We describe a real time polymerase chain reaction (PCR) technique for the detection of treponemal DNA in the vitreous of patients with suspected syphilitic uveitis.
Case 1
A 41 year old white homosexual male presented with a 1 week history of pain, redness, and reduced visual acuity in the right eye. There was a recent history of mouth ulcers and skin rashes involving the left lower limb. Corrected Snellen visual acuities were 6/60 and 6/6 in the right and left eyes respectively. In the right eye there were 4+ cells in the anterior chamber and vitreous. The right optic disc was swollen with patchy retinitis involving the inferior quadrants. The left eye was normal. Treponema specific serology tests, total antibody enzyme immunoassay assay (EIA) and Treponema pallidum particle agglutination test (TPPA) were strongly positive. Rapid plasma reagin (RPR) titre was 1:512, consistent with active treponemal infection. Subsequent cerebrospinal fluid analysis was also positive for both RPR and TPPA, with the additional finding of lymphocytosis.
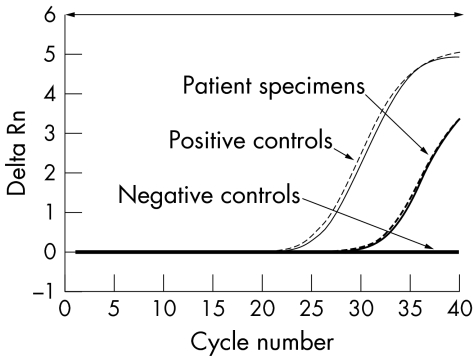
PCR evaluation of vitreous for herpes viruses and Toxoplasma revealed a positive result for Epsein‐Barr virus. An in‐house TaqMan probe based real time PCR assay (Applied biosystems, UK) targeting the Treponema pallidum repeat protein C (Tpr C) gene detected the presence of the T pallidum DNA.2 The patient's DNA extract was tested in parallel with T pallidum Nichols strain Seattle genomic DNA as a positive control and a DNA extract previously tested negative for T pallidum as negative control. All samples were tested in duplicate (fig 1). The results confirmed the presence of T pallidum DNA in the vitreous and, with serological and CSF evidence, a diagnosis of secondary syphilis with syphilitic panuveitis was confirmed.
Figure 1 Real time PCR amplifications plot of the vitreous sample of case 1 tested for treponemal DNA in duplicates. The change in normalised reporter signal (Delta Rn) was interpreted with T pallidum positive and negative controls.
Case 2
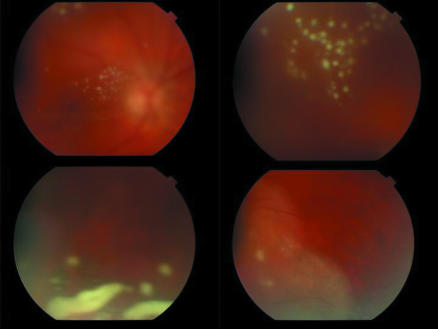
A 53 year old man presented with a 1 month history of rapidly progressive decrease in vision in the left eye. His visual acuities were 6/6 in the right eye and counting fingers in the left eye. He had a panuveitis in the left eye, with evidence of 4+ cells in the anterior chamber and vitreous. There were multiple fluffy white opacities in the vitreous and fundus examination revealed peripheral retinal infiltrates (fig 2). A working diagnosis of acute retinal necrosis was made but vitreous PCR results were negative for herpes viruses and Toxoplasma. There was a poor clinical response to systemic valaciclovir therapy and within a 3 week period, he developed multiple vitreous opacities and peripheral retinal infiltrates in the right eye. His treponemal serology was strongly positive and the RPR titre was 1:256, indicating active treponemal infection. Additionally, he was positive for treponemal IgM tested by EIA. Cerebrospinal fluid showed lymphocytosis. PCR analysis of vitreous detected treponemal DNA and a diagnosis of bilateral syphilitic panuveitis was established and appropriate therapy begun.
Figure 2 Fundus photographs showing intense vitritis, an unusual presenting sign of acute syphilitic posterior uveitis that resolved completely following a 4 week course of oral doxycyclin therapy.
Comment
Serology is the mainstay of diagnosis of syphilis.1 However, serological testing cannot identify whether there is active infection in a particular organ. This has led to the development of PCR based techniques, which specifically target difficult diagnostic areas such as neurosyphilis and congenital syphilis.2,3,4 To our knowledge, this is the first report of the use of real time PCR to detect T pallidum DNA in vitreous samples. While syphilis was adequately diagnosed by serology in both cases, the specific detection of T pallidum DNA and exclusion of other infective agents, particularly herpes simplex virus and varicella zoster virus, helped in patient management. This is particularly important in HIV infected patients who may have concurrent infection by more than one agent.5
The real time PCR method with a specific fluorescent labelled probe used in our patients is specific for T pallidum. However, none of the diagnostic methods, including DNA based tests, can distinguish between syphilis and infection with other pathogenic treponemes, including yaws, pinta, non‐venereal endemic syphilis, or bejel.4
As there is only limited experience of using this PCR test on vitreous samples, further studies are required to establish the sensitivity and specificity. Nevertheless, the test can provide confirmation of the diagnosis of ocular disease in patients with positive syphilis serology and adds to the diagnostic evaluation of patients who may have multiple infections.
Acknowledgements
The real time treponemal PCR project was supported by a research grant received from Guy's and St Thomas's charity. The positive control used in the study was kindly supplied by Professor S Lukehart, University of Washington, USA.
References
- 1.Egglestone S I, Turner A J, for the PHLS syphilis serology working group Serological diagnosis of syphilis. Commun Dis Public Health 20003158–162. [PubMed] [Google Scholar]
- 2.Noordhoek G T, Wolters E C, De Jonge M E J.et al Detection by polymerase chain reaction of Treponema pallidum DNA in cerebrospinal fluid from neurosyphilis patients before and after treatment. J Clin Microbiol 1991291976–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grimprel E, Sanchez P J, Wendel G D.et al Use of polymerase chain reaction and rabbit infectivity to detect Treponema pallidum in amniotic fluid. J Clin Microbiol 1991291711–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larsen S A, Steiner B M, Rudolph Laboratory diagnosis and interpretation for syphilis. Clin Microbiol Rev 199581–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ormerod L D, Puklin J E, Sobel J D. Syphilitc posterior uveitis: correlative findings and significance. Clin Infect Dis 2001321661–1673. [DOI] [PubMed] [Google Scholar]