain insult; a small optic disc area was only seen in children with white matter damage estimated to have occurred before 28 gestational weeks and a large cup area in a normal sized disc occurred after 28 weeks of gestation.13 While four of the five patients with small optic disc area and PVWM damage in our study had evidence of ischaemic brain insult before 28 weeks of gestation, one of the patients (patient 16 in table 5) was estimated to have sustained the injury at 30 weeks gestation. Only one patient with PVWM damage in our study (patient 5 in table 5) had a large cup in a normal sized disc. This patient had periventricular haemorrhage (IVH 4), estimated to have occurred at 28 weeks gestation, with no evidence of PVL on CUSS or on MRI at 2 years of age (fig 6). Jacobson et al hypothesised that the increased cupping reflected damage caused by a “later” lesion in the PVWM and that, as the scleral canal around the optic nerve had fully developed, the degeneration of neural tissue resulted in a loss of optic nerve substance rather than any overall change in optic disc size.13 We were unable to demonstrate a statistically significant association between disc morphology and timing of insult. The methodology used by Jacobson et al in distinguishing between PVH and PVL and in attributing a time of insult to the PVWM damage has already been questioned by Brodsky.17
This is the first study to look at the optic nerve status in premature infants using the image analysis facility on the RetCam 120. The size of the optic disc on RetCam images is influenced by the anatomical dimensions of the eye and optical aberrations. In adults, correction of optic disc measurements on fundal photographs is possible using magnification correction formulas (Bengtsson and Krakau, Littman).26,27 These corrections are based on the premise that refraction is strongly correlated to axial length. In premature babies, myopia is caused by a more curved cornea, a thicker lens and a shallower anterior chamber depth rather than an increase in axial length.28 Therefore, these adult correction methods are not applicable to premature children.29 Pach et al have shown that, unlike axial myopia, refractive myopia is not associated with magnification and therefore a correction factor is not necessary.30 Rimmer et al have shown, in an autopsy study, that growth of the normal optic disc and nerve is only 50% complete at 20 weeks gestation and 75% complete by full term.31 Our results for mean horizontal ODD at 33–34 weeks gestational age (1.03 (SD 0.12) mm) correlate well with their findings for autopsy eyes in babies of less than 40 weeks gestation, especially when allowance is made for tissue shrinkage (Sylvester and Ari reported an average of 12.3% shrinkage in the diameter of the optic nerve after formalin fixation)—Rimmer et al 0.93 (SD 0.15) mm and Rimmer et al modified for shrinkage 1.06 (SD 0.17) mm.32
Premature infants with IVH 4 represented 8.3% of our study population. Other authors have reported an incidence of 4–15% for IVH 4 in the preterm neonate.3,33 As our study was based on the analysis of fundal images taken for ROP screening, it included only those infants less than 31 weeks gestational age and/or ⩽1500 g birth weight. However, PVWM damage (IVH 4 and PVL) typically occurs between 24–34 weeks of gestation.12 Our study population, therefore, cannot be regarded as representative of all premature babies at risk for IVH.
Using the image analysis software of the RetCam 120, we have objectively measured the optic discs of premature babies and have demonstrated that 44% of babies with IVH 4 had ONH. As ONH may have significant neurological and visual implications we recommend that preterm infants with Grade 4 IVH be referred for ophthalmological evaluation even when they fall outside ROP screening criteria.
Abbreviations
CUSS - cranial ultrasound scan
IVH - intraventricular haemorrhage
MRI - magnetic resonance imaging
OCA - optic cup area
ODA - optic disc area
ODD - optic disc diameter
ONH - optic nerve hypoplasia
ORA - optic disc rim area
PVL - periventricular leucomalacia
PVWM - periventricular white matter
ROP - retinopathy of prematurity
References
- 1.Yin R, Reddihough D S, Ditchfield M R.et al Magnetic resonance imaging findings in cerebral palsy. J Pediatr Child Health 200036139–144. [DOI] [PubMed] [Google Scholar]
- 2.Hutchison A A, Barrett J M, Fleischer A C. Intraventricular haemorrhage in the premature infant. N Eng J Med 19823071272–1273. [DOI] [PubMed] [Google Scholar]
- 3.Volpe J J. Intraventricular haemorrhage in the premature infant—current concepts. Part I. Ann Neurol 1989253–11. [DOI] [PubMed] [Google Scholar]
- 4.Brodsky M C, Glasier C M. Optic nerve hypoplasia: clinical significance of associated central nervous system abnormalities on magnetic resonance imaging. Arch Ophthalmol 199311166–74. [DOI] [PubMed] [Google Scholar]
- 5.Algawi K, Beigi B, Asady M.et al Ophthalmological sequelae following post‐haemorrhagic hydrocephalus. Neuro‐ophthalmology 19951597–101. [Google Scholar]
- 6.King K M, Cronin C M. Ocular findings in premature infants with grade 4 intraventricular haemorrhage. J Pediatr Ophthalmol Strabismus 19933084–87. [DOI] [PubMed] [Google Scholar]
- 7.Burke J P, O'Keefe M, Bowell R. Optic nerve hypoplasia, encephalopathy, and neurodevelopmental handicap. Br J Ophthalmol 199175236–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frisen L, Holmegaard L. Spectrum of optic nerve hypoplasia. Br J Ophthalmol 1978627–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobson L, Hellstrom A, Flodmark O. Large cups in normal‐sized optic discs: a variant of optic nerve hypoplasia in children with periventricular leucomalacia. Arch Ophthalmol 19971151263–1269. [DOI] [PubMed] [Google Scholar]
- 10.Hoyt C S, Good W V. Do we really understand the difference between optic nerve hypoplasia and atrophy? Eye 19926201–204. [DOI] [PubMed] [Google Scholar]
- 11.Hellstrom A. Optic nerve morphology may reveal adverse events during prenatal and perinatal life‐digital image analysis. Surv Ophthalmol 199944(Suppl 1)63–73. [DOI] [PubMed] [Google Scholar]
- 12.Hellstrom A, Hard A L, Svensson E.et al Ocular fundus abnormalities in children born before 29 weeks of gestation: a population‐based study. Eye 200014324–329. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson L, Hard A L, Svensson E.et al Optic disc morphology may reveal timing of insult in children with periventricular leucomalacia and/or periventricular haemorrhage. Br J Ophthalmol 2003871345–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papile L A, Burstein J, Burstein R.et al Incidence and evolution of subependymal and intraventricular haemorrhage: a study of infants with birth weights less than 1500 g. J Pediatrics 197892529–534. [DOI] [PubMed] [Google Scholar]
- 15.Debillion T, N'Guyen S, Muet A.et al Limitations of ultrasonography for diagnosing white matter damage in preterm infants. Arch Dis Child Fetal Neonatal Ed 200388F275–F279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flodmark O, Lupton B, Li D.et al MR imaging of periventricular leucomalacia in childhood. AJNR 198910111–118. [DOI] [PubMed] [Google Scholar]
- 17.Brodsky M C. Semiology of periventricular leucomalacia and its optic disc morphology. Br J Ophthalmol 2003871309–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berger R, Bender S, Sefkow S.et al Peri/intraventricular haemorrhage: a cranial ultrasound study on 5286 neonates. Eur J Obstet Gynaecol Biol 199775191–203. [DOI] [PubMed] [Google Scholar]
- 19.Van de Bor M, Verloove‐Vanhorick S P, Brand R.et al Incidence and prediction of periventricular‐intraventricular haemorrhage in very preterm infants. J Perinat Med 198715333–339. [DOI] [PubMed] [Google Scholar]
- 20.Amato M, Pasquier S, von Muralt G.et al Incidence of retinopathy of prematurity in low birth weight infants with peri‐ventricular hemorrhage. Neuropediatrics 198617191–192. [DOI] [PubMed] [Google Scholar]
- 21.Phillips J, Christiansen S P, Ware G.et al Ocular morbidity in very low birth weight infants with intraventricular haemorrhage. Am J Ophthalmol 1997123218–223. [DOI] [PubMed] [Google Scholar]
- 22.O'Keefe M, Kafil‐Hussain N, Flitcroft I.et al Ocular significance of intraventricular haemorrhage in premature infants. Br J Ophthalmol 200185357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hungerford J, Stewart A, Hope P. Ocular sequelae of preterm birth and their relation to ultrasound evidence of cerebral damage. Br J Ophthalmol 198670463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christiansen S P, Fray K J, Spencer T. Ocular outcomes in low birth weight premature infants with intraventricular haemorrhage. J Pediatr Ophthalmol Strabismus 200239157–165. [DOI] [PubMed] [Google Scholar]
- 25.Brodsky M C, Glasier C M. Optic nerve hypoplasia. Clinical significance of associated central nervous system abnormalities on magnetic resonance imaging. Arch Ophthalmol 199311166–74. [DOI] [PubMed] [Google Scholar]
- 26.Bengtsson B, Krakau C E T. Correction of optic disc measurements on fundus photographs. Graefes Arch Clin Exp Ophthalmol 199223024–28. [DOI] [PubMed] [Google Scholar]
- 27.Littman H. Determination of the real size of an object on the fundus of the living eye. Klin Monatsbl Augenheilkd 1982180286–289. [DOI] [PubMed] [Google Scholar]
- 28.Gordon R A, Donzis P A. Refractive development of the human eye. Arch Ophthalmol 1985103785–789. [DOI] [PubMed] [Google Scholar]
- 29.Hellstrom A, Hard A L, Chen Y.et al Ocular fundus morphology in preterm children. Influence of gestational age, birth size, perinatal morbidity and postnatal growth. Invest Ophthalmol Vis Sci 199738184–192. [PubMed] [Google Scholar]
- 30.Pach J, Pennell D O, Romano P E. Optic disc photogrammetry: magnification factors for eye position, centration, and ametropias, refractive and axial; and their application in the diagnosis of optic nerve hypoplasia. Ann Ophthalmol 198921454–462. [PubMed] [Google Scholar]
- 31.Rimmer S, Keating C, Chou T.et al Growth of the human optic disc and nerve during gestation, childhood, and early adulthood. Am J Ophthalmol 1993116748–753. [DOI] [PubMed] [Google Scholar]
- 32.Sylvester P E, Ari K. The size and growth of the human optic nerve. J Neurol Neurosurg Psychiatry 19612445–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trounce J Q, Rutter N, Levene M I. Periventricular leucomalacia and intraventricular haemorrhage in the preterm neonate. Arch Dis Childhood 1986611196–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]