Abstract
Backgound/aim
The transport of radiolabelled photoreceptor outer segments (POS) lipids was investigated by cultured retinal pigment epithelial cells (RPE). Phagocytosis of POS by the RPE is essential to maintain the health and function of the photoreceptors in vivo. POS are phagocytised at the apical cell surface of RPE cells. Phagocytised POS lipids may be either recycled to the photoreceptors for reincorporation into new POS or they may be transported to the basolateral surface for efflux into the circulation.
Results
The authors have demonstrated that high density lipoprotein (HDL) stimulates efflux of radiolabelled lipids, of POS origin, from the basal surface of RPE cells in culture. Effluxed lipids bind preferentially to HDL species of low and high molecular weight. Effluxed radiolabelled phosphotidyl choline was the major phospholipid bound to HDL, with lesser amounts of phosphatidyl ethanolamine, phosphatidyl inosotol. Effluxed radiolabelled triglycerides, cholesterol, and cholesterol esters also bound to HDL. Lipid free apolipoprotein A‐I (apoA‐I) and apoA‐I containing vesicles also stimulate lipid efflux.
Conclusion
The findings suggest a role for HDL and apoA‐I in regulating lipid and cholesterol transport from RPE cells that may influence the pathological lipid accumulation associated with age related macular degeneration.
Keywords: Bruch's membrane, retinal pigment epithelium, lipid, age related macular degeneration
Age related macular degeneration (AMD) is the leading cause of visual loss in the Western world.1,2,3 Most visual loss in AMD develops secondary to neovascularisation beneath the retina that leads to haemorrhage, accumulation of subretinal fluid and, inevitably, replacement of macular tissue with a scar.4 Before visual loss from AMD, there is progressive accumulation of lipids in Bruch's membrane, a multilayered extracellular tissue separating the retina from its choroidal blood supply.5,6 Progressive lipid deposition in Bruch's membrane reduces diffusional transport from the choroid to the retina and is thought to impair retinal function.7,8 There has been considerable debate over whether the lipid deposits in Bruch's membrane are of circulatory or retinal origin. Recent evidence suggests the predominant source of this lipid is from the retina, deriving from residues of degraded photoreceptor outer segments (POS) effluxed from the retinal pigment epithelium (RPE) into Bruch's membrane.9 Although cholesteryl ester and apolipoprotein B deposition in Bruch's membrane suggests contribution from plasma lipids, analysis of lipids and apolipoproteins from tissue and RPE cell cultures indicates that these cells may account for most of the deposits.10,11 Mechanisms by which lipids efflux from the RPE across Bruch's membrane and into the choroidal circulation are incompletely understood. RPE cells express apolipoprotein E (apoE),12,13 scavenger receptor BI (SR‐BI),14 and ATP binding cassette transporter A1 (ABCA1),15 all recognised components of reverse cholesterol transport (RCT).16 Similar to macrophages, apoE expression is regulated by nuclear hormone receptor ligands.13
AMD shares risk factors with atherosclerosis, such as smoking, hypertension, and elevated C reactive protein (CRP) levels.17,18,19 The relation between AMD and hyperlipidaemia is not consistent.20,21,22,23,24,25,26 Investigators have speculated that since the main source of Bruch's membrane lipids is the retina and RPE, and not the circulation, serum lipids levels would not necessarily correlate with the extent of lipid deposition in Bruch's membrane.8,9,11 Serum HDL levels have also not been associated consistently with AMD. Several studies showed a positive correlation between serum HDL levels and advanced stages of AMD.24,25,26 Other studies have not confirmed these results.22,23 Recently, in a case‐control study of a Veterans Affairs Medical Center cohort, HDL levels correlated negatively with the development of neovascularisation in patients with AMD.27
The atheroprotective properties of HDL include promotion of RCT, antioxidative, and anti‐inflammatory effects (reviewed by Assmann and Nofer28 and Nofer et al29). The Age‐Related Eye Disease Study demonstrated that high dose supplementation with anti‐oxidant vitamins C and E, β carotene, and zinc reduces visual loss in patients with macular degeneration.30 The importance of anti‐oxidants may be attributed to protection against lipid peroxidation owing to the high content of oxygen, polyunsaturated fatty acids, and light irradiation in the retina.31 The anti‐oxidative and anti‐inflammatory attributes of HDL may protect against visual loss associated with AMD. The presence of CRP, complement components, and macrophages in Bruch's membrane deposits is suggestive of a chronic inflammatory response in AMD.18
To determine whether HDL may be involved in RCT from RPE cells, we have studied human RPE cells in culture incubated with radiolabelled POS. We demonstrate that labelled lipids of POS origin are transported through RPE for efflux from the cell at the basolateral surface. The effluxed labelled lipids (primarily phospholipids) are bound preferentially to HDL of both low and high molecular weight species in a process that is stimulated by HDL and apolipoprotein A‐I (apoA‐I).
Materials and methods
Cell culture and POS labelling
Primary cultures of normal human RPE cells from a 35 year old male donor were grown as described.14,32 RPE cells (passage 5–10) were propagated to confluence on laminin coated six well or 12 well Costar Transwell tissue culture plates (Fischer Scientific, Los Angeles, CA, USA) with DMEM H21 containing 5% FBS, 2 mM glutamine, 5 mg/ml gentamycin, 100 IU/ml penicillin, 100 mg/ml streptomycin, 2.5 mg/ml fungizone, 1 ng/ml bFGF, and 1 ng/ml EGF in the top and bottom chambers. POS were prepared from bovine retinas as described33 and stored at −70°C for use. POS were labelled with 1,4,7,10,13,16,19‐[1‐14C] docosahexanoic acid (DHA) (ICN Life Sciences, 49 Ci/mol) as described.34 For lipid efflux experiments, cell monolayers were washed three times with Dulbecco's phosphate buffered saline (PBS) and medium containing 14C labelled POS (50 µg/ml) and 5% lipoprotein free fetal calf serum was added to the top chambers. Bottom chambers contained apolipoprotein and lipoprotein acceptors in serum free medium.
Lipoprotein purification and analyses
Low density lipoprotein (LDL) (d = 1.019–1.063 g/ml) and HDL (d = 1.063–1.210 g/ml) were purified from human plasma by KBr density gradient ultracentrifugation as described.35 ApoA‐I was purified from human HDL as described.36 RPE media samples were adjusted to d = 1.25 g/ml with solid potassium bromide, underlayered with a KBr solution (d = 1.21 g/ml), and ultracentrifuged (Beckman 50.2 Ti rotor) at 45 000 rpm for 24 hours at 10°C. The lipoprotein containing d<1.21 g/ml fraction was transferred to a centrifugal ultrafilter (5K MCO, Viva Sciences, Hannover, Germany), buffer exchanged to 0.15 M NaCl, 1 mM EDTA (pH 7.4), 0.025% NaN3 (Sal‐EN), and concentrated.
Lipoprotein fractions were analysed by non‐denaturing PAGE. Briefly, samples were electrophoresed in linear 0–30% gradient PAG at 200 V at 10°C for 3000 V hours. Gels were calibrated to the mobilities of calibrator proteins (HMW kit, Amersham Pharmacia, Piscataway, NJ, USA) supplemented with LDL and ovalbumin, Stokes diameter, 25 nm and 6.0 nm, respectively. Distribution of 14C label was determined by fractionating Coomassie stained gel into 2 mm slices. Gel samples were treated with 0.2 ml TS‐1 reagent (Research Products International, Mt. Prospect, IL, USA) at 50°C, overnight in a shaking waterbath, cooled; 0.04 ml glacial acetic acid added before radioactivity was determined by liquid scintillation counting (LSC).
Discoidal HDL composed of purified human plasma apoA‐I, DMPC, and cholesterol were produced by the sodium cholate dialysis method37 and purified by FPLC on two tandemly connected columns (Superdex 200, Amersham Pharmacia, Piscataway, NJ, USA).
Thin layer chromatography (TLC)
14C labelled lipids were extracted from HDL by the Bligh‐Dyer method38 and separated by one dimensional TLC by sequential development; first in solvent 1: chloroform/methanol/acetic acid/water (25:15:4:2) until the solvent front had progressed half way up the plate; then in solvent 2: n‐hexane/diethylether/acetic acid (65:35:2), until the solvent front reached the top of the plate. Lipid species were detected by acid charring. Plates were immersed in 7.5% copper acetate, 2.5% copper sulfate, 8% phosphoric acid, and heated on a hotplate for 1 hour. Lipid spots identified by charring were cut out and subjected to liquid scintillation counting.
Results
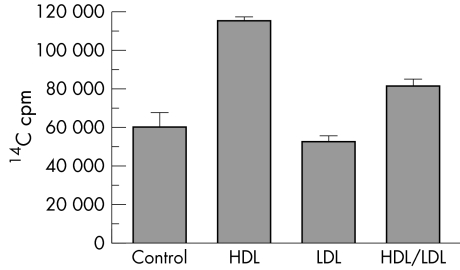
Since HDL has been demonstrated to facilitate lipid and cholesterol efflux in macrophages, we sought to determine whether HDL has similar effect on lipid efflux from RPE cells. RPE cells were cultured in Transwell plates and fed 14C‐DHA labelled bovine POS in the apical chambers in the presence or absence of purified lipoproteins added to the bottom media. Lipoprotein acceptors included LDL (100 µg/ml), HDL (100 µg/ml), and LDL+HDL (50 µg/ml each). After 36 hours 14C in basal media was determined by liquid scintillation counting. As shown in figure 1, total 14C in basal media was significantly increased by HDL (p = 0.0027, two tailed t test). HDL stimulated basal 14C labelled lipid efflux 1.9‐fold compared to no lipoprotein acceptor. LDL did not significantly increase basal efflux of 14C labelled lipids (p = 0.4293, two tailed t test). When LDL and HDL were present together, stimulation of 14C labelled lipid efflux was about half that of HDL alone (1.4‐fold), although this was not significantly different from the control (p = 0.0719, two tailed t test).
Figure 1 HDL stimulates efflux of 14C labelled lipids from RPE cells in culture (p = 0.0027, t test, n = 3). Total 14C cpm in basal medium (mean (SEM)) is shown.
In order to determine whether basally effuxed 14C labelled lipids associated with lipoproteins, like samples were combined and lipoproteins were purified from basal media by ultracentrifugation at a density of 1.21 g/ml. The amount of 14C in the d<1.21 g/ml density fraction for each sample was determined by liquid scintillation and is given in table 1.
Table 1 14C labelled lipid associated with lipoprotein.
| 14C cpm bound | |
|---|---|
| Control | 1270 |
| HDL | 71 800 |
| LDL | 5860 |
| HDL/LDL | 31 400 |
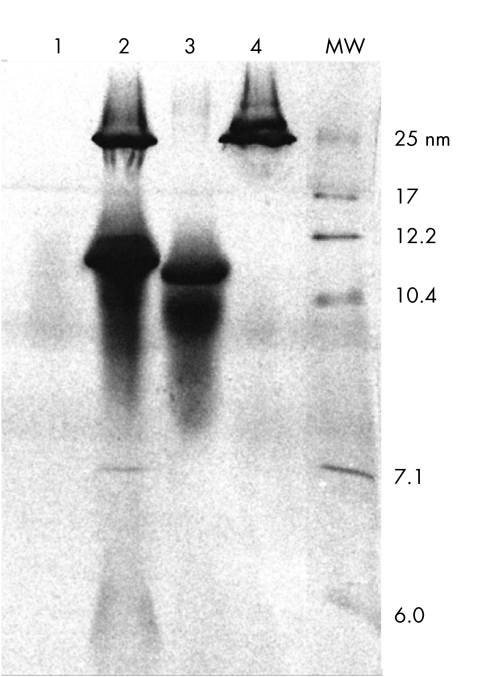
HDL bound about 14‐fold more 14C labelled lipids than did LDL. When both LDL and HDL were present, 14C in the d<1.21 g/ml fraction was intermediate to the amount when either HDL or LDL were present alone. In the absence of added lipoproteins, control media had low, but measurable, levels of radioactivity in the d<1.21 g/ml fraction. The ultracentrifuged media lipoprotein fractions were resolved by non‐denaturing PAGE (fig 2). The Coomassie stained components observed in HDL (fig 2, lane 3) and LDL (fig 2, lane 4) are typical lipoprotein profiles expected of pure LDL and HDL. For purposes of comparison control basal medium (fig 2, lane 1) and purified plasma lipoprotein (fig 2, lane 2) profiles are also shown.
Figure 2 Repurification of HDL and LDL following incubation with RPE cells fed 14C labelled POS. Shown are Coomassie stained gel lanes containing samples: control medium (lane 1), purified plasma lipoproteins (lane 2), repurified HDL (lane 3), and repurified LDL (lane 4). Calibrator proteins of known Stokes diameter (nm) in lane labelled MW.
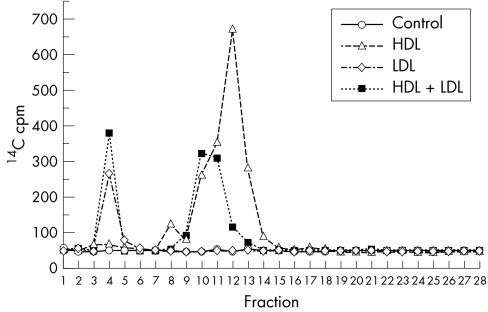
The distribution of 14C labelled lipids among the lipoproteins in HDL and LDL samples was determined. Gel lanes (fig 2, lanes 3 and 4) were fractionated and counted. As shown in figure 3, radioactivity was confined to the lipoproteins present in each sample: HDL (1783 cpm), LDL (266 cpm). HDL+LDL was separated on another gel (not shown) and yielded 966 cpm in the HDL band and 380 cpm in the LDL band. Again, HDL was a better acceptor (sixfold to sevenfold) than LDL when tested as a pure lipoprotein and in plasma. When purified LDL and HDL were combined, HDL exhibited a twofold to threefold higher affinity for basally effluxed 14C labelled lipids.
Figure 3 Distribution of radioactivity in repurified lipoproteins. Polyacrylamide gel lanes were fractionated from the top (fraction 1) to the bottom (fraction 28). 14C was quantified by liquid scintillation counting. Coomassie blue stained fractions 3–5 (LDL) and fractions 9–14 (HDL).
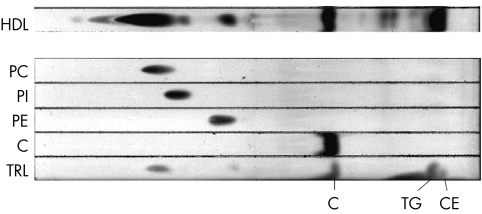
Lipids were extracted from the HDL fraction, purified as above, and partially purified by one dimensional TLC. As shown in figure 4 several lipid spots could be identified. Most of the 14C label was in phosphatidyl choline (PC) and cholesterol (C), with lesser amounts in phosphatidyl inosotol (PI), phosphatidyl ethanolamine (PE), triglycerides (TG) and cholesterol esters (CE) (table 2). The remaining 14C label was in a dozen other, as yet unidentified, spots.
Figure 4 TLC separation and identification of some lipids bound to HDL. Following incubation with RPE cells fed 14C labelled POS, HDL bound lipids were extracted and separated by TLC (bottom of plate at the left). Standards for pure phosphatidyl choline (PC), phosphatidyl inosotol (PI), phosphatidyl ethanolamine (PE), and cholesterol (C) were run, as well as triglyceride rich lipids (TRL) which contains triglycerides (TG) and cholesterol esters (CE) were run.
Table 2 14C labelled lipid bound to HDC.
| Lipid | % cpm |
|---|---|
| PC | 26.0 |
| PI | 3.0 |
| PE | 3.0 |
| C | 21.0 |
| TG | 2.0 |
| CE | 2.0 |
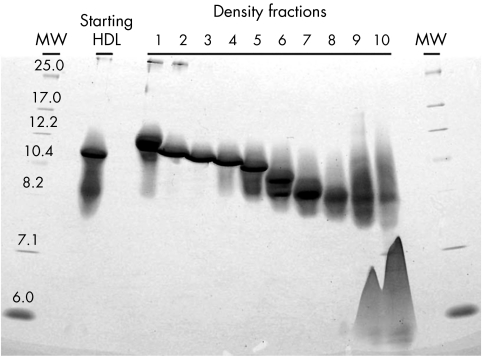
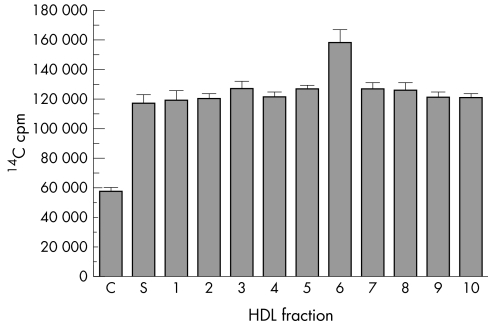
As a first step in determining which HDL fraction was the most potent stimulator of 14C labelled lipid efflux, we fractionated plasma HDL (1.063<d<1.210) by ultracentrifugation in a continuous KBr density gradient. Ten HDL fractions ranging in density (1.07–1.18 g/ml) and particle size (6–11 nm, Stoke's diameter) (fig 5) were tested in equivalent protein concentrations (100 µg/ml). All HDL fractions stimulated basal efflux of 14C labelled lipids more than twofold (p<0.0005, t test) (fig 6). In addition, all HDL fractions bound effluxed 14C labelled lipids (not shown).
Figure 5 Isolation of HDL subspecies. HDL was separated by KBr ultracentrifugation, fractionated and analysed by non‐denaturing PAGE. Shown is a Coomassie stained gel. Densities of each fraction are: F1 (d = 1.077), F2 (d = 1.086), F3 (d = 1.096), F4 (d = 1.105), F5 (d = 1.116), F6 (d = 1.127), F7 (d = 1.141), F8 (d = 1.154), F9 (d = 1.176), F10 (d = 1.191). Calibrator proteins of known Stoke's diameter (nm) are in lanes labelled MW.
Figure 6 All HDL subspecies stimulate efflux of 14C labelled lipids from RPE cells in culture (p<0.0005, t test, n = 3). Total 14C cpm in basal medium (mean (SEM)) is shown.
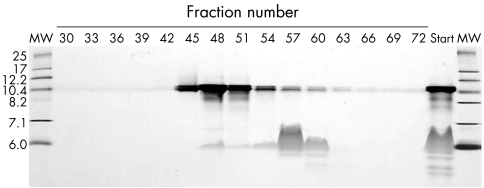
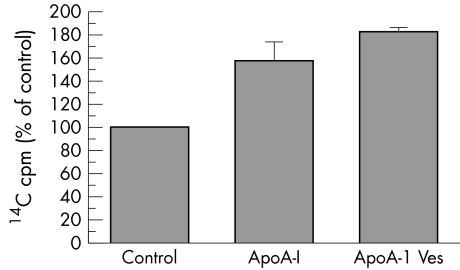
As a first step in identifying the components of HDL necessary and sufficient for stimulating basal efflux of 14C labelled lipids, an artificial HDL, consisting of purified apoA‐I, cholesterol, and DMPC, was synthesised as described in Methods. Purified artificial HDL (apoA‐I vesicles), average Stoke's diameter of 10 nm, is shown in figure 7, fractions 44–49. The ability of purified apoA‐I and apoA‐I vesicles (fractions 44–49), to stimulate basal 14C labelled lipid efflux was tested. Both purified apoA‐I and apoA‐I vesicles stimulated 14C labelled lipid efflux by about 1.5‐fold to 2‐fold (p = 0.0079, Mann‐Whitney test) (fig 8).
Figure 7 Purification of synthetic HDL (apoA‐I vesicles). Discrete lipid particles from sodium cholate dispersions of DMPC, cholesterol, and apo A‐I were purified by FPLC and fractions were analysed by non‐denaturing PAGE followed by Coomassie blue staining. Starting material (Start), and calibrator proteins of known Stoke's diameter (nm) (MW) are shown.
Figure 8 Purified apoA‐I (ApoA‐I) and synthetic HDL (ApoA‐I Ves) stimulate efflux of 14C labelled lipids from RPE cells in culture (p = 0.0079, Mann‐Whitney test, n = 5). Results are the combination of two separate experiments normalised to control levels of 14C cpm in basal medium. Control is 100%.
Discussion
In non‐ocular cell types, where RCT and its regulation has been studied extensively,16 nascent HDL particles containing apoA‐I bind to ABCA1, promoting phospholipid and cholesterol efflux. Binding of these lipids to HDL forms pre‐beta migrating HDL, which is then converted to larger alpha migrating HDL through esterification of cholesterol by lecithin:cholesterol acyltransferase (LCAT). In macrophages, incubation with apoA‐I, the major apolipoprotein component of HDL, increases efflux, probably mediated by direct binding of apoA‐I to ABCA1.39 Recent evidence suggests that apoA‐I binding to ABCA1 may reduce ABCA1 turnover, effectively increasing overall efflux mediated by this transporter.40
Lipid efflux from RPE may be mediated, as it is in macrophages, by SR‐BI and ABCA1. We have previously demonstrated expression of these proteins by cultured human RPE cells and, in the case of ABCA1, have localised expression to the basal aspect of the cell.14,15 Increased lipid efflux by RPE in the presence of HDL and apo A‐I is probably mediated by binding of the lipoproteins to ABCA1. To bind ABCA1 in the basal RPE plasma membrane, a lipoprotein acceptor must traverse Bruch's membrane from the choriocapillaris. With ageing, there is progressive thickening of Bruch's membrane. This thickening is associated with a reduction in macromolecular permeability and hydraulic conductivity across Bruch's membrane.7,41 Moore and Clover have reported a 10‐fold reduction in macromolecular permeability of Bruch's membrane from the first to the ninth decades of life.41 They show that proteins of molecular weight >200 kDa could traverse a young patient's Bruch's membrane, while elderly patients had an exclusion limit of between 100–200 kDa.
We have demonstrated that various species of HDL bind 14C labelled lipids basally effluxed by human RPE. Analyses of these density subclasses show that they range in size of 6.0–12.2 nm Stokes diameter. Their corresponding apparent molecular weights (Mr 43–440 kDa) are consistent with the possibility that HDL may affect lipid transport in vivo. The potential functional differences of different HDL subspecies or their abilities to traverse Bruch's membrane have not been extensively studied. Serum levels of HDL2 have been demonstrated to be negatively correlated with risk for coronary disease.42 Gordiyenko et al have demonstrate that rhodamine labelled LDL can traverse Bruch's membrane in the mouse.43 However, little is known of the permeability of human submacular Bruch's membrane to LDL and HDL in vivo. It is possible that, with ageing, some of the larger molecular weight HDL species may not traverse Bruch's membrane efficiently. This might lead to increased lipid accumulation in the RPE and Bruch's membrane. Furthermore, there is no known mechanism, other than diffusion across Bruch's membrane, for removing lipids from Bruch's membrane if they were effluxed by other means—for example, SR‐BI or as large apoB containing lipoproteins.10 The inability of larger molecular weight lipoprotein acceptors to fully traverse Bruch's membrane might contribute to progressive lipid accumulation that occurs with ageing in Bruch's membrane. In the present study, apo A‐I increased lipid efflux by approximately 50% in cultured human RPE. The molecular weight of apo A‐I is 28 kDa and may be better at traversing a thickened Bruch's membrane in older subjects. Thus, nascent HDL particles such as pre‐beta HDL (6.0 nm Stokes diameter, personal communication, B Ishida 2005) may be particularly important in removing lipids from RPE and Bruch's membrane in older individuals.
Reducing access of some HDL species to Bruch's membrane and the RPE may have other consequences in ageing. HDL's atheroprotective effects derive not only from its role in reverse cholesterol transport, but also its anti‐oxidative properties.28 HDL bound enzymes, paraoxonase, and platelet activating factor acetylhydrolase inhibit lipid peroxidation. Because lipid peroxidation has been implicated in both the pathogenesis of AMD and identified as a potential therapeutic target, HDL's potent anti‐oxidants may play a part in slowing the progression of AMD. A Bruch's membrane barrier to HDL diffusion may effectively diminish the anti‐oxidant properties of this lipoprotein.
Since lipid accumulation in Bruch's membrane (basal linear deposit) is one of the best histopathological correlates with AMD,44,45 an understanding of the mechanisms of RCT in the RPE is particularly important. The present study demonstrates that HDL is a preferred lipoprotein acceptor for effluxed residues derived from phagocytised POS. The changes that occur in ageing and AMD may impair access of HDL and apoA‐I to the basal surface of the RPE and the inner aspect of Bruch's membrane. A resultant decrease in RCT may contribute to the pathological deposition of lipid and cholesterol observed in AMD. Furthermore, pharmaceutical strategies to increase RCT in RPE may be useful in treating the early stages of AMD.
Acknowledgements
This study was supported by a gift from That Man May See, Inc (DMS); and grants from: Merit Review Grant from the Veterans Affairs Medical Center (DMS), and National Institutes of Health (R01‐HL31210) (JPK).
Abbreviations
ABCA1 - ATP binding cassette transporter A1
AMD - age related macular degeneration
apoE - apolipoprotein E
apoA‐I - apolipoprotein A‐I
C - cholesterol
CE - cholesterol esters
CRP - C reactive protein
DHA - docosahexanoic acid
HDL - high density lipoprotein
LCAT - lecithin:cholesterol acyltransferase
LDL - low density lipoprotein
LSC - liquid scintillation counting
PBS - phosphate buffered saline
PC - PI, phosphatidyl choline
phosphatidyl inosotol -
PE - phosphatidyl ethanolamine
POS - photoreceptor outer segments
RCT - reverse cholesterol transport
RPE - retinal pigment epithelium
SR‐BI - scavenger receptor BI
TG - triglycerides
TLC - thin layer chromatography
Footnotes
Competing interests: There are no competing interests to report for any of the authors.
References
- 1.National Advisory Eye Council Vision research—a national plan: 1999–2003. Vol 1. A report of the National Advisory Eye Council. Bethesda, MD: National Institutes of Health, NIH publication 199998–4120.
- 2.Leibowitz H M, Krueger D E, Maunder L R.et al The Framingham Eye Study monograph: An ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973–1975. Surv Ophthalmol 198024(Suppl)335–610. [PubMed] [Google Scholar]
- 3.Rosenberg T, Klie F. The incidence of registered blindness caused by age‐related macular degeneration. Acta Ophthalmol Scand 199674399–402. [DOI] [PubMed] [Google Scholar]
- 4.Bressler N M, Bressler S B, Fine S L. Age‐related macular degeneration. Surv Ophthalmol 198832375–413. [DOI] [PubMed] [Google Scholar]
- 5.Sheraidah G, Steinmetz R, Maguire J.et al Correlation between lipids extracted from Bruch's membrane and age. Ophthalmology 199310047–51. [DOI] [PubMed] [Google Scholar]
- 6.Spaide R F, Ho‐Spaide W C.et al Characterization of peroxidized lipids in Bruch's membrane. Retina 199919141–147. [DOI] [PubMed] [Google Scholar]
- 7.Moore D J, Hussain A A, Marshall J. Age‐related variation in the hydraulic conductivity of Bruch's membrane. Invest Ophthalmol Vis Sci 1995361290–1297. [PubMed] [Google Scholar]
- 8.Ruberti J W, Curcio C A, Millican C L.et al Quick‐freeze/deep‐etch visualization of age‐related lipid accumulation in Bruch's membrane. Invest Ophthalmol Vis Sci 2003441753–1759. [DOI] [PubMed] [Google Scholar]
- 9.Holz F G, Sheraidah G, Pauleikhoff D.et al Analysis of lipid deposits extracted from human macular and peripheral Bruch's membrane. Arch Ophthalmol 1994112402–406. [DOI] [PubMed] [Google Scholar]
- 10.Li C M, Presley J B, Zhang X.et al Retina expresses microsomal triglyceride transfer protein: implications for age‐related maculopathy. J Lipid Res 200546628–640. [DOI] [PubMed] [Google Scholar]
- 11.Malek G, Li C M, Guidry C.et al Apolipoprotein B in cholesterol‐containing drusen and basal deposits of human eyes with age‐related maculopathy. Am J Pathol 2003162413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson D H, Ozaki S, Nealon M.et al Local cellular sources of apolipoprotein E in the human retina and retinal pigmented epithelium: implications for the process of drusen formation. Am J Ophthalmol 2001131767–781. [DOI] [PubMed] [Google Scholar]
- 13.Ishida B Y, Bailey K R, Duncan K G.et al Regulated expression of apolipoprotein E by human retinal pigment epithelial cells. J Lipid Res 200445263–271. [DOI] [PubMed] [Google Scholar]
- 14.Duncan K G, Bailey K R, Kane J P.et al Human retinal pigment epithelial cells express scavenger receptors BI and BII. Biochem Biophys Res Commun 20022921017–1022. [DOI] [PubMed] [Google Scholar]
- 15.Stewart J M, Bailey K R, Duncan K G.et al Expression of reverse cholesterol transport protiens SR‐BI, SR‐BII and ABCA1 in the human retinal pigment epithelium. Abstract 1711 in Association for Research in Vision and Ophthalmology (ARVO) Annual Meeting, May 4–9, 2003, Fort Lauderdale, FL.
- 16.Rader D J. Regulation of reverse cholseterol transport and clinical implications. Am J Cardiol 200392(suppl)42J–49J. [DOI] [PubMed] [Google Scholar]
- 17.Evans J R. Risk factors for age‐related macular degeneration. Prog Retin Eye Res 200120227–253. [DOI] [PubMed] [Google Scholar]
- 18.Seddon J M, Gensler G, Milton R C.et al Association between C‐reactive protein and age‐related macular degeneration. JAMA 2004291704–710. [DOI] [PubMed] [Google Scholar]
- 19.Snow K K, Seddon J M. Do age‐related macular degeneration and cardiovascular disease share common antecedents? Ophthalmic Epidemiol 19996125–143. [DOI] [PubMed] [Google Scholar]
- 20.Age‐Related Eye Disease Study Research Group Risk factors associated with age‐related macular degeneration: a case‐control study in the age‐related eye disease study: age‐related eye disease study report number 3. Ophthalmology 20001072224–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Eye Disease Case‐Control Study Group Risk factors for neovascular age‐related macular degeneration Arch Ophthalmol19921101701–1708. [DOI] [PubMed] [Google Scholar]
- 22.Abalain J H, Carre J L, Leglise D.et al Is age‐related macular degeneration associated with serum lipoprotein and lipoparticle levels? Clin Chim Acta 200232697–104. [DOI] [PubMed] [Google Scholar]
- 23.Blumenkranz M S, Russell S R, Robey M G.et al Risk factors in age‐related maculopathy complicated by choroidal neovascularization. Ophthalmology 198693552–558. [DOI] [PubMed] [Google Scholar]
- 24.Delcourt C, Michel F, Colvez A.et al Associations of cardiovascular disease and its risk factors with age‐related macular degeneration: the POLA study. Ophthalmic Epidemiol 20018237–249. [DOI] [PubMed] [Google Scholar]
- 25.Hyman L, Schachat A P, He Q.et al Hypertension, cardiovascular disease, and age‐related macular degeneration. Age‐Related Macular Degeneration Risk Factors Study Group. Arch Ophthalmol 2000118351–358. [DOI] [PubMed] [Google Scholar]
- 26.Klein R, Klein B E, Tomany S C.et al The association of cardiovascular disease with the long‐term incidence of age‐related maculopathy: the Beaver Dam Eye Study. Ophthalmology 2003110636–643. [DOI] [PubMed] [Google Scholar]
- 27.Wilson H L, Schwartz D M, Bhatt H R.et al Statin and aspirin therapy are associated with decreased rates of choroidal neovascularization among patients with age‐related macular degeneration. Am J Ophthalmol 2004137615–624. [DOI] [PubMed] [Google Scholar]
- 28.Assmann G, Nofer J R. Atheroprotective effects of high‐density lipoproteins. Annu Rev Med 200354321–341. [DOI] [PubMed] [Google Scholar]
- 29.Nofer J R, Kehrel B, Fobker M.et al HDL and arteriosclerosis: beyond reverse cholesterol transport. Atherosclerosis 20021611–16. [DOI] [PubMed] [Google Scholar]
- 30.Age‐Related Eye Disease Study Research Group A randomized, placebo‐controlled, clinical trial of high‐dose supplementation with vitamins C and E, beta carotene, and zinc for age‐related macular degeneration and vision loss. Arch Ophthalmol 20011191417–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young R W. Pathophysiology of age‐related macular degeneration. Surv Ophthalmol 198731291–306. [DOI] [PubMed] [Google Scholar]
- 32.Song M K, Lui G M. Propagation of fetal human RPE cells: preservation of original culture morphology after serial passage. J Cell Physiol 1990143196–203. [DOI] [PubMed] [Google Scholar]
- 33.Reid D M, Laird D W, Molday R S. Characterization and application of an in vitro detection system for studying the binding and phagocytosis of rod outer segments by retinal pigment epithelial cells. Exp Eye Res 199254775–783. [DOI] [PubMed] [Google Scholar]
- 34.Guisto N M, de Boschero M I, Sprecher H.et al Active labeling of phosphatidylcholines by[1–14C]docosahexaenoate in isolated photoreceptor membranes. Biochim Biophys Acta 1986860137–148. [DOI] [PubMed] [Google Scholar]
- 35.Havel R J, Eder H A, Bragdon J H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest 1955341345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scanu A M, Edelstein C. Solubility in aqueous solutions of ethanol of the small molecular weight peptides of the serum very low density and high density lipoproteins: relevance to the recovery problem during delipidation of serum lipoproteins. Anal Biochem 197144576–588. [DOI] [PubMed] [Google Scholar]
- 37.Li H H, Thomas M J, Pan W.et al Preparation and incorporation of probe‐labelled apoA‐I for fluorescence resonance energy transfer studies of rHDL. J Lipid Res 2001422084–2091. [PubMed] [Google Scholar]
- 38.Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 195937911–917. [DOI] [PubMed] [Google Scholar]
- 39.Wang N, Tall A R. Regulation and mechanisms of ATP‐binding cassette transporter A1‐mediated cellular cholesterol efflux. Arterioscler Thromb Vasc Biol 2003231178–1184. [DOI] [PubMed] [Google Scholar]
- 40.Wang N, Chen W, Linsel‐Nitschke P.et al A PEST sequence in ABCA1 regulates degradation by calpain protease and stabilization of ABCA1 by apoA‐I. J Clin Invest 200311199–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore D J, Clover G M. The effect of age on the macromolecular permeability of human Bruch's membrane. Invest Ophthalmol Vis Sci 2001422970–2975. [PubMed] [Google Scholar]
- 42.Morgan J, Carey C, Lincoff A.et al High‐density lipoprotein subfractions and risk of coronary artery disease. Curr Atheroscler Rep 20046359–365. [DOI] [PubMed] [Google Scholar]
- 43.Gordiyenko N, Campos M, Lee J W.et al RPE cells internalize low‐density lipoprotein (LDL) and oxidized LDL (OxLDL) in high quantities in vitro and in vivo. Invest Ophthalmol Vis Sci 2004482822–2829. [DOI] [PubMed] [Google Scholar]
- 44.Curcio C A, Millican C L. Basal linear deposit and large drusen are specific for early age‐related maculopathy. Arch Ophthalmol 1999117329–339. [DOI] [PubMed] [Google Scholar]
- 45.Green W R, Enger C. Age‐related macular degeneration histopathologic studies. The 1992 Lorenz E. Zimmerman Lecture. Ophthalmology 19931001519–1535. [DOI] [PubMed] [Google Scholar]