Abstract
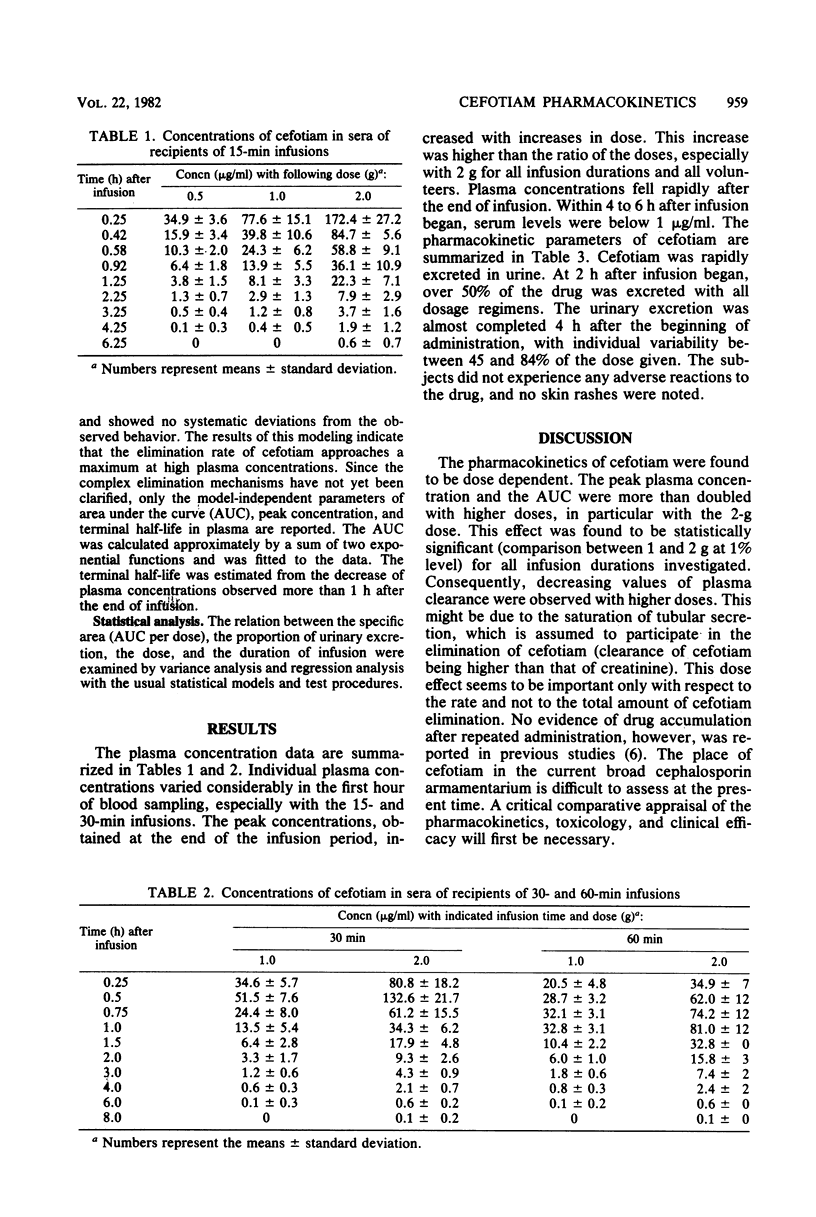
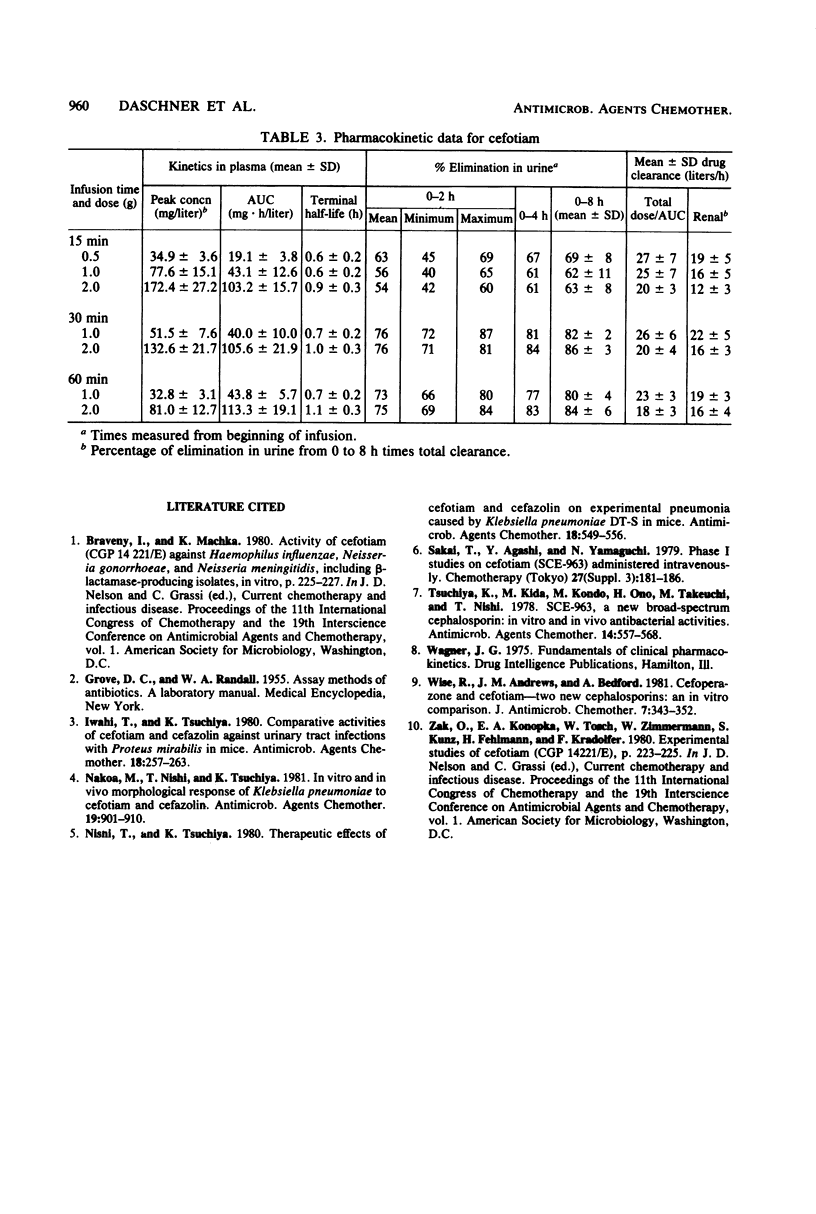
Doses of 0.5, 1.0, and 2.0 g of cefotiam were infused intravenously over a 15-min period in a crossover fashion to eight volunteers. Doses of 1.0 and 2.0 g were infused intravenously over periods of 30 and 60 min in a double crossover fashion to another eight volunteers. Serum concentrations fell rapidly from peak levels between 30 and 170 micrograms/ml at the end of the infusion to less than 1.0 micrograms/ml within 6 h after all regimens. The terminal half-life in plasma varied between 0.6 and 1.1 h. The slopes of the time-concentration curves with the different regimens showed different half-life data. The urinary excretion of cefotiam was mostly completed within 4 h of the beginning of drug administration. The pharmacokinetics of cefotiam were dose dependent. With the 2-g dose, the peak plasma concentrations and the values for the area under the curve were more than twice the values observed with 1 g; decreasing values of plasma clearance were observed with higher doses. Injection of cefotiam caused no immediate discomfort or reaction at the infusion site.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Iwahi T., Tsuchiya K. Comparative activities of cefotiam and cefazolin against urinary tract infections with Proteus mirabilis in mice. Antimicrob Agents Chemother. 1980 Aug;18(2):257–263. doi: 10.1128/aac.18.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao M., Nishi T., Tsuchiya K. In vitro and in vivo morphological response of Klebsiella pneumoniae to cefotiam and cefazolin. Antimicrob Agents Chemother. 1981 May;19(5):901–910. doi: 10.1128/aac.19.5.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi T., Tsuchiya K. Therapeutic effects of cefotiam and cefazolin on experimental pneumonia caused by Klebsiella pneumoniae DT-S in mice. Antimicrob Agents Chemother. 1980 Oct;18(4):549–556. doi: 10.1128/aac.18.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya K., Kida M., Kondo M., Ono H., Takeuchi M., Nishi T. SCE-963, a new broad-spectrum cephalosporin: in vitro and in vivo antibacterial activities. Antimicrob Agents Chemother. 1978 Oct;14(4):557–568. doi: 10.1128/aac.14.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Andrews J. M., Bedford K. A. Cefoperazone and cefotiam--two new cephalosporins: an in-vitro comparison. J Antimicrob Chemother. 1981 Apr;7(4):343–352. doi: 10.1093/jac/7.4.343. [DOI] [PubMed] [Google Scholar]


