Abstract
Aim
To report a novel technique using amniotic membrane to cover exposed glaucoma tube shunts.
Methods
A consecutive series of three cases that underwent drainage tube shunt surgery with the Ahmed valve for intractable glaucoma. All three patients developed exposure of the tube secondary to necrosis of the overlying bovine pericardial patch and conjunctiva. Repair of the defect was carried out with a double layer of amniotic membrane, the inner one acting as a graft and the outer as a patch. Autologous serum was used to promote epithelial growth.
Results
Successful lasting closure of the conjunctival defect was achieved in all cases.
Conclusion
Erosion of the drainage tube following shunt surgery is a potentially serious problem. It can be successfully managed using a double layer of amniotic membrane.
Keywords: amniotic membrane, glaucoma, tube shunt
Glaucoma drainage devices remain an effective option for management of refractory glaucoma.1,2,3 However, this form of management is not without its problems. It is estimated that 2–7% of patients undergoing this procedure develop melting of the overlying scleral/bovine pericardial patch with erosion of the tube through the conjunctiva,2,3,4,5,6,7 thus placing the eye at risk of intraocular infection. Repair of these eroded tubes normally requires replacement of the scleral patch and covering of this patch with conjunctiva. In eyes with multiple previous ocular surface surgeries, however, achieving closure of the conjunctival covering can be very difficult or impossible because of the fragile nature of the tissue and the firm adherence to underlying scar tissue.
We present three such cases of tube exposure and describe our approach to dealing with conjunctival deficiency with a novel use for amniotic membrane transplant.
Case reports
Case 1
A 65 year old female patient presented to the clinic on routine review with a conjunctival defect and tube exposure. She had a 40 year history of glaucoma and had undergone three previous glaucoma filtering procedures culminating in an Ahmed glaucoma valve implant tube shunt (New World Medical, Inc, Rancho Cucamonga, CA, USA) procedure with bovine pericardial patch and penetrating keratoplasty for a decompensated cornea 4 years previously. The pericardial patch had subsequently been removed because of patch necrosis with conjunctival erosion and exposure of the patch.
An initial attempt was made to repair the conjunctiva by direct closure; however, she developed further tube exposure 4 months later, which was treated by a conjunctival pedicle autograft. This too did not last, with tube erosion occurring 1 month later. Further treatment with a scleral patch over the tube and a two layered amniotic membrane patch graft to the conjunctival defect was carried out (figs 1 and 2).
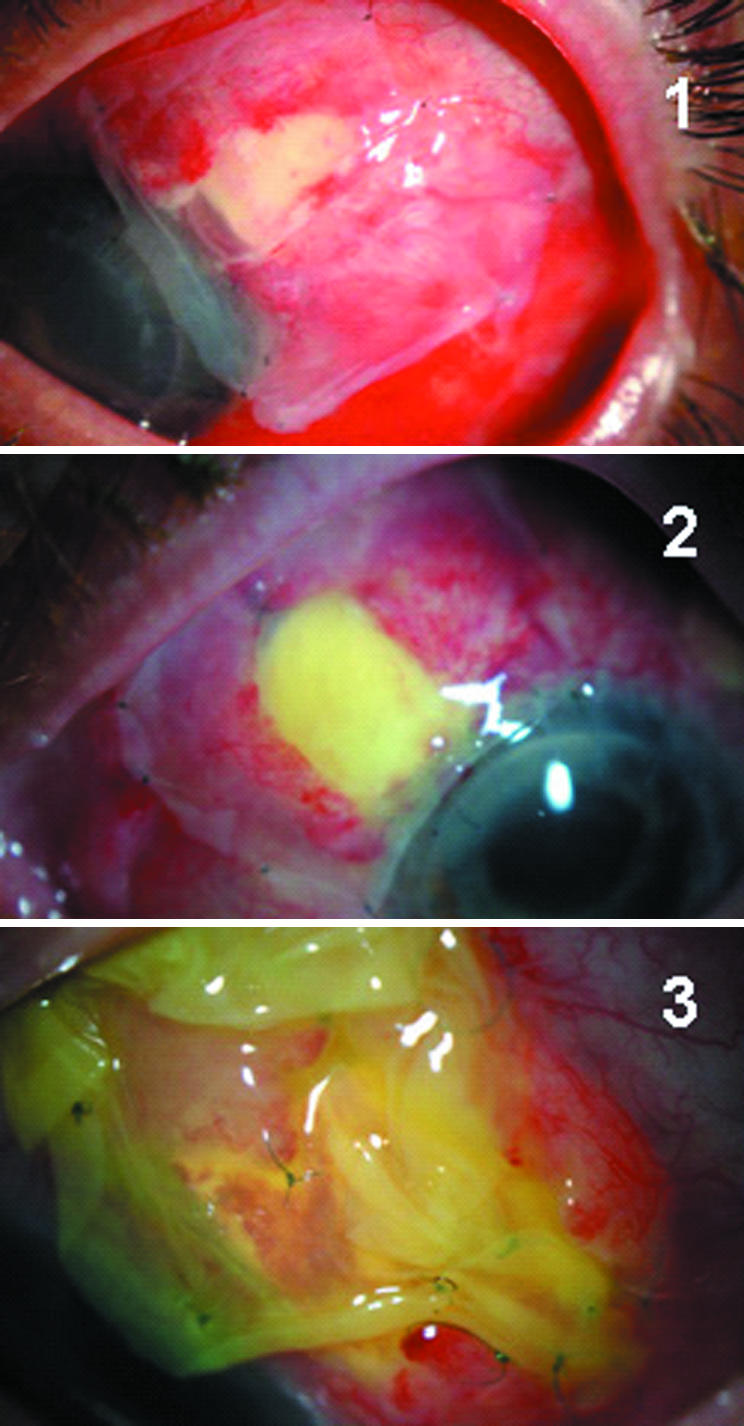
Figure 1 Showing the conjunctival defects covered with amniotic membrane on the first postoperative day for cases 1 and 2 and day 6 for case 3, respectively.
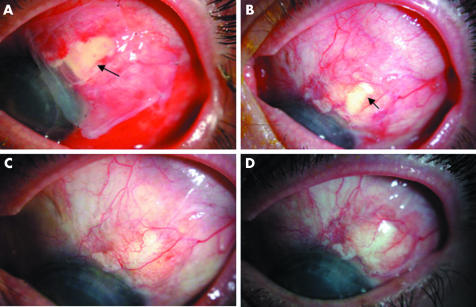
Figure 2 Chronological re‐epithelialisation of epithelial defect following conjunctival erosin repair. (A) Amniotic membrane (outer, epithelial surface down) covering epithelial defect (arrow) day 1 following surgery. (B) Remaining epithelial defect at 2 weeks (arrow) migrating over inner amniotic membrane remnant, outer amniotic membrane has come off. (C) Re‐epithelialisation complete at 4 weeks. (D) Stable re‐epithelialisation at 6 months following surgery.
Two and a half years after surgery the conjunctiva remains healthy with no further incidents of tube erosions with well controlled intraocular pressure.
Case 2
A 58 year old female patient presented with exposure of her tube from previous Ahmed tube shunt procedure. She had previously required three penetrating keratoplasties, initially for keratoconus and had suffered two episodes of corneal rejection. Her other eye had been enucleated because of painful phthisis from repeat corneal surgeries. Her intraocular pressure was poorly controlled despite maximal medical therapy and two applications of cyclodiode ciliary body laser ablation and a prior failed Ahmed glaucoma valve implant tube shunt operation. Her second Ahmed tube shunt operation was augmented with mitomycin C and a bovine pericardial patch was used. Eleven months postoperatively she developed a large conjunctival defect over the patch graft which became necrotic and required removal. The conjunctival defect was closed with a conjunctival autograft, but further tube erosion occurred after 12 months. This was managed by direct closure, but once again the tube eroded after 3 weeks. A scleral patch graft to cover the tube and a two layered amniotic membrane patch graft, carried out as in case 1, was performed to close the conjunctival defect (fig 1).
The superficial amniotic membrane transplant sloughed off at 3 weeks and the defect totally epithelialised after 4 months. The defect remains epithelialised over 2½ years later.
Case 3
A 62 year old female patient with Fuchs' heterochromic cyclitis presented to the emergency eye clinic with a painful left eye and acutely necrotic patch overlying an Ahmed glaucoma valve implant tube shunt.
Her ophthalmic history included three previous failed glaucoma filtering procedures and an aqueous Ahmed tube shunt with bovine pericardial patch 2 years previously. At this presentation, the patch appeared infected and topical cefuroxime and gentamicin antibiotics were started. However, the inflammation failed to resolve and the patch began to melt. The pericardial patch was removed and conjunctival closure attempted. Immediately postoperatively there was a small area of tube exposure, which did not heal over the following 4 weeks. In order to cover the tube a scleral patch, with two layered amniotic membrane patch graft was performed (fig 1). The superficial amniotic membrane transplant fell off at 4 weeks and the defect progressively healed completely over 3 months. One year later a small defect in the conjunctiva with tube exposure appeared where a nylon suture had eroded through the scleral patch and conjunctiva. The suture was trimmed but the defect failed to heal.
The patient has undergone further amniotic membrane patch grafting and scleral patch replacement. Re‐epithelialisation was completed after 4 weeks and remains intact at 6 months.
Method of amniotic patching (fig 3)
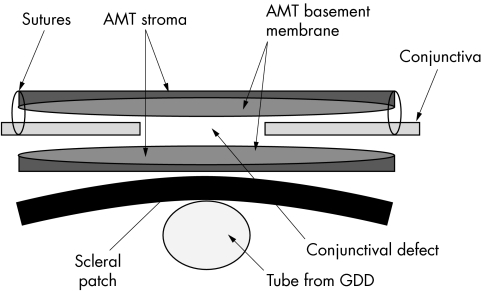
Figure 3 Schematic demonstrating layering of free conjunctival edges between subconjunctival (graft) and extraconjunctival (patch) amniotic membrane (AMT, amniotic membrane transplant; GDD, glaucoma drainage device).
The conjunctiva is reflected from around the exposed tube. Extensive undermining of the conjunctiva to enhance as much as possible the mobility of existing conjunctiva is carried out. Following this a full thickness scleral patch is fashioned and secured over the tube with 4×9/0 nylon interrupted buried sutures. The first layer of amniotic membrane is then applied over the scleral patch with the epithelial side up; this is shaped to be larger than the existing epithelial defect so that excess membrane is tucked in under the free edge of the undermined conjunctiva. The conjunctiva is secured to the underlying episclera/amniotic membrane with 4×10/0 nylon sutures. A second layer of amniotic membrane is then applied over the epithelial defect; this is placed epithelial side down and again shaped to be larger than the conjunctival defect. This is secured to the underlying conjunctiva/episclera with 4−6×10/0 nylon sutures. This second layer of amniotic membrane acts as a bandage (patch) to protect the underlying membrane (graft) and aid underlying epithelial regrowth.
Amniotic membrane used for these procedures was harvested and processed locally before being stored frozen. The membrane was subsequently defrosted before surgery.8
Postoperatively the patients received autologous 20% serum drops four times a day for 1 week as well as preservative free chloramphenicol drops. The outer amniotic membrane transplant normally sloughed off after 5–14 days. Complete re‐epithelisation normally occurred in 3–4 months depending on the size of the conjunctival defect.
Discussion
Tube shunt surgery is a useful adjunct in the treatment of refractory glaucoma; however, it is not without its complications. In order to avoid the use of scleral or pericardial patching some clinicians fashion a tunnel or flap to cover the tube of the glaucoma drainage device. However when such materials are used one such complication is tube erosion where the underlying tube connecting anterior chamber to drainage device erodes through the scleral patch or pericardium and conjunctiva.4 This becomes a possible entry site for infection and potential endophthalmitis and blindness. Patch grafts have been used to cover the tube and then cover the patch with conjunctiva. Materials in use today for patch grafting include human sclera, bovine pericardium, and autologous fascia lata. The advent of patches has decreased the exposure rate from 30% to less than 5%.4 However, when tube erosion and exposure do occur, successful closure of the defect can be difficult to obtain.
Previous methods for closing these defects in the conjunctiva have been described. These include direct sutured closure of the defect and the use of conjunctival autografts.4 These modalities were previously tried on the three cases reported but failed and the tube eroded again.
Amniotic membrane has been used widely in the treatment of ocular surface disease,8 and it has been used in glaucoma for the repair of leaking glaucoma filtering blebs.9,10,11
The technique for repair is as described above. The two pieces of amniotic membrane sandwich the conjunctival edges of the defect. The underlying epithelial side upward membrane provides a substrate for epithelial migration from the conjunctiva and closure of the defect. The underlying amniotic graft becomes incorporated into the conjunctiva, while the overlying amniotic patch, which provides protection for the underlying process, falls off after several weeks.
Our technique, which to the authors' knowledge has not been described before, has given good long term results. It is important to elucidate the causative factor for tube erosion and eliminate this. Common causes include increased tension on the conjunctiva overlying the tube causing a mechanical breakdown, or through compression on the conjunctival small vessels causing localised ischaemia and thus necrosis. Foreign bodies—for example, suture material, may act as a source of inflammation and localised tissue melt. This was the cause of failure in one of our patients (case 3). In addition, in cases where there is ongoing inflammation or infection at the erosion site amniotic membrane grafting should not be attempted until these problems are resolved.
The use of amniotic membrane to promote re‐epithelialisation is well recognised; however, the additional property of suppression of inflammation and inhibition of scarring12 is also beneficial when dealing with eyes having undergone numerous previous surgical procedures and on which tissue dissection of already scarred tissue is likely to generate further inflammation and scarring.
Autologous serum for the treatment of corneal surface disease and persistent epithelial defects has been previously described.13,14,15,16 Autologous serum is believed to provide a growth factor rich environment which promotes healing and epithelial regrowth.14,15,16 All three patients received autologous serum for 1 week following repair of eroded tube defect with amniotic membrane. This was to aid in re‐epithelialisation on the amniotic membrane.
Both autologous serum14,15,16 and amniotic membrane grafts17 alone have been shown to promote epithelial regrowth. Autologous serum was used to maximise the success of this procedure; however, it is likely that the use of amniotic membrane alone as a substrate for epithelial growth would be sufficient to promote re‐epithelialisation when access to autologous serum is limited.
Pericardial patch grafts in this case series did not perform well as all three cases suffered a patch graft melt, requiring removal and scleral patch graft to be used instead. This melt of pericardial path grafts has been previously described.18
It is the authors' opinion that this novel use of amniotic membrane and the technique used provide good long term results for the treatment and closure of tube exposure in aqueous tube shunt surgery and provide an additional approach to management of an often difficult problem.
References
- 1.Broadway D C, Iester M, Schulzer M.et al Survival analysis for success of Molteno tube implants. Br J Ophthalmol 200185689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayyala R S, Zurakowski D, Smith J A.et al A clinical study of the Ahmed glaucoma valve implant in advanced glaucoma. Ophthalmology 19981051968–1976. [DOI] [PubMed] [Google Scholar]
- 3.Heuer D K, Lloyd M A, Abrams D A.et al Which is better? One or two? A randomized clinical trial of single‐plate versus double‐plate Molteno implantation for glaucomas in aphakia and pseudophakia. Ophthalmology 1992991512–1519. [DOI] [PubMed] [Google Scholar]
- 4.Heuer D K, Budenz D, Coleman A. Aqueous shunt tube erosion. J Glaucoma 200110493–496. [DOI] [PubMed] [Google Scholar]
- 5.Huang M C, Netland P A, Coleman A L.et al Intermediate‐term clinical experience with the Ahmed glaucoma valve implant. Am J Ophthalmol 199912727–33. [DOI] [PubMed] [Google Scholar]
- 6.Coleman A L, Mondino B J, Wilson M R.et al Clinical experience with the Ahmed glaucoma valve implant in eyes with prior or concurrent penetrating keratoplasties. Am J Ophthalmol 199712354–61. [DOI] [PubMed] [Google Scholar]
- 7.Siegner S W, Netland P A, Urban R C., Jret al Clinical experience with the Baerveldt glaucoma drainage implant. Ophthalmology 19951021298–1307. [DOI] [PubMed] [Google Scholar]
- 8.Dua H S, Gomes J A, King A J.et al The amniotic membrane in ophthalmology. Surv Ophthalmol 20044951–77. [DOI] [PubMed] [Google Scholar]
- 9.Kee C, Hwang J M. Amniotic membrane graft for late‐onset glaucoma filtering leaks. Am J Ophthalmol 2002133834–835. [DOI] [PubMed] [Google Scholar]
- 10.Barton K, Budenz D L, Khaw P T.et al Glaucoma filtration surgery using amniotic membrane transplantation. Invest Ophthalmol Vis Sci 2001421762–1768. [PubMed] [Google Scholar]
- 11.Budenz D L, Barton K, Tseng S C. Amniotic membrane transplantation for repair of leaking glaucoma filtering blebs. Am J Ophthalmol 2000130580–588. [DOI] [PubMed] [Google Scholar]
- 12.Tseng S C, Li D Q, Ma X. Suppression of transforming growth factor‐beta isoforms, TGF‐beta receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J Cell Physiol 1999179325–335. [DOI] [PubMed] [Google Scholar]
- 13.Lagnado R, King A J, Donald F.et al A protocol for low contamination risk of autologous serum drops in the management of ocular surface disorders. Br J Ophthalmol 200488464–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsumoto Y, Dogru M, Goto E.et al Autologous serum application in the treatment of neurotrophic keratopathy. Ophthalmology 20041111115–1120. [DOI] [PubMed] [Google Scholar]
- 15.Tsubota K, Goto E, Shimmura S.et al Treatment of persistent corneal epithelial defect by autologous serum application. Ophthalmology 19991061984–1989. [DOI] [PubMed] [Google Scholar]
- 16.Tsubota K, Goto E, Fujita H.et al Treatment of dry eye by autologous serum application in Sjogren's syndrome. Br J Ophthalmol 199983390–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen H J, Pires R T, Tseng S C. Amniotic membrane transplantation for severe neurotrophic corneal ulcers. Br J Ophthalmol 200084826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King A J, Azuara‐Blanco A. Pericardial patch melting following glaucoma implant insertion. Eye 200115(Pt 2)236–237. [DOI] [PubMed] [Google Scholar]