Abstract
Aim
To survey existing ophthalmic follow up protocols in the United Kingdom for very low birthweight (VLBW) children. In addition, relative risk analysis was performed using data from a cohort study to assess which factors (birth weight, gestational age, retinopathy of prematurity (ROP) status) led to a high risk of developing amblyogenic factors.
Methods
Questionnaires were sent to every orthoptic department in the United Kingdom (n = 288) for information on their policy on the follow up of VLBW children.
Results
Responses were received from 125 departments (43%). There was a large variation in criteria used for follow up; 21% of respondents using birth weight (BW) and gestational age (GA), 22% using stage 3 or treated ROP, the remainder using a combination of these factors. There was no consensus regarding when follow up should commence (from 3 months to 3 years) or cease (1–8 years). Relative risk analysis revealed that birth weight under 1500 g, GA under 33 weeks, and the presence of severe ROP were significant risk factors for developing one or more amblyogenic factors.
Conclusion
There is no consensus on whether VLBW children need to be reviewed. There is a greatly increased risk of ophthalmic deficits in those with severe ROP or severe neurological disorders, and also in those with mild or no ROP. Children in the latter group who are not routinely followed up, have a high risk of developing treatable refractive errors and strabismus. This raises the question of whether an additional screening examination is merited.
Keywords: birth weight, ophthalmic follow up
Current vision screening guidelines by the Child Health Sub‐Group of the National Screening Committee, recommend that all UK children should be assessed by an orthoptist between the ages of 4 years and 5 years (cited by Hall and Elliman1). The main reasons underlying this recommendation are that screening should be undertaken when a high uptake is easily achieved,2 at an age when most children can be reliably tested,3 and treatment is still effective.4,5,6,7 Targeted conditions for current childhood vision screening are amblyopia and its associations (refractive error that causes reduced vision, and strabismus). Children born prematurely have an increased risk of developing amblyogenic factors such as strabismus, anisometropia, and/or high myopia8,9,10 but, in addition have an increase in milder refractive errors11 and perceptual problems12,13 that may not be identified by standard orthoptic screening.11 Currently, no additional recommendations have been made for the screening of this group of children; however, it is stated that “the increased risk of other eye problems including myopia, squint, and cortical visual impairment should be remembered.”1 This raises the question of whether, in addition to the current screening recommendations for this ex‐preterm population, a targeted screening protocol should be implemented at an earlier age.
The screening guidelines for retinopathy of prematurity (ROP) in the United Kingdom recommend that all infants of birth weight under 1500 g and less than 32 weeks gestational age should be screened by an ophthalmologist.14 However, follow up beyond the neonatal period is only recommended for infants with stage 3 ROP and those who have undergone ROP treatment. In practice though, these guidelines are rather imprecise, and adherence is problematic, as no age at follow up or type of assessment are stated.
The aim of this study was to determine the current vision screening/follow up policies for low birthweight children in the United Kingdom. This was administered by means of a questionnaire. In addition, the published data on the ophthalmic outcome of low birthweight children were examined to determine whether a particular subgroup had a significantly greater risk of long term visual disorders. This comparison is based on data from a population based study of children of birth weight less than1701 g, assessed at 10–12 years of age (n = 293).15,16 In this study only a few cases of severe ROP occurred so this population is biased towards mild ROP; therefore, current literature on the outcome of severe ROP will also be evaluated to determine the prevalence of ophthalmic disorders in this population.
Methods
The study was divided into two parts. In part 1 data were collected by questionnaires that were sent to all 288 orthoptic units (to be completed by the orthoptists and ophthalmologists) in the United Kingdom, identified from the list compiled by the British and Irish Orthoptic Society. The questionnaire was divided into six sections seeking information on the following:
Section 1: Arrangements for follow up of children born preterm.
This was a yes/no tick box.
Section 2: The follow up criteria.
The options given were:
All preterm
Defined by a particular BW/GA (to be specified)
All who had any ROP
All stage 3 ROP
All treated ROP
Section 3: The age of children at follow up.
This was completed for each of the categories stated above.
Section 4: The frequency of follow up examinations.
This was completed for each of the categories stated above.
Section 5: Age at which follow up ceased and discharge criteria.
The respondent was asked to state whether this was by age (to be specified), ability to perform vision tests (to be specified) or other criteria (to be specified).
Section 6: Who carried out the follow up assessments?
This was completed for each of the categories stated in section 2.
In part 2, data on birth weight, gestational age and ROP status from the prospective study of 293 children (birth weight <1701 g)10,16 were analysed to determine which components led to the highest risk of developing one of the three main amblyogenic factors—strabismus (any type of manifest deviation both constant and intermittent), anisometropia (more than 1 dioptre), or other significant refractive errors (any myopia or hypermetropia more than 3 dioptres).
Statistical analysis
Statistical analysis has shown that low birthweight children have a significant increase in the prevalence of ophthalmic deficits such as strabismus; however, a single p value does not indicate how great the risk is for a low birthweight child to develop strabismus. Therefore, a relative risk (RR) analysis for the amblyogenic factors strabismus, anisometropia and refractive errors was performed.17
Results
Part 1
Questionnaire responses were received from 125 departments, a response rate of 43%.
Section 1: Arrangements for follow up of children born preterm
A total of 78 units (62% of respondents) reported to routinely follow up prematurely born infants after their discharge from the special care baby unit (there is no standard definition of prematurity). Of these 78 units only eight had written guidelines in the form of a protocol which was submitted with the questionnaire. All results reported here represent these 78 units, percentages being a proportion of this number.
Section 2: The follow up criteria
In 1% of respondents all preterm children were followed up. For the remaining 99% the criteria for follow up varied greatly; birth weight (BW), gestational age (GA), the presence of ROP, stage 3 ROP, treated ROP, or a combination of these factors were used to determine which children were eligible for long term follow up (as shown in table 1). The data in table 1 are a combination of all the various BW and GA criteria, the majority of respondents reported using <1500 g (n = 35) as their criterion, whereas two units used <1250 g, and a further two used <1000 g. The GA criteria were more variable; <32 weeks (n = 22), <31 weeks (n = 10), <30 weeks (n = 8), and <28 weeks (n = 3).
Table 1 Combinations of criteria for follow up.
| BW | GA | Any ROP | Stage 3 ROP | Treated ROP | % |
|---|---|---|---|---|---|
| ✓ | 1 | ||||
| ✓ | ✓ | 21 | |||
| ✓ | ✓ | ✓ | 20 | ||
| ✓ | ✓ | ✓ | ✓ | 11 | |
| ✓ | ✓ | ✓ | 2 | ||
| ✓ | ✓ | ✓ | 2 | ||
| ✓ | ✓ | 1 | |||
| ✓ | ✓ | ✓ | 1 | ||
| ✓ | 11 | ||||
| ✓ | 2 | ||||
| ✓ | 5 | ||||
| ✓ | ✓ | 22 |
Other criteria used included requests from a paediatrician, parental concern, infants with prolonged stage 2 ROP, family history of ophthalmic problems and neurological co‐morbidity (not included in table 1).
Section 3: The age of children at follow up
This ranged from 6 weeks to 36 months. There was no single factor that determined that follow up should commence at an earlier age (table 2). For a large number the age at which follow up commenced was ill defined and gave no indication of the possible age range. There is data overlap with table 2 where different age at follow up was specified according to the different follow up criteria when multiple criteria were used.
Table 2 Summary of age when follow up commenced with respect to follow up criteria.
| Criteria for follow up | Age follow up commenced (months) | |||
|---|---|---|---|---|
| (N = number of departments) | ||||
| 1.5 to <6 | 6–12 | >12 to 36 | Ill defined | |
| Preterm | 4 | 1 | 1 | 1 |
| BW | 12 | 22 | 2 | 1 |
| GA | 13 | 20 | 2 | 1 |
| Any ROP | 13 | 11 | 0 | 3 |
| Stage 3 ROP | 30 | 12 | 1 | 8 |
| Treated ROP | 35 | 9 | 1 | 8 |
| Categories combined (earliest age) | 49 | 24 | 3 | 5 |
Section 4: The frequency of follow up examinations
Of the 71.2% (n = 56) who responded to this question the frequency of follow up assessments was most commonly 6–12 months (44.9% of respondents, n = 35) with 29.5% (n = 23) assessed more regularly (varied from 2 weeks to 3 months). Only one department reported that assessments were carried out every 24 months and 24.4% (n = 19) stated that the frequency depended upon clinical findings. There was a variation in the frequency of follow up assessments within each category of specified criteria for follow up.
Section 5: Age at which follow up ceased and discharge criteria
This section explored the criteria that determined when follow up could cease in the absence of any ophthalmic problem.
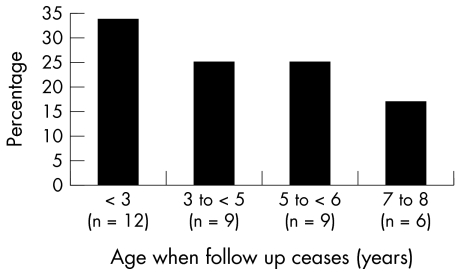
Most respondents stated that age was the main factor, although this ranged from 1–8 years (as shown in fig 1).
Figure 1 Ages at which follow up ceased in each orthoptic department.
However, 68% (n = 53) of respondents stated that stereopsis and stable visual acuity were the criteria used for discharge, but standards differed. Twelve units specified a vision test for follow up assessment; these included Cardiff cards, Kay pictures, Sheridan Gardner, Snellen chart and logMAR tests (EDTRS, single and crowded Keeler logMAR). This may reflect the wide age range at discharge. One respondent also required the assessment to include a Goldmann visual field test before discharge.
Section 6: Who carried out the follow up assessments?
Seventy two per cent of respondents (n = 56) gave information relating to the question of which healthcare professional assessed the child at follow up. A combination of the orthoptist and ophthalmologist were involved in the care of 92.8% (n = 52) of respondents. The patient was seen only by the ophthalmologist in 7.1% of cases (n = 4) and in one instance the orthoptist only. However, 10.7% (n = 6) also reported that for at least one of the referral categories the orthoptist alone would perform the assessment, but the categories did not include treated ROP which was always assessed by an ophthalmologist with or without an orthoptist.
Part 2
Relative risk analysis
The aim of this analysis was to determine whether BW (grouped into 1700–1501 g, 1500–1001 g and <1001 g), GA (grouped into <33 weeks and <29 weeks) or the presence/severity of ROP significantly increased the risk of any of the three amblyogenic factors.
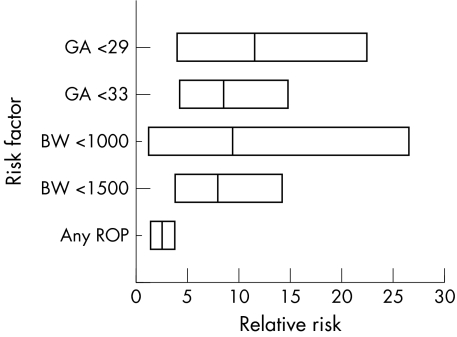
Figure 2 shows the relative risk (line through box) and 95% confidence intervals (edges of the box) for each neonatal factor on the development of strabismus. Only the factors that significantly indicate an increased risk of the development of strabismus are included in the graph. The rate of strabismus in this population was 20.1% (59/293). Analysis showed that birth weight under 1500 g was a significant risk factor for the development of strabismus, compared to the full term population (BW>2500 g). However, when comparing those with birth weight under 1000 g to the other low birthweight children (1001–1700 g) there was no increased risk of developing strabismus. There was a similar pattern when analysing the effect of GA as the infants with the lowest GA had the highest risk but those with GA 29–33 weeks also had a significant increase in the risk of developing strabismus. This highlights that all low birthweight children are at increased risk of developing strabismus, and it is not a problem confined to the most premature infants. Also, the presence of ROP increases the risk of developing strabismus, however the presence of severe ROP did not increase that risk (but there are low numbers in this group).
Figure 2 Relative risk factor analysis. Relative risk (line through box) and 95% confidence intervals (edges of box) for the development of strabismus.
From the neonatal factors analysed there were no identified risk factors for the development of anisometropia.
In addition to strabismus and anisometropia very low birthweight children also have an increased prevalence of refractive errors, particularly myopia. Therefore, a relative risk analysis was carried out to determine which factors increased the risk of the development of myopia or hypermetropia above 3 dioptres. Analysis showed that birth weight <1500 g was associated with a relative risk of 2.53 for the development of myopia (95% CI 1.51 to 3.95) and a high risk (4.98) for the development of significant hypermetropia (95% CI 1.94 to 11.44). Gestational age (<33 weeks) was also a significant risk factor for myopia (RR 2.5, 95% CI 1.5 to 3.91) and hypermetropia (RR 4.9, 95% CI 1.96 to 11.03) with lower gestational age (<29) only increasing the risk for hypermetropia (RR 6.12, 95% CI 1.24 to 20.57). ROP, mild or severe, was not a significant risk factor for the development of myopia or hypermetropia above 3 dioptres. As mentioned above there were low numbers of babies with severe ROP.
Discussion
The primary aim of this study was to examine the ophthalmic screening policies for low birthweight children currently adopted in the United Kingdom. Although the response rate was relatively low (43%) the sample obtained allowed us to fulfil the remit which was to examine the variation in follow up of VLBW children. The main finding from the data gathered is a large variation in many respects in the protocols implemented for the follow up care of preterm infants. Of the 78 units that do continue to assess preterm infants after discharge from the neonatal unit only 24.4% comply with the national recommendations of monitoring those children with treated or stage 3 ROP. The differences in practice are such that on a countrywide basis they can be neither effective nor efficient, but they are needed. The age at which follow up examinations commenced also varied considerably, although it is possible that the ambiguous wording of the questionnaire failed to differentiate screening for acute phase ROP and long term follow up.
The prevalence of amblyogenic factors in the VLBW population, and full term controls, have been summarised in table 3 from the most recent epidemiological studies of VLBW children over the age of 3 years. While these studies differ in their inclusion criteria, ethnicity, previous treatment for amblyopia, prevalence of ROP, and many other factors, they all highlight the increase in refractive errors and amblyogenic factors relative to children born at full term and of “normal” weight.
Table 3 Rates of amblyogenic factors in low birthweight and full term children.
| Authors | No | Inclusion criteria | Age at follow up | Strabismus | Anisometropia >1DS | Myopia | Hypermetropia >3DS |
|---|---|---|---|---|---|---|---|
| Darlow et al8 | 313 | BW<1500 g | 7 years | 22% | Not stated | 21% (any detected by photorefraction) | 18% |
| Holmstrom et al18 | 260 | BW<1500 g | 3.5 years | 13.5% | Not stated | Not stated | Not stated |
| Larsson et al11* | 213 | BW<1500 g | 10 years | Not stated | 8.9% | 15.1% (<0DS) | 4.2% |
| 217 | Full term | 0.9% | 11.1% (<0DS) | 0.9% | |||
| O'Connor et al10,26 | 293 | BW <1701 g | 10–12 years | 20.1% | 9% | 22.4% (<0DS) | 6.6% |
| 169 | Full term | 3% | 8.9% (<0DS) |
*The Larsson paper is a follow up study of the Holmstrom study so represents the same children.
The high incidence of amblyogenic factors in children born preterm is acknowledged.10,18,19,20 This poses the question: should there be a targeted screening programme for children who are born low birth weight in addition to that recommended for all children? The current national screening guidelines recommend orthoptic screening between 4 years and 5 years of age, when the vast majority of children are in school and at an age where treatment of amblyopia is considered still to be effective. However it is not known whether the amblyopia of preterm infants differs in severity, time course, or treatability, particularly as there may be a neurological element impacting on the visual development. This also assumes that primary screening at age 4–5 years is in place, but it is known that these guidelines are not comprehensively applied in many areas of the country.
Considering when screening should be undertaken, it is necessary to know the age at onset and stability of the target conditions. The onset and stability is known for high myopia associated with ROP. This has an early onset and is relatively stable during early childhood.21 Low myopia, associated with preterm birth, but not specifically with ROP, that is “myopia of prematurity,” is thought to have an onset later in childhood.16 Strabismus in VLBW children may develop from a few months after birth. While the presence of strabismus at 6 months was still maintained at age 10 years, 50% of the cases developed after infancy.10 It is acknowledged that cosmetically obvious squints will be noticed; however, this does not account for all cases of strabismus which is why it was included as a target condition.
Severe ROP is known to be highly correlated with strabismus22; however, in our population based study (upon which these data are based) there was a bias towards mild ROP with only a small number developing ROP of stage 3 and above (n = 10), which may account for the finding that ROP was not shown to be a risk factor. If the relative risk analysis had found any factors to be good predictors of amblyogenic factors this would have allowed a highly specific targeted group to be identified for follow up, substantially reducing the number of children needing to be assessed and therefore minimising the cost. However, the analysis did not identify any individual risk factors suggesting that as with ROP, the refractive errors and strabismus that follow have a multifactorial aetiology.
When considering a targeted screening programme for VLBW children, it may be pertinent to subdivide these children into two groups: firstly, those who had severe ROP or had perinatal neurological insults and, secondly, those who had either no or mild ROP (stage 1 or 2) and no clinically obvious neurological dysfunction. The former group are likely to be under ophthalmic care based on our clinical experience, therefore additional screening would not be required under these circumstances. However, those in the latter group who are likely to have been discharged from ophthalmic care still have an increased risk of amblyopia/strabismus, refractive error compared to full term controls.16,23 It is this group that is the focus of our attention in this study and raises the question—is additional screening required, over and above what is received by the whole population at 4–5 years?
There is a paucity of data on the development of amblyopia in low birthweight children which would allow a decision to be made with respect to prophylactic refractive correction. However, reports suggest that correcting significant refractive errors in any population is beneficial for instance for school performance.24,25 As a large proportion of low birthweight children develop refractive errors warranting treatment before the age at which screening is currently recommended (4.5 years) this would suggest that an additional earlier targeted screening programme would be beneficial. However, as the time course of the development of the target disorders is not clearly understood in the LBW population it is not possible to identify an ideal time for this examination to occur.
To conclude, screening of children born of very low birth weight is haphazard and patchy and varies in content, such diverse practice being difficult to support. The high incidence of amblyogenic factors, in VLBW children does raise the issue of an additional focused assessment of children before the age of 4 years, or the use of targeted screening in areas where there is no primary screening between 4 years and 5 years. In the meantime parents should be informed that their child has an increased risk of developing a number of ophthalmic problems and self referral is to be encouraged.
Abbreviations
BW - birth weight
GA - gestational age
ROP - retinopathy of prematurity
RR - relative risk
VLBW - very low birth weight
References
- 1.Hall D M B, Elliman D.Health for all children. 4th ed: Oxford University Press, 2002
- 2.Allen J W, Bose B. An audit of preschool vision screening. Arch Dis Child 1992671292–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kupl M T, Dobson V, Peskin E.et al The electronic visual acuity tester: testability in preschool children. Optom Vis Sci 200481238–244. [DOI] [PubMed] [Google Scholar]
- 4.Clarke M P, Wright C M, Hrisos S.et al Randomised controlled trial of treatment of unilateral visual impairment detected at preschool vision screening. BMJ 20033271251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holmes J M, Kraker R T, Beck R W.et al A randomized trial of prescribed patching regimens for treatment of severe amblyopia in children. Ophthalmology 20031102075–2087. [DOI] [PubMed] [Google Scholar]
- 6.Stewart C E, Fielder A R, Stephens D A.et al Treatment of unilateral amblyopia: factors influencing visual outcome. Invest Ophthalmol Vis Sci 2005463152–3160. [DOI] [PubMed] [Google Scholar]
- 7.Pediatric Eye Disease Investigative Group The clinical profile of moderate amblyopia in children younger than 7 years. Arch Ophthalmol 2002120281–287. [PubMed] [Google Scholar]
- 8.Darlow B A, Clemett R S, Horwood J.et al Prospective study of New Zealand infants with birth weight less than 1500g and screened for ROP: visual outcome at age 7–8 years. Br J Ophthalmol 199781935–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmstrom G, el Azazi M, Kugelberg U. Ophthalmological long term follow up of preterm infants: a population based, prospective study of the refraction and its development. Br J Ophthalmol 1998821265–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Connor A R, Stephenson T J, Johnson A.et al Strabismus in children of birth weight less than 1701 g. Arch Ophthalmol 2002120767–773. [DOI] [PubMed] [Google Scholar]
- 11.Larsson E K, Rydberg A C, Holmstrom G E. A population‐based study of the refractive outcome in 10‐year‐old preterm and full‐term children. Arch Ophthalmol 20031211430–1436. [DOI] [PubMed] [Google Scholar]
- 12.Mackay T L, Jakobson L S, Ellemberg D.et al Deficits in the processing of local and global motion in very low birthweight children. Neuropsychologia 2005431738–1748. [DOI] [PubMed] [Google Scholar]
- 13.Vicari S, Caravale B, Carlesimo G A.et al Spatial working memory deficits in children at ages 3–4 who were low birth weight, preterm infants. Neuropsychology 200418673–678. [DOI] [PubMed] [Google Scholar]
- 14.The report of a Joint Working Party of The Royal College of Ophthalmologists and the British Association of Perinatal Medicine Retinopathy of prematurity: guidelines for screening and treatment. Early Hum Dev 199646239–258. [PubMed] [Google Scholar]
- 15.Ng Y K, Fielder A R, Shaw D E.et al Epidemiology of ROP. Lancet 198821235–1238. [DOI] [PubMed] [Google Scholar]
- 16.O'Connor A R, Stephenson T J, Johnson A.et al Long term ophthalmic outcome of low birth weight children with and without retinopathy of prematurity. Pediatrics 200210912–18. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Yu K F. What's the relative risk? JAMA 19982801690–1691. [DOI] [PubMed] [Google Scholar]
- 18.Holmstrom G, el Azzazi M, Kugelberg U. Ophthalmological follow up of preterm infants: a population based, prospective study of visual acuity and strabismus. Br J Ophthalmol 199983143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmstrom G E, Larsson E K. Development of spherical equivalent refraction in prematurely born children during the first 10 years of life: a population‐based study. Arch Ophthalmol 20051231404–1411. [DOI] [PubMed] [Google Scholar]
- 20.O'Connor A R, Stephenson T J, Johnson A.et al Visual function in low birthweight children. Br J Ophthalmol 2004881149–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinn G E, Dobson V, Kivlin J.et al Prevalence of myopia between 3 months and 5½ years in preterm infants with and without retinopathy of prematurity. Ophthalmol 19981051292–1300. [DOI] [PubMed] [Google Scholar]
- 22.Bremer D L, Palmer E A, Fellows R R.et al Strabismus in premature infants in the first year of life. Arch Ophthalmol 1998116329–333. [DOI] [PubMed] [Google Scholar]
- 23.O'Connor A R, Fielder A R, Birch E E. Long term ophthalmic outcome of low birth weight children who did not have retinopathy of prematurity. Riv Ital Pediatr 200228359–365. [DOI] [PubMed] [Google Scholar]
- 24.Atkinson J, Anker S, Nardini M.et al Infant vision screening predicts failures on motor and cognitive tests up to school age. Strabismus 200210187–198. [DOI] [PubMed] [Google Scholar]
- 25.Williams W R, Latif A H, Hannington L.et al Hyperopia and educational attainment in a primary school cohort. Arch Dis Child 200590150–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Connor A R, Stephenson T J, Johnson A.et al Change of refractive state and eye size in children of birth weight less than 1701 g. Br J Ophthalmol 200690456–460. [DOI] [PMC free article] [PubMed] [Google Scholar]