Congenital stationary night blindness (CSNB) is a non‐progressive retinal disorder characterised by defective night vision from birth1 that is clinically and genetically heterogeneous. It is most commonly inherited as an X linked disorder, but autosomal dominant (OMIM #163500) and recessive (OMIM#258100) forms have been described. A recent report describes mutations of the GRM6 gene in three patients with autosomal recessive CSNB.2GRM6 encodes the glutamate receptor MGluR6, which is expressed by the rod and cone ON bipolar cells.3 Pharmacological block of this receptor in primates4,5 leads to a similar CSNB phenotype as that described in subjects with GRM6 mutations.2 We have previously identified mutations in CACNA1F and NYX genes in males with X linked CSNB,6,7 and have encountered several female patients with a similar phenotype. We screened these affected females for mutations in RHO which can cause autosomal dominant CSNB,8,9,10 but failed to identify mutations. Recently, following the publication of GRM6 mutations in CSNB2 we have identified nonsense mutations in one female with CSNB.
Case report
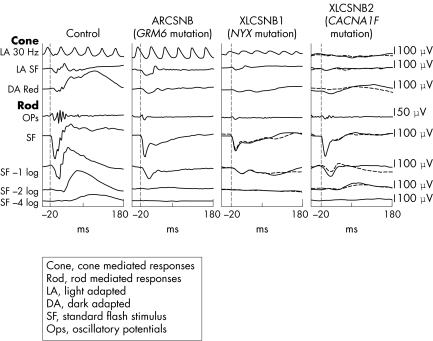
The patient was 7 years of age at the time of clinical examination. Her parents thought that she had always had poor night vision and had noticed horizontal nystagmus since early infancy. She had worn spectacle correction for myopic astigmatism since the age of 3 (right eye: −2.50DS/−3.5DC×85, left eye: −2.50DS/−4.00DC×100). She was otherwise fit and well, and had no family history of ocular disorders. Her distance visual acuity was logMAR +0.3 (6/12, 20/40) in each eye and she was able to read N5 print. Colour vision was full (Ishihara and City University plates). She had no nystagmus in primary position but a small amplitude horizontal nystagmus was noted in lateral gaze. She had a small angle esophoria and 500 seconds of arc stereoacuity. Funduscopy showed inferotemporally tilted discs with mild hypopigmentation in the inferotemporal fundus. There was no ophthalmoscopic evidence of foveal hypoplasia. The patient underwent ISCEV standardised electroretinographic testing using contact gold foil electrodes (protocol previously described)7 (fig 1). Subjects with AR or XLCSNB characteristically have a “negative wave” electroretinogram (ERG) with grossly subnormal scotopic (rod photoreceptor pathway) b‐wave amplitudes. The patient's ERG waveform was more similar to that in XLCSNB1 (“complete”) rather than XLCSNB2 (“incomplete”): the differences being the absence of recordable scotopic oscillatory potentials differences and the presence of a 30 Hz flicker response in this and the XLCSNB1 subjects. Blood was taken, with informed consent, from the patient and DNA extracted using standard techniques. Using polymerase chain reaction (PCR) we amplified each of her GRM6 gene exons2 and sequenced the coding region and intron‐exon boundaries. We identified two nonsense mutations: R238X and Y409X, in exons 2 and 4 respectively (fig 2). These mutations specific have not been previously reported in CSNB but other nonsense mutations are described.2
Figure 1 Comparison of ERG waveforms. The absence of oscillatory potentials and the presence of a 30 Hz flicker response make the ERG in this case more similar to that seen in subjects with the X linked CSNB1 genotype than those with CSNB2.
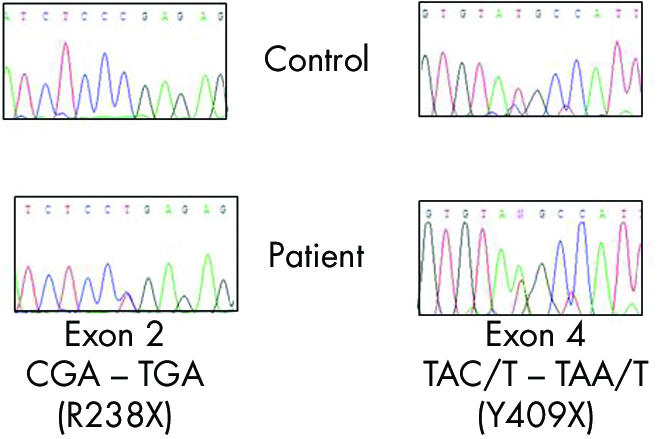
Figure 2 Mutations in GRM6 gene.
Comment
Mutations in GRM6 cause some cases of ARCSNB. In darkness, glutamate is tonically released from the photoreceptor presynaptic terminal while light induced photoreceptor hyperpolarisation diminishes its release. The reduced stimulation of the “on” bipolar cell in light conditions results in it depolarisation. Absence of the MgluR6 receptor as a result of GRM6 gene mutation causes the “on” bipolar cells to be relatively depolarised (light adapted) in darkness, causing the symptom of night blindness. Both this form of ARCSNB and XLCSNB1 appear to profoundly affect the “on” bipolar cell retinal pathway only and therefore have similar waveforms: specifically grossly subnormal, often absent scotopic b‐wave response, an absence of scotopic oscillatory potentials but a relatively well preserved 30 Hz flicker response. In subjects with XLCSNB2, in which abnormalities in function of the L‐type calcium channel appears to impair both the ON and “off” bipolar pathway: scotopic b‐waves and oscillatory potentials are usually recordable but the 30 Hz flicker response is grossly sub‐normal.
The striking similarities between GRM6 CSNB and CSNB1 caused by NYX mutations suggest that nyctalopin (encoded by NYX) plays an important part in signalling through the “on” bipolar pathway. Since little is known about the function of nyctalopin these clinical observations may help to guide further studies of its function and any interaction with mGluR6 in bipolar cell function.
References
- 1.Jacobi F K.et al Phenotypic expression of the complete type of X‐linked congenital stationary night blindness in patients with different mutations in the NYX gene. Graefes Arch Clin Exp Ophthalmol 2002240822–828. [DOI] [PubMed] [Google Scholar]
- 2.Dryja T P.et al Night blindness and abnormal cone electroretinogram ON responses in patients with mutations in the GRM6 gene encoding mGluR6. Proc Natl Acad Sci USA 20051024884–4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakajima Y.et al Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for L‐2‐amino‐4‐phosphonobutyrate. J Biol Chem 199326811868–11873. [PubMed] [Google Scholar]
- 4.Slaughter M M, Miller R F. 2‐amino‐4‐phosphonobutyric acid: a new pharmacological tool for retina research. Science 1981211182–185. [DOI] [PubMed] [Google Scholar]
- 5.Schiller P H, Sandell J H, Maunsell J H. Functions of the ON and OFF channels of the visual system. Nature 1986322824–825. [DOI] [PubMed] [Google Scholar]
- 6.Zito I.et al Mutations in the CACNA1F and NYX genes in British CSNBX families. Hum Mutat 200321169. [DOI] [PubMed] [Google Scholar]
- 7.Allen L E.et al Genotype‐phenotype correlation in British families with X linked congenital stationary night blindness. Br J Ophthalmol 2003871413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sieving P A.et al Dark‐light: model for nightblindness from the human rhodopsin Gly‐90→Asp mutation. Proc Natl Acad Sci USA 199592880–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al‐Jandal N.et al A novel mutation within the rhodopsin gene (Thr‐94‐Ile) causing autosomal dominant congenital stationary night blindness. Hum Mutat 19991375–81. [DOI] [PubMed] [Google Scholar]
- 10.Dryja T P.et al Heterozygous missense mutation in the rhodopsin gene as a cause of congenital stationary night blindness. Nat Genet 19934280–283. [DOI] [PubMed] [Google Scholar]