Abstract
Aim
To evaluate the role of optical coherence tomography (OCT) in determining choroidal neovascularisation (CNV) activity before and after photodynamic therapy (PDT) in patients with pathological myopia.
Methods
33 patients (33 eyes) with pathological myopia and being treated with PDT were included. Every 3 months all patients were evaluated and presence or absence of leakage on fluorescein angiography, presence of intraretinal or subretinal fluid on OCT, and macular and choroidal neovascular complex thickness on OCT, were determined at each examination.
Results
The macular thickness decreased significantly after PDT at 6 months (p = 0.001) and at 12 months follow up (p = 0.01). However, no significant changes in CNV thickness were measured after PDT at 6 months of follow up (p = 0.418) and at 12 months of follow up (p = 0.521). Once the diagnosis of CNV associated with pathological myopia was established, before treatment, OCT had a sensitivity of 96.96% for detecting CNV activity. After treatment, OCT had a good sensitivity (95.23%) and a moderate specificity (69,69%) in determining CNV activity, which resulted in a diagnostic efficiency (proportion of correct results) of 79.62%.
Conclusions
OCT appears to be useful for indicating CNV activity. Therefore, it may serve as a complementary technique for deciding the need for PDT and re‐treatment in patients with pathological myopia.
Keywords: photodynamic therapy, myopia, choroidal neovacularisation, optical coherence tomography, fluorescein angiography
Pathological myopia is a major cause of blindness,1,2 and in many cases the vision loss is the result of the development of choroidal neovascularisation (CNV).3
The results of the Verteporfin in Photodynamic (VIP) studies4,5 showed that photodynamic therapy (PDT) is beneficial for subfoveal CNV related to pathological myopia. Currently, fluorescein leakage from CNV on fluorescein angiography (FA) is the main criterion in the decision to treat and re‐treat using PDT.4,5
We recently found that OCT is useful for identifying CNV activity and that OCT may be a complementary technique for determining the need for PDT and re‐treatment in patients with age related macular degeneration (AMD).6
The aim of the current study was to assess the sensitivity and specificity of OCT in detecting the presence of subretinal or intraretinal fluid for identifying CNV activity before and after PDT in patients with CNV associated with pathological myopia. We also evaluated the role of OCT in detecting changes associated with PDT in these patients.
Methods
A total of 33 consecutive patients who presented with signs of pathological myopia were included in this prospective observational case study.
The research followed the tenets of the Declaration of Helsinki; and the study was approved by the university clinic research committee. The best corrected visual acuity (BCVA) was evaluated using the Snellen chart by the same certified optometrist. During disease evolution, when the BCVA reached 20/400, ETDRS at 2 metres was used and the visual acuity (VA) was expressed as the Snellen equivalent.
The same two independent observers determined the presence or absence of leakage on the angiograms in each case. When the two observers did not agree, the judgment of the senior retina specialist was accepted as valid.
Optical coherence tomograms were obtained with a commercially available unit (OCT3, Humphrey‐Zeiss, Dublin, CA, USA) using the previously reported methodology.6,7,8 The same experienced technician performed all OCT evaluations. Another independent observer, who was masked to the patient status, evaluated the OCT scans on each occasion. The following data were recorded: retinal thickness at the fovea, CNV complex thickness, and the presence or absence of subretinal and intraretinal fluid. Measurements of the retinal thickness at the fovea were performed using a manually assisted technique in the system software (version A 1.1; Zeiss‐Humphrey). Foveal fixation and landmark functions were used to perform every scan in the same macular region.
CNV was considered present in cases in which well defined thickness and fragmentation of the retinal pigment epithelium (RPE)/choriocapillaris reflection were observed.9 Only the well defined CNV on the OCT scans were measured manually to determine thickening. If subretinal or intraretinal fluid was present on the OCT scan, that remark was considered positive. FA was considered the standard reference for these two findings on OCT and was considered positive when leakage was present.
Statistical analysis
Sensitivity, specificity, diagnostic efficiency, and the likelihood ratio for a positive and a negative test were calculated for OCT, with FA as the standard reference. The Student t test was used to assess differences between paired means (macular and CNV thickness change). Two tailed p values less than 0.05 were considered significant. The association between the pretreatment macular thickness and the pretreatment CNV thickness with BCVA evolution (post‐treatment minus pretreatment BCVA) was calculated with Pearson's correlation coefficient.
Results
Thirty three consecutive white patients (11 men, 22 women) were included in this study. The average age was 52.06 (SD 9.49) years.
Macular thickness was measured using OCT at the pretreatment examination and at the 6 month follow up visit in these 33 eyes. At the 12 month follow up examination, the macular thickness was measured in 29 eyes. The mean number of treatments in the group followed for 12 months (n = 29) was 2.06 (SD 1.24, range 1–5). Table 1 shows the data regarding macular thickness and CNV thickness. A statistically significant difference was observed between the macular thickness at 6 months and the pretreatment macular thickness (paired t test; p = 0.001). A significant difference also was found between the macular thickness at the 12 month follow up visit and the pretreatment macular thickness (paired t test, p = 0.01).
Table 1 Change in macular and choroidal neovascularisation (CNV) thickness after photodynamic therapy.
| Pretreatment | 6 Months | 12 Months | |
|---|---|---|---|
| Retinal thickness at fovea (SD) (μm) | 363 (102) (n = 33) | 310 (93)∗ (n = 33) | 310 (96)† (n = 29) |
| CNV thickness (SD) (μm) | 175 (43) (n = 24) | 168 (39) (n = 24) | 170 (50) (n = 20) |
∗Statistically significant difference with respect to the pretreatment measure (p = 0.001); †statistically significant difference with respect to the pretreatment measure (p = 0.01).
The thickness of the CNV complex also was obtained at the same examinations. At pretreatment a well defined CNV on the OCT scans was identified and measured in 24 of 33 eyes (72.72%). At the 6 month follow up visits, in the same 24 of 33 eyes, the CNV complex was again identified. At the 12 month follow up visit, well defined CNV on the OCT scans was measured in 20 of 29 eyes (68.96%). In the rest of the scans, the CNV could not be clearly identified clearly. No statistically significant change in CNV thickness was observed at the 6 month follow up examination compared with the pretreatment CNV thickness (paired t test, p = 0.418) (table 1). Likewise, the difference between the CNV thickness at the 12 month follow up visit and pretreatment was not statistically significant (paired t test, p = 0.521).
No significant correlation was found between the pretreatment macular thickness and changes in the BCVA after 6 months (r = 0.167; p = 0.368) or 12 months (r = 0.024; p = 0.907). Likewise, no significant correlation was found between the pretreatment CNV thickness and changes in BCVA after 6 months (r = 0.091; p = 0.672) or 12 months (r = 0.288; p = 0.218).
There was agreement on the presence of leakage between the two observers in 119 of 141 angiograms interpreted.
Before treatment, FA leakage was seen in all eyes, and OCT detected the presence of either intraretinal or subretinal fluid (fig 1) in 32 of 33 eyes (96.96%). Therefore, with FA as the reference standard, OCT had a sensitivity of nearly 97% in detecting CNV activity. Only one case (3.03%) in which leakage was present on pretreatment FA was considered negative on OCT.
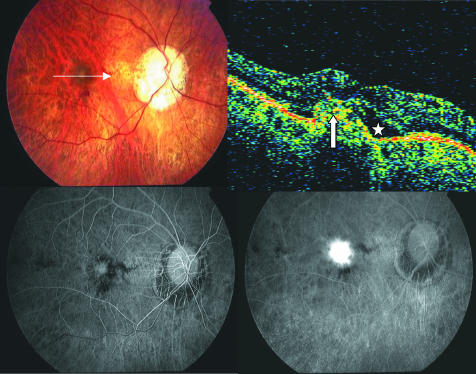
Figure 1 Pretreatment of the right eye of a patient with pathological myopia. Top left, fundus photograph shows characteristic of pathological myopia and signs of choroidal neovascularisation (CNV). Top right, optical coherence tomography (OCT) shows subretinal fluid (asterisk). The CNV complex also is identifiable in OCT scan (white arrow). Bottom left, early phase of angiogram shows a classic CNV membrane. Bottom right, leakage on a late phase angiogram.
After treatment leakage was observed on 42 angiograms; in 40 (95.23%) of them OCT showed intraretinal or subretinal fluid (fig 2). Nevertheless, of a total of 66 angiograms in which no leakage was observed, only 46 (69.69%) had no intraretinal or subretinal fluid (fig 3). With FA as the standard, OCT achieved a sensitivity of 95.23% in determining CNV activity after treatment. However, OCT obtained a moderate specificity of 69.69%. The likelihood ratio for a positive test was 3.06, and the likelihood ratio for a negative test was 0.07. OCT had a diagnostic efficiency of 79.62% to detect post‐treatment CNV activity in the presence of intraretinal or subretinal fluid.
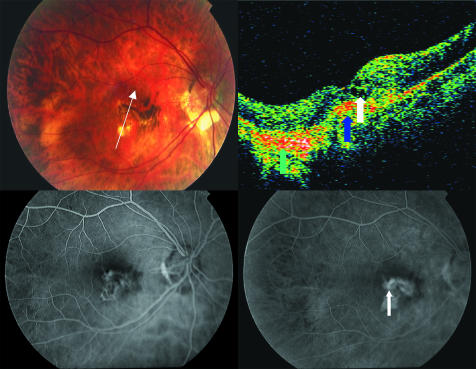
Figure 2 The right eye of a patient 3 months after the second photodynamic therapy sesion. Top left, fundus photograph shows ophthalmoscopic signs of CNV, chorioretinal atrophy and hyperpigmentation. Top right, intraretinal fluid (white arrow), signs of chorioretinal atrophy (green arrow) and choroidal neovascular complex (blue arrow) are present on the OCT scan. Bottom left and right, early and late phases of angiogram, respectively. In the late phase a minimun leakage is seen (white arrow).
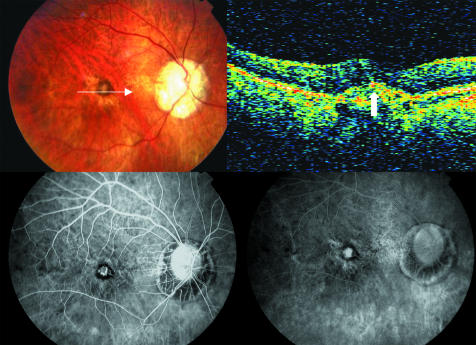
Figure 3 The right eye of the same patient of figure 1 after photodynamic therapy. Top left, the fundus photograph does not show ophthalmoscopic signs of lesion activity. Top right, an hyper‐reflective lesion related to the CNV complex identified on fluorescein angiogram is identifiable on OCT (white arrow), but no intraretinal or subretinal fluid is seen. Bottom left and right, early and late phases of angiogram respectively, show staining of the lesion but no leakage.
Discussion
In the present study, we demonstrated that when CNV secondary to pathological myopia was diagnosed with intraretinal and subretinal fluid, OCT seemed to have excellent sensitivity and moderate specificity in detecting CNV activity, with FA leakage as the standard test.
Our results indicated that the macular thickness decreased significantly at the 6 month and 12 month examinations. We hypothesise that the reduction in macular thickness in our series of eyes resulted from partial or total resolution of the subretinal fluid, intraretinal fluid, or both.10
We identified the CNV complex in 72.72% of eyes at pretreatment. These percentages are only slightly higher that those we obtained in a series of eyes with predominantly classic CNV secondary to ARMD.6 Hee et al9 described that the CNV complex was better defined by OCT in classic lesions. Because most of the CNV lesions in pathological myopia are only classic and located above the RPE, it was easier to detect the suspected CNV complex by OCT. However, because OCT cannot differentiate blood from fibrosis on CNV, OCT alone cannot be used to detect CNV. Currently, posterior pole biomicroscopy and FA are needed to correctly establish a diagnosis of CNV associated with pathological myopia.
In the current study, using OCT, we did not find significant changes in CNV thickness after PDT (table 1). On one hand, although we used foveal fixation and landmark functions to achieve good image registration, it is possible that the difficulty of precisely replicating the scan location could have introduced errors in the interpretation of the changes in lesion thickness over time.
On the other hand, PDT was reported to have a vaso‐occlusive mechanism that affects both the CNV and the normal choroid.11 However, compared with other treatments such as laser photocoagulation, CNV does not completely resolve after PDT.12 Other authors,13 who reported histological findings of surgically excised myopic choroidal neovascular membrane after PDT, concluded that PDT induced only temporary occlusion.
We did not find a significant correlation between BCVA changes and pretreatment macular thickness or pretreatment CNV thickness, which may indicate that pretreatment macular thickness is not associated with the final success of PDT. Sahni et al also reported that there was no statistically significant association between the presence or absence of intraretinal fluid and subretinal fluid and VA in patients with ARMD who had undergone PDT.14
We reached the decision to retreat patients based on BCVA and FA. The presence of fluorescein leakage from CNV was the main criterion for retreating patients during follow up, as reported previously.4,5,15 Nevertheless, we found that FA is sometimes difficult to interpret.16,17,18 In the present study, there was agreement on the presence of leakage between the two observers in 84.39% of the angiograms with CNV associated with pathological myopia. This agreement was slightly less than the agreement we achieved when interpreting the angiograms of patients with ARMD.6 In our experience, FA was inconclusive in a higher percentage of eyes with CNV related to pathological myopia than in cases with exudative AMD.6
In our study, OCT detected intraretinal or subretinal fluid in 20 (30.3%) of the cases in which FA did not show clear leakage. Recently, we found that in patients with ARMD, OCT detected intraretinal or subretinal fluid in 14.2% of the cases in which FA did not show clear leakage.6 We think that in some cases of pathological myopia, leakage is even more difficult to identify than in patients with AMD. CNV in pathological myopia is often diagnosed in younger patients in whom the RPE is involved with the neovascular complex, and fluid is difficult to estimate with slit lamp biomicroscopy and leakage is not always easily evaluated. Nevertheless, we do not propose that the presence of intraretinal or subretinal fluid in OCT indicates that treatment or re‐treatment is always required. The decision to treat or re‐treat in each case also must be based on slit lamp biomicroscopy, BCVA, and the presence of leakage on FA. However, in accordance with other authors, we propose that OCT may assist in therapeutic decision making,6,14,19 especially when there are doubts based on the results of other techniques.
In conclusion, OCT may be a good complementary imaging technique in the decision making process regarding treatment or re‐treatment with PDT of CNV in pathological myopia. This may be especially useful when FA is inconclusive15 or the general condition of the patient does not allow frequent FA examinations.20,21
Abbreviations
AMD - age related macular degeneration
BCVA - best corrected visual acuity
CNV - choroidal neovascularisation
FA - fluorescein angiography
OCT - optical coherence tomography
PDT - photodynamic therapy
RPE - retinal pigment epithelium
VIP study - Verteporfin in Photodynamic study
References
- 1.Ghafour I M, Allan D, Foulds W S. Common causes of blindness and visual handicap in the west of Scotland. Br J Ophthalmol 198367209–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sperduto R D, Seigel D, Roberts J.et al Prevalence of myopia in the United States. Arch Ophthalmol 1983101405–407. [DOI] [PubMed] [Google Scholar]
- 3.Tano Y. Pathologic myopia: where are we now? Am J Ophthalmol 2002134645–660. [DOI] [PubMed] [Google Scholar]
- 4.Verteporfin in Photodynamic Therapy (VIP) Study Group Photodynamic Therapy of Subfoveal Choroidal neovascularization in pathologic myopia with verteporfin. 1‐Year results of a randomized clinical trial—VIP report No 1. Ophthalmology 2001108841–852. [DOI] [PubMed] [Google Scholar]
- 5.Verteporfin in Photodynamic Therapy (VIP) Study Group Verteporfin therapy of subfoveal choroidal neovascularization in pathologic myopia. 2‐year Results of a randomized clinical trial—VIP Report No 3. Ophthalmology 2003110667–673. [DOI] [PubMed] [Google Scholar]
- 6.Salinas‐Alamán A, García‐Layana A, Maldonado M J.et al Using optical coherence tomography to monitor photodynamic therapy in age related macular degeneration. Am J Ophthalmol 200514023–28. [DOI] [PubMed] [Google Scholar]
- 7.Sánchez‐Tocino H, Álvarez‐Vidal A, Maldonado M J.et al Retinal thickness study with optical coherence tomography in patients with diabetes. Invest Ophthalmol Vis Sci 2002431588–1594. [PubMed] [Google Scholar]
- 8.Hee M R, Puliafito C A, Wong C.et al Quantitative assessment of macular edema with optical coherence tomography. Arch Ophthalmol 19951131019–1029. [DOI] [PubMed] [Google Scholar]
- 9.Hee M R, Baumal C R, Puliafito C A.et al Optical coherence tomography of age‐related macular degeneration and choroidal neovascularization. Ophthalmology 19961031260–1270. [DOI] [PubMed] [Google Scholar]
- 10.Rogers A H, Martidis A, Greenberg P B.et al Optical coherence tomography findings following photodynamic therapy of choroidal neovascularization. Am J Ophthalmol 2002134566–576. [DOI] [PubMed] [Google Scholar]
- 11.Costa R A, Farah M E, Cardillo J A.et al Immediate indocyanine green angiography and optical coherence tomography evaluation after photodynamic therapy for subfoveal choroidal neovascularization. Retina 200323159–165. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt‐Erfurth U, Michels S, Barbazetto I.et al Photodynamic effects on choroidal neovascularization and physiological choroid. Invest Ophthalmol Vis Sci 200243830–841. [PubMed] [Google Scholar]
- 13.Scupola A, Ventura L, Tiberti A C.et al Histological findings of a surgically excised myopic choroidal neovascular membrane after photodynamic therapy. A case report. Graefes Arch Clin Exp Ophthalmol 2004242605–610. [DOI] [PubMed] [Google Scholar]
- 14.Sahni J, Stanga P, Wong D.et al Optical coherence tomography in photodynamic therapy for subfoveal choroidal neovascularization secondary to age related macular degeneration: a cross sectional study. Br J Ophthalmol 200589316–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Treatment of Age‐Related Macular Degeneration with Photodynamic Therapy (TAP) and Verteporfin in Photodynamic Therapy (VIP) study groups Photodynamic therapy of subfoveal choroidal neovascularization with verteporfin. Fluorescein angiographic guidelines for evaluation and treatment—TAP and VIP. Report No 2. Arch Ophthalmol 20031211253–1268. [DOI] [PubMed] [Google Scholar]
- 16.Holz F G, Jorzik J, Schutt F.et al Agreement among ophthalmologists in evaluating fluorescein angiograms in patients with neovascular age‐related macular degeneration for photodynamic therapy eligibility (FLAP‐Study). Ophthalmology 2003110400–405. [DOI] [PubMed] [Google Scholar]
- 17.Kaiser R S, Berger J W, Williams G A.et al Variability in fluorescein angiography interpretation for photodynamic therapy in age‐related macular degeneration. Retina 200222683–690. [DOI] [PubMed] [Google Scholar]
- 18.Friedman S M, Margo C E. Choroidal neovascular membranes: reproducibility of angiographic interpretation. Am J Ophthalmol 2000130839–841. [DOI] [PubMed] [Google Scholar]
- 19.Coscas G, Coscas F, Soubrane G. Monitoring the patient after treatment: angiographic aspects of recurrence and indications for retreatment. J Fr Ophtalmol 20042781–92. [DOI] [PubMed] [Google Scholar]
- 20.Yannuzzi L A, Rohrer K T, Tindel L J.et al Fluorescein angiography complication survey. Ophthalmology 198693611–617. [DOI] [PubMed] [Google Scholar]
- 21.Karhunen U, Raitta C, Kala R. Adverse reactions to fluorescein angiography. Acta Ophthalmol 198664282–286. [DOI] [PubMed] [Google Scholar]