Abstract
Aim
To evaluate the relative diagnostic strength of cup to disc (C/D) ratio, clinical disc damage likelihood scale (DDLS), a new clinical method of documenting glaucomatous optic disc changes, and Heidelberg retina tomograph (HRT‐II) in patients with glaucoma, glaucoma suspects, and normal controls.
Method
Consecutive observational case series. 110 eyes from 110 patients categorised as glaucoma, glaucoma suspect, or normal were examined clinically to grade the DDLS score. HRT‐II examination was performed by an examiner masked to the clinical examination findings. Optic disc parameters and Moorfields regression analysis findings were recorded. Stereophotographs of the optic disc were examined independently by two glaucoma specialists in masked fashion to determine the C/D ratio. Zeiss SITA Standard 24‐2 visual fields were obtained within 3 months of HRT‐II and clinical examination. For each patient, the eye with the worse mean deviation of the visual field test was enrolled in the study, and each field was additionally graded by the four level Hodapp‐Parrish‐II‐Anderson staging. Specificity and sensitivity were calculated by receiver operating characteristic (ROC) curves.
Results
Mean patient age was 58 years (SD 13.3) with 45 glaucoma patients, 23 glaucoma suspects, and 42 normals. The mean deviation on Humphrey visual field assessment using SITA‐Standard was −4.95 D (SD 5 D) Clinical examination using DDLS had the best predictive power with an area under the ROC curve value of 0.95 when glaucoma patients and suspects were separated from borderline or normals. This was followed by clinical examination of C/D ratio (0.84), and HRT‐II Moorfields analysis (0.68). The order of diagnostic strength did not change when definite glaucoma was compared to borderline and normals.
Conclusions
The DDLS grading performs well compared to C/D ratio and HRT‐II evaluation. Attention to disc diameter and to rim width may increase the value of clinical optic disc examination.
Keywords: disc damage likelihood scale, glaucoma, C:D ratio, Heidelberg retina tomograph
Glaucoma is characterised by progressive loss of retinal ganglion cells, manifest clinically by loss of optic disc neuroretinal rim tissue, defects in the retinal nerve fibre layer, and deficits on functional visual field testing. In order to detect early glaucomatous changes, clinicians need to identify these changes and distinguish them from variations of normal. In practice, clinicians evaluate the disc and nerve fibre layer by ophthalmoscopy and test the visual field to determine damage and to assess progression.
The cup/disc (C/D) ratio was introduced by Armaly1,2 as a standardised method to evaluate the optic nerve and to communicate the results. It has been shown that the larger the C/D ratio, the more severe the field damage is likely to be.3,4 However, the C/D ratio does not take into consideration the diameter of the optic disc, nor does it directly describe focal changes in the neuroretinal rim. It has been long recognised that focal rim loss, particularly at the vertical poles of the disc are characteristic of glaucoma.5,6 Several investigators have pointed out that small discs have fewer nerve fibres and smaller C/D ratios than do larger discs.7,8,9
The disc damage likelihood scale (DDLS) was devised by Spaeth et al to incorporate the evaluation of disc size and rim width in clinical grading of the disc.10 It has high interobserver reproducibility and correlates strongly with the degree of glaucomatous visual field damage.10,11
Optic disc examination has been automated with a variety of imaging instruments, including the Heidelberg retina tomograph II (HRT‐II), a confocal scanning laser ophthalmoscope. The HRT‐II evaluates disc topography with analysis software and determines optic disc parameters (such as C/D ratio), generates estimates of rim area and disc diameter, and grades the disc as normal, borderline, or abnormal compared to a normative database. Wollstein et al12 have shown a high level of accuracy of the Moorfields regression software to distinguish normal from abnormal discs in a European population, and found that the instrument in their study outperformed grading of stereophotographs by consensus of five experts.
The following study aims to compare the sensitivity and specificity of three methods of examination in glaucoma diagnosis: C/D ratio measured from stereophotographs, DDLS grading performed during clinical examination, and HRT‐II imaging parameters.
Methods
The research adhered to the tenets of the Declaration of Helsinki and the study was approved by the Auckland ethics committee. After informed consent, 110 consecutive patients were enrolled from a university glaucoma specialty clinic. Each patient had a diagnosis of definite glaucoma (n = 42), glaucoma suspect (n = 23), or no glaucoma (n = 45). The diagnosis was based on a full clinical evaluation by a glaucoma specialist, including medical and ophthalmic history, slit lamp examination, intraocular pressure, gonioscopy, funduscopy, and standard automated perimetry Swedish Interactive Thresholding Algorithm (SITA) Standard 24‐2 (Humphrey Field Analyser Model 740, Zeiss Meditec, Dublin, CA, USA).
Normal eyes were defined as those with no family history of glaucoma in a first degree relative, no history or evidence of intraocular surgery, and no retinal pathological features. Normal eyes also had a best corrected visual acuity of 20/40 or better, with refractive error between +3.00 dioptres and −6.00 dioptres, normal appearing optic nerve head, and normal visual field (VF) tests. Glaucoma suspects had no history or evidence of intraocular surgery, no evidence of retinal pathological features, and normal VF test results. Glaucoma suspects also had intraocular pressures (IOP) between 22 mm Hg and 30 mm Hg and/or asymmetric cupping (difference in vertical cupping greater than 0.2 between eyes), large cupping, abnormal appearing optic nerve head, or a family history of glaucoma in a first degree relative. Glaucomatous eyes were defined as those with abnormal VF test results with evidence of glaucomatous optic neuropathy. Glaucomatous optic neuropathy was defined as asymmetry between fellow eyes of greater than 0.2, rim thinning, notching, excavation, or retinal nerve fibre layer defects.
An abnormal visual field test was defined as an abnormal glaucoma hemifield test (“outside normal limits”) and at least three points in the same hemifield that exceeded a probability value of 0.5% in the pattern deviation plot, and an optic disc that was judged to be compatible with glaucoma and with the degree and type of visual field damage in that eye. In addition, there could be no signs of other diseases of the retina or optic nerve that could have led to the disc or field findings. The diagnosis was not directly based on the examination features to be tested in this study (DDLS grading, colour stereophotograph grading, or HRT‐II tests), each of which was examined in masked fashion at a different time from the diagnostic determination. It should be noted, however, that features of the disc were taken into account by the glaucoma specialist in the diagnostic criteria, and these features are also evaluated in one form or another in the clinical, photographic, and imaging studies. The diagnosis of glaucoma was made without reference to the untreated level of the intraocular pressure; nor was the intraocular pressure level at the time of photography, examination, or imaging measured in this study. The eye with the worse mean deviation (MD) on its visual field test was enrolled in the study. This produced the broadest range of damage for the tests, and assured that each eye included as glaucoma would exhibit damage; some people with glaucoma have one damaged and one normal eye, and inclusion of the latter would be a confounding feature in the study. Each eye underwent colour disc stereophotography and the vertical C/D ratio in each stereopair was examined independently using Canon CF60U camera (Canon, Tokyo, Japan) at the 30° setting using 35 mm Kodak Ektachrome EPR 150 film by two glaucoma specialists (HDM and MD). All stereo disc photographs were taken within 3 months of the study.
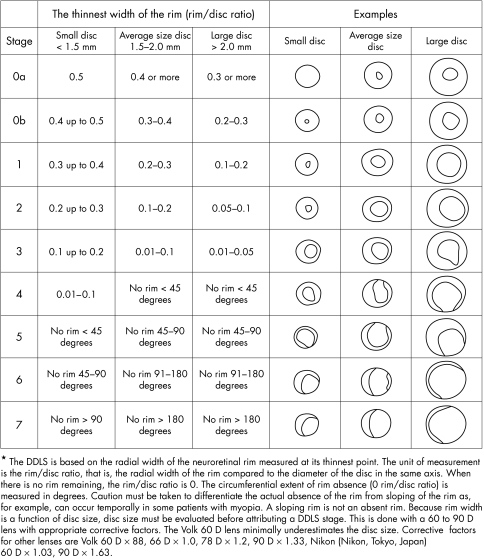
For DDLS grading, eyes were examined by a glaucoma specialist (HDM) masked to the clinical diagnosis using a Nikon 60 dioptre non‐contact fundus lens at a slit lamp. The DDLS stage descriptions were described by Bayer et al.3 Briefly, staging of DDLS involves slit lamp measurement of disc size and assessment of neuroretinal rim to disc ratio in whichever axis the rim is thinnest. In the absence of rim tissue, the angular distance (in degrees) of absent rim is measured. The disc is then staged from 0 to 7, as read from the DDLS table (fig 1).
Figure 1 Disc damage likelihood scale.
Visual fields were performed with SITA‐Standard 24‐2 within 3 months of the clinical assessment. An observer, who was masked to findings of the clinical examination and diagnosis, recorded MD, pattern standard deviation (PSD), and graded the field using modified Hodapp‐Parrish‐Anderson (HPA) staging (the original staging was applied to 30‐2 field tests).13 This staging categorises each field into one of four groups: normal, early change, moderate change, or severe change. It should be noted that an abnormal hemifield test is part of the grading system of the HPA staging.
Each eye was also examined using the HRT‐II, which was performed on the day of the clinical examination. The HRT‐II uses a 670 nm diode laser as a light source. Sixty four confocal images, each 384×384 pixels are converted to a single topographic image. An experienced operator masked to the diagnosis and clinical assessment performed the examinations and marked out the disc margin on all eyes examined. All individual disc parameters as well as Moorfields regression analysis results were recorded for each eye. All standard deviations were less than 20 μm.
Subjects with a refractive error of more than 5 dioptres from emmetropia or 2.5 dioptres of astigmatism, history of neurological diseases, intraocular trauma, intraocular surgery (except subjects who had undergone cataract surgery more than 9 months before the examination), or other optic neuropathies that may affect visual fields or colour vision were excluded from the study. Patients with unreliable visual field tests (fixation losses, false positive, and false negatives >25%) were excluded.
Statistical methods included univariate Spearman's rank correlation coefficient to explore the linear relation between variables and multivariate models in which a variety of iterative logistic regressions (forward, backward, and stepwise selection) were used to examine the predictive value of variables in the separation of glaucoma from suspect or normal. Sensitivity and specificity were calculated for each examination method. Firstly, we tested how well each method discriminated between glaucoma and those classified as suspects or normal. Then, calculations were repeated looking at the separation of glaucoma from normal with suspects excluded from analysis.
The receiver operating characteristic (ROC) curves were drawn for a variety of variables, and the area under the curve (AOC) was used as a statistic in non‐parametric analysis to estimate the value of each method in identifying glaucoma eyes.14 All tests were two tailed and a 5% significance level was maintained throughout. The procedures of the analysis program SAS (v 8.2 SAS Institute Inc) were used.
Results
The study group consisted of people with an average age of 58 years, nearly equally divided between men and women (table 1).
Table 1 Demographics of study patients.
| Sex | ||
| Male/female | 53/57 | |
| Age (years) | ||
| Mean | 58 (SD 13.3) | |
| Median | 59 | |
| Range | 11–88 | |
| Diagnosis | ||
| No glaucoma | 42 | |
| Glaucoma suspect | 23 | |
| Definite glaucoma | 45 | |
| Visual field | ||
| Mean deviation | −4.95 (SD 5) | |
| Range | −20.9 to 1.4 | |
| Optic nerve parameters | ||
| Vertical C:D ratio | 0.63 (SD 0.19) |
They represented the spectrum of glaucoma damage, with an average MD in the mild category (−4.95). Clinical examination using DDLS had the highest AUC, 0.91 predictive values of variables in the separation of glaucoma from suspect or normal. (table 2).
Table 2 Area under curve for receiver operator characteristics for diagnostic tools in glaucoma.
| Glaucoma v borderline and normal (CI) | Glaucoma and borderline v normal (CI) | ||
|---|---|---|---|
| HRT‐II parameters | 0.51 to 0.62 | ||
| Rim area | 0.62 (0.47 to 0.67) | ||
| Rim volume | 0.58 (0.47 to 0.64) | ||
| Cup shape measure | 0.58 (0,48 to 0.64) | ||
| Moorfields regression analysis | 0.54 (0.45 to 0.68) | 0.68 (0.61 to 0.87) | |
| Visual field HPA | 0.75 (0.63 to 0.87) | 0.81(0.70 to 0.93) | |
| Visual field mean deviation | 0.78 (0.72 to 0.91) | 0.84 (0.63 to 0.89) | |
| Visual field pattern standard deviation | 0.80 (0.70 to 0.93) | 0.84 (0.73 to 0.91) | |
| Clinical examination C:D | 0.81(61 to 0.93) | 0.84 (0.74 to 0.92) | |
| Clinical examination DDLS | 0.91 (−.84 to 0.98) | 0.95 (0.80 to 0.98) |
HRT‐II, Heidelberg retina tomograph; HPA, Hodapp‐Parrish‐Anderson VF score; DDLS, disc damage likelihood score; C:D, cup to disc ratio; CI, confidence intervals.
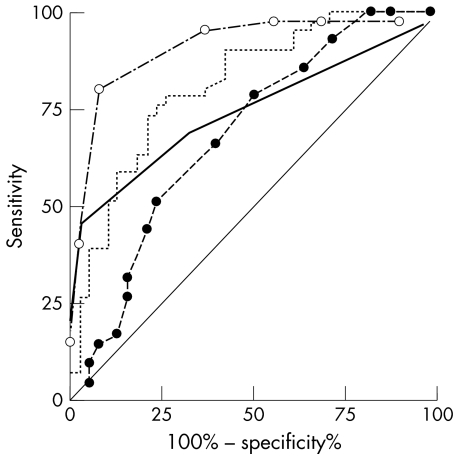
This was followed by vertical C/D ratio (AUC = 0.81), MD on visual field examination (0.78), HPA scoring (0.75) and HRT‐II rim area (0.62), and Moorfields regression analysis (0.54). When calculations were repeated using the less stringent discrimination of definite glaucoma versus no glaucoma, the order of diagnostic strength did not change. Figure 2 illustrates the receiver operator curves for these parameters.
Figure 2 Receiver operator curves for mean deviation (dotted line), Hodapp‐Parrish‐Anderson scoring (solid line), disc damage likelihood score (broken line, open dots), and cup to disc ratio (broken line, solid dots) for glaucoma versus borderline and normals.
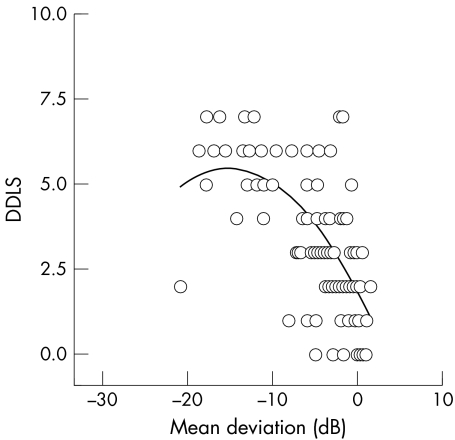
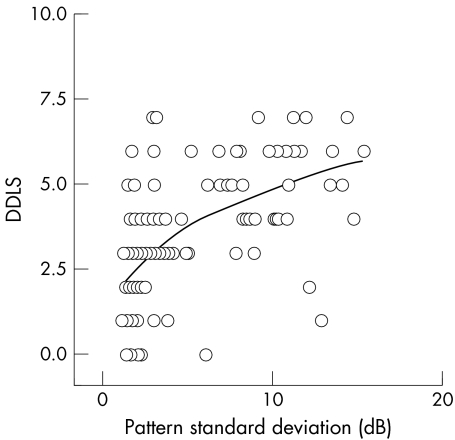
Scatter plots were obtained for the relation of DDLS with field MD and PSD. There was a strong inverse correlation between DDLS stage and MD (fig 3) and a positive correlation between DDLS stage and PSD (fig 4). Regression analysis found that both linear and curvilinear fits of the data had similar r2 values. The DDLS stage was seen to follow the HPA visual field score.
Figure 3 Scatter plot of relation between mean deviation of the visual field and disc damage likelihood scale (DDLS). Linear regression analysis showed a statistically significant correclation with a correlation coefficient r, = −0.62, p<0.0001.
Figure 4 Scatter plot of relation between pattern standard deviation of the visual field and disc damage likelihood scale (DDLS). Linear regression analysis showed a statistically significant correclation for DDLS with a correlation coefficient r, = −0.61, p<0.0001, r2 = 0.32.
When the DDLS stage was regressed against HRT‐II parameters, HRT‐II rim area was a significant independent predictor of DDLS, adjusting for disc size (p<0.0001), the partial r2 value was 16%). Additionally, disc size is also a significant independent predictor of DDLS, though it explained only an additional 4% of the variance in DDLS (p = 0.02).
Discussion
Our results demonstrate that DDLS is an excellent method to distinguish between glaucoma and normal eyes, and in the present study, it outperformed C/D ratio. The C/D ratio has several shortcomings. It only indirectly examines the neuroretinal rim tissue, concentrating on the width of the hole rather than the surrounding rim tissue that determine its border. Also, the examiner is focused on the ratio in the vertical disc axis and may overlook focal thinning in an oblique axis. The C/D ratio does not take into consideration the optic disc size. Hence, large discs which are likely to have larger C/D ratio (but may have normal neuroretinal rims) are more likely to be classified as glaucomatous,15 while small discs with small C/D ratio are more likely to be classified as normal, whether they actually have glaucoma or not.16
Previous research has demonstrated that neuroretinal rim area correlates more strongly with field damage than C/D ratio or cup volume.17 The two major advantages of DDLS are, firstly, that it considers disc size and, secondly, that it focuses attention on how much neuroretinal rim tissue is present. Consideration of disc size is central to the DDLS process. By categorising discs as small, medium, or large, the expectation of rim thickness is adjusted. This reduces misclassification bias based on disc size. The introduction of DDLS formalises the process of examining the neuroretinal rim. The examiner is forced to examine the rim throughout its circumference, documenting the area of greatest thinning.
DDLS has some limitations. It is theoretically possible that a patient with a static DDLS grade may have advancing damage—for example, if focal notching of the disc was followed by generalised atrophy. For this reason patients should be monitored longitudinally with other modalities. It is also not easy to apply to tilted optic nerves or to those with sloped temporal rims. Furthermore, DDLS does not offer a system to document progression in more than one region or a new region of the optic disc until it is more severely involved than the originally documented area. The method requires some effort to learn and is best carried out with the table of stages at hand during slit lamp evaluation of the fundus. While the C/D ratio is ingrained as a clinical tool, the additional discipline of systematic DDLS grading adds value to clinical observation.
HRT‐II software attempts to distinguish normal discs from early glaucoma discs, using a normative database consisting of a white, English population. In one study, the HRT‐II linear regression technique was found to separate the two groups with a specificity and sensitivity of 96% and 84%, respectively, and even to outperform clinician evaluation of disc photographs.18 Others have also found that the HRT‐II is accurate and repeatable in assessing optic nerve head anatomy.19,20 We did not find that the HRT‐II performed as well in our group of patients under the conditions of this study. The differences between our findings and other reports may derive from differences in the patient population or from the diagnostic criteria used. Clearly, laser imaging provides large amounts of information, including rim evaluation and disc size measures. Further refinements in the use of HRT‐II testing may further increase its clinical value.
We compared the MD in visual field tests to clinical disc measures for its predictive value. Because we used the hemifield test to categorise the patients as having glaucoma, we could not use this measure in the analysis. MD is known to be relatively non‐specific, declining with general sensitivity loss related to cataract and other factors. It was therefore not surprising that both DDLS and C/D ratio outperformed MD.
The relation of DDLS to visual field indices in our data shows a generally curvilinear relation, with many having advanced stages in DDLS with minimal field change. Other comparisons of the structural and functional changes in glaucoma eyes have suggested that this may indicate that structural measures of glaucoma injury are detected before functional measures21,22 with present technology. Histological studies of human glaucoma eyes show loss of 25–35% of retinal ganglion cells before clinically significant abnormalities are found in the same eyes by automated perimetry.23,24 Garway‐Heath has recently pointed out that when both structural and functional measures are plotted on logarithmic scales (or both on linear scales), the structure‐function relation is seen to be linear.25 While this might suggest that functional change should be detected as early as structural change, the methods now available for visual sensitivity have too high a variability to allow them to call the eye defective in functional testing as early as our structural examinations do.26
A limitation of this study, as with other studies of glaucoma diagnostic accuracy, is that they are confounded by some degree by how to define glaucoma. If a study wishes to evaluate structural changes, how valid is it to include structure in the definition? Yet, if it is not included, one risks placement of non‐glaucoma eyes in the study population. On the other hand, use of disc and nerve fibre layer examination alone could allow the inclusion of eyes that are not at a stage of functional loss—or inclusion of eyes at the extreme of the distribution of C/D ratio that are only anatomical variants. Many reports,11,27,28 including this one, use an experienced glaucoma clinician with a predetermined definition of glaucoma as the arbiter of what is “characteristic” glaucoma damage. Until a detailed understanding what objective and independent parameters of the optic nerve head can be called glaucoma can be agreed upon this potential bias will need to be acknowledged in studies that attempt to identify diagnostic accuracy of glaucoma. To minimise this, in the present study the patients were classified as glaucoma, suspects or normal before recruitment and independent of the assessment of the stereodisc photographs, DDLS score, and HRT‐II imaging. Furthermore, the stereodisc photographs were assessed by an independent examiner who had not been involved in the previous classification of the patients.
In conclusion, the DDLS staging system appears to be superior to C/D ratio as a clinical approach to optic disc evaluation. Its systematic estimation of disc size and rim narrowing is apparently at least as sensitive and specific as laser imaging by HRT‐II, at lower cost. It continues to be important to carefully examine the optic disc for glaucoma management.
Acknowledgements
This research was supported through unrestricted funds by Alcon New Zealand for the NZ Alcon Optic Nerve Research Fellowship. The authors thank Dr HA Quigley for editorial review.
Abbreviations
AOC - area under the curve
C:D ratio - cup to disc ratio
DDLS - disc damage likelihood scale
HPA staging - Hodapp‐Parrish‐Anderson staging
HRT - Heidelberg retina tomography
IOP - intraocular pressure
MD - mean deviation
PSD - pattern standard deviation
ROC - receiver operating characteristic
VF - visual field
References
- 1.Armaly M. Genetic determination of cup/disk ratio of the optic nerve. Arch Ophthalmol 19677835–43. [DOI] [PubMed] [Google Scholar]
- 2.Armaly M, Sayeghi R. The cup/disk ratio. Arch Ophthalmol 196982191–196. [DOI] [PubMed] [Google Scholar]
- 3.Douglas G, Drance S, Schulzer M. A correlation of fields and disks in open angle glaucoma. Can J Ophthalmol 19749391–398. [PubMed] [Google Scholar]
- 4.Hart W J, Yablonski M, Kass M.et al Multivariate analysis of the risk of glaucomatous visual field loss. Arch Ophthalmol 1979971455–1458. [DOI] [PubMed] [Google Scholar]
- 5.Kirsch R E, Anderson D R. Clinical recognition of glaucomatous cupping. Am J Ophthalmol 197375442–454. [DOI] [PubMed] [Google Scholar]
- 6.Quigley HA I I. Changes in the appearance of the optic disc. Surv Ophthalmol 198530117–126. [DOI] [PubMed] [Google Scholar]
- 7.Caprioli J. Discrimination between normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 1992;33153–9. [PubMed]
- 8.Jonas J B, Schmidt A M, Müller‐Bergh J A.et al Human optic nerve fiber count and optic disc size. Invest Ophthalmol Vis Sci 1992332012–2018. [PubMed] [Google Scholar]
- 9.Quigley H A, Coleman A L, Dorman‐Pease M E. Larger optic nerve heads have more nerve fibers in normal monkey eyes. Arch Ophthalmol 19911091441–1443. [DOI] [PubMed] [Google Scholar]
- 10.Bayer A, Harasymowycz P, Henderer J D.et al Validity of a new disk grading scale for estimating glaucomatous damage: correlation with visual field damage. Am J Ophthalmol 2002133758–763. [DOI] [PubMed] [Google Scholar]
- 11.Henderer J D, Liu C, Kesen M.et al Reliability of the disk damage likelihood scale. Am J Ophthalmol 200313544–48. [DOI] [PubMed] [Google Scholar]
- 12.Wollstein G, Garway‐Heath D F, Hitchings R A. Identification of early glaucoma cases with the scanning laser ophthalmoscope. Ophthalmology 19981051557–1563. [DOI] [PubMed] [Google Scholar]
- 13.Hodapp E, Parish R, Anderson D.Clinical decision in glaucoma. St Louis: CV Mosby, 199311–63.
- 14.DeLong E R, DeLong D M, Clarke‐Pearson D L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach 1988. Biometrics 44837–845. [PubMed] [Google Scholar]
- 15.Jonas J B, Zach F M, Gusek G C.et al Pseudoglaucomatous physiologic large cups. Am J Ophthalmol. 1989;107;137–44. [DOI] [PubMed]
- 16.Heijl A, Molder H. Optic disc diameter influences the ability to detect glaucomatous disc damage. Acta Opthalmol 199371122–129. [DOI] [PubMed] [Google Scholar]
- 17.Caprioli J, Miller J M. Correlation of structure and function in glaucoma. Quantitative measurements of disc and field. Ophthalmology 198895723–727. [DOI] [PubMed] [Google Scholar]
- 18.Wollstein G, Garway‐Heath D F, Fontana L.et al Identifying early glaucomatous changes. Comparison between expert clinical assessment of optic disc photographs and confocal scanning ophthalmoscopy. Ophthalmology 20001072272–2277. [DOI] [PubMed] [Google Scholar]
- 19.Chauhan B C, LeBlanc R P, McCormick T A.et al Test‐Retest variability of topographic measurements with confocal scanning laser tomography in patients with glaucoma and control subjects. Am J Ophthalmol 19941189–15. [DOI] [PubMed] [Google Scholar]
- 20.Rohrschneider K, Burk R O, Kruse F E.et al Reproducibility of the optic nerve head topography with a new laser tomographic scanning device. Ophthalmology 19941011044–1049. [DOI] [PubMed] [Google Scholar]
- 21.Zeyen T G, Caprioli J. Progression of disc and field damage in early glaucoma. Arch Ophthalmol 199311162–65. [DOI] [PubMed] [Google Scholar]
- 22.Jonas J B, Grundler A E. Correlation between mean visual field loss and morphometric optic disk variables in the open‐angle glaucomas. Am J Ophthalmol 1997124488–497. [DOI] [PubMed] [Google Scholar]
- 23.Quigley H A, Addicks E M, Green W R.et al Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch Ophthalmol 198199635–649. [DOI] [PubMed] [Google Scholar]
- 24.Kerrigan‐Baumrind L A, Quigley H A, Pease M E.et al Numbers of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same person. Invest Ophthalmol Vis Sci 200041741–748. [PubMed] [Google Scholar]
- 25.Quigley H A, Dunkelberger G R, Green W R. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmology 1989107453–464. [DOI] [PubMed] [Google Scholar]
- 26.Harwerth R S, Quigley H A. Visual field defects and retinal ganglion cell losses in human glaucoma patients. Arch Ophthalmol. (accepted for publication) [DOI] [PMC free article] [PubMed]
- 27.Correnti A J, Wollstein G, Price L L.et al Comparison of optic nerve head assessment with a digital stereoscopic camera (Discam), scanning laser ophthalmoscopy, and stereophotography. Ophthalmology 20031101499–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kesen M R, Spaeth G L, Henderer J D.et al The Heidelberg Retina Tomograph vs clinical impression in the diagnosis of glaucoma. Am J Ophthalmol 2002133613–616. [DOI] [PubMed] [Google Scholar]