Abstract
Aim
To investigate the mechanical properties (stress‐strain relation, elasticity, hysteresis, response to stress spikes and drops) of isolated human Bruch's membrane‐choroid, as well as the effect of ageing and aged related macular degeneration (AMD).
Methods
13 Bruch's membrane‐choroid complexes were obtained from human donors (21–97 years). Two samples (aged 85 and 95) showed signs of AMD including large, soft drusen, choroidal neovascularisation, and/or disciform scars. Various hydrostatic pressures (stress) were applied to the choroidal surface of mid‐peripheral samples mounted in a modified open Ussing chamber. Linear scans of the tissue were recorded by optical coherence tomography (OCT) and the pressure induced deformation (strain), elasticity, hysteresis, and response to pressure spikes and drops measured.
Results
The elasticity of human Bruch's membrane‐choroid complex decreased linearly with ageing (p<0.001) after the age of 21 with an approximate reduction of 1% per year. The decrease was not exaggerated in AMD. The recoil capacity of Bruch's membrane‐choroid was not affected by ageing. The response to pressure spikes/drops was similar in age matched normal and AMD eyes. The results suggest that although the aged induced decrease in Bruch's membrane elasticity may contribute to breaks in this membrane in AMD leading to neovascularisation this is not sufficient. The presence of other factors is required for its development.
Conclusion
The elasticity of Bruch's membrane‐choroid complex decreases with age while recoil capacity does not. The decrease was not exaggerated in AMD.
Keywords: Bruch's membrane, elasticity, recoil, aged related macular degeneration
Bruch's membrane, the thin acellular lamina of extracellular matrix (ECM) found between the retinal pigment epithelium (RPE) and the choroid is under constant cycles of pressure induced stress as a result of the choroidal flow changes occurring with the cardiac cycle. The mechanical properties of Bruch's membrane are critical determinants of its physiology and pathophysiology. Thus, the stress‐strain relation of Bruch's membrane may determine transport across this tissue by altering the porosity of the membrane, thereby influencing its hydraulic conductivity and diffusional characteristics. Moreover, the elastic properties of Bruch's membrane will determine its ability to sustain potentially damaging stress and strain perturbations in situations like laser treatment.
Bruch's membrane is composed of five layers: (1) inner basal lamina of RPE, (2) outer collagenous zone, (3) elastic zone comprising a meshwork of elastic fibres, (4) inner collagenous zone, and (5) outer basal lamina of choriocapillaris.1 This highly organised meshwork of connective tissue elements provides Bruch's membrane with the capacity to deform as pressure (stress) is applied (elasticity) and to return to its original shape after the stress is removed (resilience). Ageing of Bruch's membrane is known to be associated with thickening,2 increased cross linking of fibres, deposition of debris, reduced porosity, an exponential decrease in hydraulic conductivity,3,4 and a linear decline of diffusional transport.5 These changes may be associated with a reduction in elasticity and/or resilience. To our knowledge, no direct measurements have been carried out to evaluate the response of human Bruch's membrane to varying external pressures as a function of age or in aged related macular degeneration (AMD).
In the present experimental study, we examined the stress‐strain relation, elasticity, hysteresis (lagging of strain behind its cause), and the response to pressure spikes and drops of human Bruch's membrane‐choroid complexes of different ages and in AMD. Bruch's membrane inner border is well demarcated, whereas the outer limit is very ill defined with expansions of Bruch's membrane in the choroid forming intercapillary pillars.6 We used a Bruch's membrane‐choroid complex, which allowed us to maintain the efficacy of our preparations and record physiologically relevant measurements. A new experimental model for measuring the stress‐strain relation in isolated human Bruch's membrane‐choroid complex is described. The apparatus is based on a modified Ussing chamber for pressure application, the pressure induced deformation being examined by optical coherence tomography (OCT) scanning.
Any difference between the responses of ageing and AMD samples would suggest a role of Bruch's membrane mechanical properties in the pathophysiology of this ocular disorder of the elderly.
Materials and methods
Tissue preparation
Thirteen pairs of human eyes (age range 21–97 years) were obtained from the Bristol Eye Bank (UK), and one eye from each donor was used in this study. After removal of the corneas for grafts, the eyes reached the laboratory on ice in saline moistened sterile containers within 48 hours of death. The globes were dissected in sterile conditions within a laminar flow cabinet (Grade II; MDH Ltd, Hampshire, UK) in a Petri dish lined with filter paper (Grade 50; Whatman, Maidstone, UK) moistened with 0.1 M phosphate buffered saline (PBS) containing 100 U/ml penicillin (Sigma) and 100 μg/ml streptomycin (Sigma). A circumferential incision was made 4 mm posterior to the corneoscleral sulcus. The anterior segment, lens, and vitreous were discarded. Two eyes (aged 85 and 95) with large, soft drusen, signs of choroidal neovascularisation, and/or disciform scars were included in the AMD group. The remaining 11 samples (21–97 years) were considered normal. The neuroretina was separated from the underlying RPE, cut at the optic nerve head and removed. Samples of RPE, Bruch's membrane, and choroid were obtained from the mid‐periphery with a 6 mm trephine (Steifel Laboratories, Bucks, UK). Except for the freezing/thawing versus unfrozen tissue experiments (where two samples were taken from the same eye) only one 6 mm specimen was used from each eye. The RPE was carefully removed using a fine sable hairbrush, and the Bruch's membrane‐choroid complex was gently separated from the sclera and flattened over a fresh piece of moistened filter paper. The isolated Bruch's membrane‐choroid complex was rapidly frozen in a eutetic mixture of isopentane precooled to −96°C in liquid nitrogen and stored at −30°C until use. On the day of an experiment given samples were immersed in cold PBS to defrost and were allowed to reach room temperature before being mounted in a chamber.
Two control samples that had not been subjected to freezing‐thawing were also examined in order to establish whether the reactions taking place during the freezing‐thawing processes altered the mechanical responses of human Bruch's membrane choroid complexes. One quadrant from one eye of each donor (aged 38 and 74) was cut in half from the optic nerve towards the periphery. A 6 mm sample from one half was mounted into the tissue cassette and experiments carried out immediately. The second half was frozen as previously described and thawed 48 hours later for parallel experiments.
Chamber
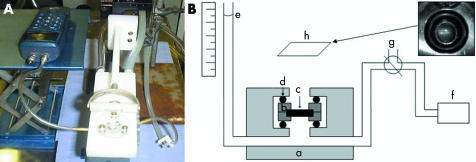
A modified Ussing chamber was used to measure the elasticity of Bruch's membrane‐choroid complex (fig 1). The samples were mounted in a polytetrafluoro‐ethlylene cassette consisting of two plates with a central 4 mm aperture (fig 1B, b). Polytetrafluoro‐ethlylene was used because it has a low coefficient of friction and forms a watertight seal when two pieces are clamped together by a small force, without the need for grease or another sealant. The 6 mm Bruch's membrane‐choroid complex (fig 1B, c) was mounted between the plates of the cassette, spanning the 4 mm central aperture, in PBS. The cassette was then inserted in the chamber. A seal was maintained between the cassette and the acrylic blocks of the chamber by means of integral rubber O‐rings (fig 1B, d) and the whole assembly was clamped together by three bolts tightened to a torque of 30 cN/m. The tissue mounting procedure typically took less than 10 minutes.
Figure 1 Photograph (A) and schematic representation (B) of the system used to apply different hydrostatic pressures (stress) to human Bruch's membrane‐choroid complex. The chamber (B, a) was constructed of acrylic. A polytetrafluoroethylene cassette (B, b) spanning a 4 mm diameter central aperture was used to mount the 6 mm diameter Bruch's membrane‐choroid complex sample (B, c). Leak‐free seals were achieved between the cassette and the chamber by rubber O‐rings (B, d). Pressure was applied to the choroidal surface of the tissue by a column of buffer (B, e) and the system connected to a manometer (B, f). A three way tap (B, g) was used to prime the system but was isolated during experiments. Above the horizontally mounted sample there was a mirror (B, h) at 45° in order to displace the horizontal light beam of the OCT and direct it towards the specimen. Superior right hand corner inset: digital photograph of the superior view of the cassette with Bruch's membrane‐choroid mounted horizontally and the laser beam positioned across the sample helped by the HeNe laser light.
Application of stress (hydrostatic pressure)
The experiments were performed at room temperature. Once the cassette was inserted and the assembly was clamped, the chamber was slowly filled with PBS with great care taken to avoid bubbles. A positive pressure was applied to the choroidal surface to the samples using a column of buffer connected to a manometer (fig 1B, f). The initial loading pressure of approximately 80–100 Pascals (Pa) was necessary to straighten the loose sample. The hydrostatic pressure applied varied up to a maximum of 4900 Pa, depending on the experiment. The deformation or strain responses were measured by scanning the samples at set intervals of time.
Strain (deformation) measurement using OCT scanning
The RPE surface of the horizontally mounted sample was scanned with the OCT 2000 scanner (Humphrey Instruments, San Leandro, CA, USA). This had been modified by placing a plane mirror above and at 45° to the objective of the lens, thus allowing the acquisition of images of horizontally oriented samples (fig 1B, h), as previously described.7 Their studies demonstrated that the scans obtained with this modification were identical in pattern and dimensions to those taken in a conventional manner. All scans on each specimen were performed at the same focal plane using the same power and polarisation settings. Each composite OCT image consisted of a linear array of a 100 juxtaposed individual z‐axis scans. Helped by the HeNe laser light viewed on the monitor, the laser beam was positioned across the centre of the sample (fig 1B, inset) and a 5 mm linear scan taken. The acquisition of each image took 0.9 seconds. The images were processed by the commercial software into a logarithmic pseudo‐colour scale in which white, red, yellow, green, blue, and black represented the range of signal intensity from high to low. The signal was dependent on the optical properties of the tissue sample in the z‐plane.
Data analysis
After calibration of the images in millimetres, the processed pseudo‐coloured images were imported into an image processing programme (Paint). The colours were changed to black and white and then inverted, without changing the original image dimensions (fig 2). The cross sectional profiles of the OCT images were analysed in order to calculate the arc length. The elevation (y, mm) of the membrane surface above the clamping horizontal was measured at given intervals (x, mm) along the diameter of the sample. This was then fitted to the following polynomial using non‐linear regression analysis provided by the Fig.P statistical package (FIG.P Software Corporation, Durham, NC USA):
Figure 2 The cross sectioned images of the OCT scanning after calibration in mm were processed with the manufacturer's software. The pseudo‐colours (left) were changed to black and white (middle) and then inverted (right) before fitting a polynomial equation in order to calculate the arc length.
y = a + bx + cx2 + dx3
The arc length of the sample was obtained by integrating the following standard equation using the facilities of Mathcad 7 (MathSoft, Inc, Cambridge, MA, USA):
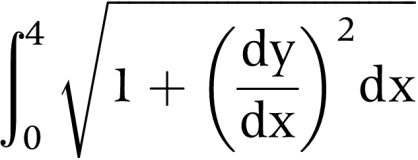 |
The boundary conditions of 0 and 4 represent the diameter of the sample in the cassette. The elasticity modulus (E) or ratio of the change in arc length or strain (ε) to the applied stress (P) of our samples was used in our analyses.
Results
Effect of freezing‐thawing on the mechanical properties of human Bruch's membrane‐choroid
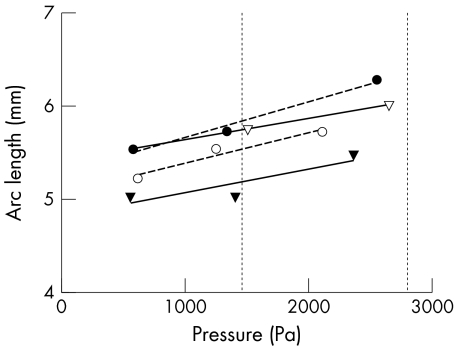
Experimental data of the stress‐strain relation (fig 3) and elasticity of frozen and thawed human Bruch's membrane‐choroid complexes from two donors (aged 38 and 74) demonstrated no significant difference when comparing to unfrozen samples from the same eye. The mechanisms of freezing and defrosting do not seem to alter the mechanical properties of human Bruch's membrane‐choroid.
Figure 3 Mechanical responses of unfrozen and frozen and thawed human Bruch's membrane‐choroid complexes. The process of freezing and defrosting do not seem to change the stress‐strain relation of Bruch's membrane‐choroid. Open symbols, fresh tissue; solid symbols, frozen‐thawed tissue. Circles, 38 year old; triangles, 74 year old. Broken line, regression for 38 year old; solid line, regression for 74 year old donor. Vertical dotted lines represent physiological intraocular pressures (1 mm Hg = 133.32 Pa).
Stress‐strain relation of normal and AMD human Bruch's membrane‐choroid complex
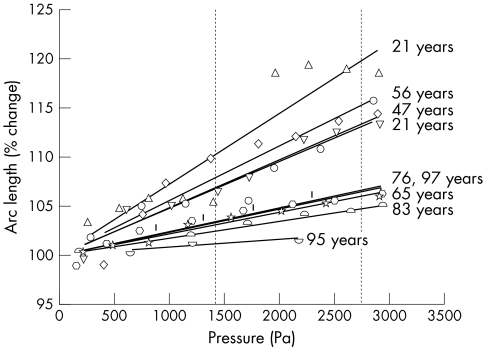
Nine human Bruch's membrane‐choroid samples without AMD and two with signs of AMD were subjected to increasing hydrostatic pressures (in 1000 Pa steps and up to a maximum of 5000 Pa). Exposure of a round area (4 mm in diameter) to this stress resulted in tissue deformation (strain) with an increase in the sample arc length (figs 4 and 5). The stress‐strain relation was monitored by OCT scanning, which generated cross sectioned images of high signal and very good resolution (fig 4).
Figure 4 Cross sectioned OCT scans of Bruch's membrane‐choroid complex from a 21 year old donor, exposed to increasing hydrostatic pressures (stress) in 1000 Pa steps and up to a maximum of 5000 Pa. A progressive increase in arc length was induced by the pressure elevations.
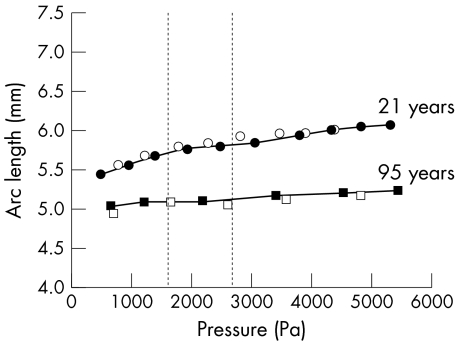
Figure 5 Stress‐strain relation of Bruch's membrane‐choroid preparations in human donors aged 21–97 years. The hydrostatic pressure (stress) applied to the choroidal side of the samples was increased in 250–500 Pa steps up to a maximum of 3000 Pa. Measurements were obtained after a 5 minute stabilisation period. The strain (change in arc length) is presented as percentage of the initial arc length. Vertical dotted lines represent physiological intraocular pressures (1 mm Hg = 133.32 Pa).
Modulus of elasticity as a function of age and in AMD
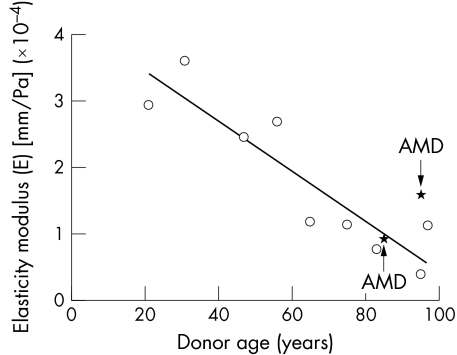
Analysis of the images with the manufacturer's software showed a linear decrease in elasticity after the age of 21 (figs 5 and 6) with an approximate decrease of 1% per year and the AMD Bruch's membrane‐choroid complexes falling within the normal range to their age (fig 6).
Figure 6 The modulus of elasticity (Ε) (strain/stress) of human Bruch's membrane‐choroid as a function of age in nine samples without AMD (same specimens as in fig 5) and two samples with AMD. Ε was calculated by linear regression analysis of measurements for each donor preparation in figure 5. The data show a linear age dependent decline in the elasticity (p<0.001) after the age of 21. AMD samples fell within the normal range for their age.
Stretch‐recoil capacity of human Bruch's membrane‐choroid with age
Two normal Bruch's membrane‐choroid complexes were distended with increasing hydrostatic pressures (loading) and they recovered their original conformation without any residual stretch when the stress was stopped (unloading) (fig 7). There was no lag of distension behind changes in pressure (also known as hysteresis) in either 21 or 95 year old donors, indicating that the ability to recoil was not affected by age.
Figure 7 Assessment of the capacity of normal Bruch's membrane‐choroid to stretch and recoil in response to increases (loading) followed by decreases (unloading) in pressure. The sample strain (arc length) was monitored during staged ascending (filled symbols) and descending (open symbols) stress (pressure) changes. Measurements were obtained after a 5 minute stabilisation period. Stress‐strain curves did not show any evidence for lag of strain (deformation) behind the stress (pressure) reduction (also known as hysteresis) in either a 95 or 21 year old donors. Dotted lines represent physiological intraocular pressures (1 mm Hg = 133.32 Pa).
Normal and AMD Bruch's membrane‐choroid responses to pressure spikes and drops
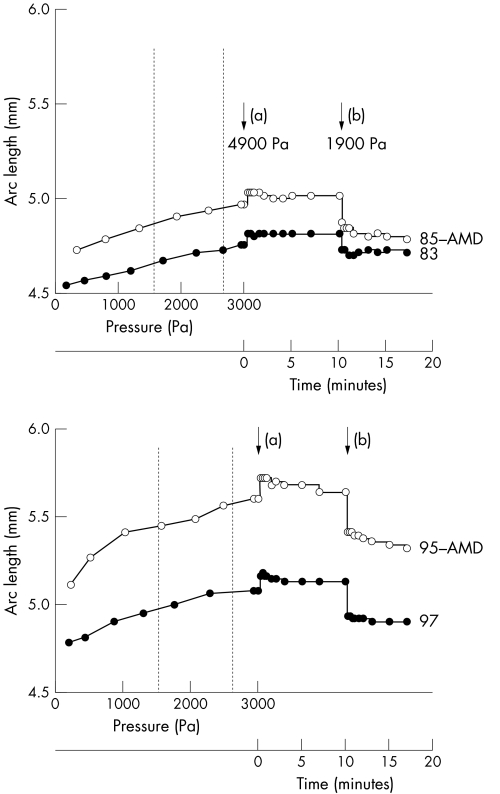
Following the strain induced by 250–500 Pa step pressure increases up to a maximum of 3000 Pa, samples from two normal (aged 83 years and 97 years) and two AMD (aged 85 years and 95 years) donors were exposed to a pressure spike of 1900 Pa and this pressure was maintained for 10 minutes. This caused a large increase in arc length which was stable for 10 minutes. The pressure was then dropped by 3000 Pa and this change produced a sharp reduction in arc length (fig 8). The responses were very similar in both normal and AMD human Bruch's membrane‐choroid complexes with the majority of the stabilisation occurring within the first 15 seconds, although there was a slight suggestion of a possible delay of stabilisation in AMD eyes. These results demonstrate that the ability of Bruch's membrane‐choroid to adapt to sudden pressure rises and falls is not different in AMD from normal individuals of similar age.
Figure 8 Elasticity and resilience properties of Bruch's membrane‐choroid samples from normal and AMD donors in response to pressure spikes and drops. For each donor sample, the increase in arc length to ascending 250–500 Pa steps of applied pressure was quantified up to a maximum of 3000 Pa. Pressure was then raised to 4900 Pa (a) and the corresponding changes in arc length followed as a function of time over 10 minutes. Pressure was then reduced by 3000 Pa (b) and responses recorded for a further 7 minutes. There was no difference in the response of normal or AMD samples of similar age. The major part of the arc length stabilisation occurred within 15 seconds of the pressure rise. There is a suggestion of a slightly slower stabilisation in AMD samples. Vertical dotted lines represent physiological intraocular pressures (1 mm Hg = 133.32 Pa).
Discussion
This study has demonstrated that the elasticity of Bruch's membrane‐choroid complex decreased with the age of the donor, whereas the recoil capacity was not affected by ageing. The age dependent reduction in elasticity was not exaggerated in AMD; what is more, the ability of AMD tissue to expand and recoil in response to pressure spikes and drops was no different from normal tissue of similar age.
The stretch‐recoil properties of Bruch's membrane are endowed by the constant conformational changes of the elastic fibres in the middle elastic zone of this pentalaminated membrane. These elastic fibres,8 comprising an elastin core surrounded by a mantle of fibrillin rich microfibrils,9 extend beyond Bruch's membrane into the inner choroid.6 Bruch's membrane elastic forces exert their influence on the inner choroid and they both function as a single entity. In this series of experiments, we used Bruch's membrane‐inner choroid complexes that would simulate the in vivo situation in a much more accurate manner. Bruch's membrane‐choroid specimens were mounted in a modified Ussing chamber3 and different hydrostatic pressures (stress) were applied to the choroidal side. This experimental method was found to be reliable for the investigation of the elasticity and resilience. However, the elasticity values presented here might be underestimated given that the area exposed to pressure was 4 mm in diameter. Within the globe the entire elastic network would be able to expand to a much greater extent.
The stress induced strain on Bruch's membrane‐choroid was monitored with optical coherence tomography. This high resolution imaging technique allowed us to obtain multiple cross sectioned images of the tissue during the experiments in a non‐invasive way. OCT imaging depends on the optical properties of the tissue (scatter, reflection, and absorption of incident light) and there are several potential factors that could limit the overall resolution of the image, as demonstrated by previous investigations carried out in our laboratory.7 The OCT images obtained were of high intensity and very good resolution. After fitting a polynomial equation to the processed images calibrated in millimetres, the arc length of the cross sections were calculated.
The aged related decrease in human Bruch's membrane‐choroid elasticity has clinical implications in relation to the expansion of this complex in response to changes in choroidal blood flow. The choroid has a high pulsatile blood flow with mean values ranging from 444 μl/min to 803 μl/min, estimated by measuring IOP pulses using Langham ocular blood flow computerised tonometry.10,11 The ability of Bruch's membrane‐choroid to undergo long range deformability and passive recoil is, therefore, essential. This high rate of blood flow is of great importance for nutrient supply to the outer retina, as well as for retinal protection from thermal damage. If the elasticity of Bruch's membrane‐choroid is reduced the choroidal blood flow would also be diminished rendering the retina more vulnerable to damage by high temperatures after exposure to normal or laser light. In this context, choroidal blood flow has been found to be decreased with age in normal subjects.12
Reduced Bruch's membrane‐choroid elasticity could potentially impair the bidirectional movement of water and dissolved substances between the choroid and RPE by altering its dynamic effect on pore size. This idea is supported by previous work determining the hydraulic conductivity of Bruch's membrane‐choroid preparations as a function of age, showing that there is an exponential increase in resistance to water movement3,4 and a reduction in the diffusion of various amino acids.5 We propose that this age related reduction in hydraulic conductivity may be in part a consequence of a decrease in elasticity.
The age dependent reduction in the elasticity of Bruch's membrane‐choroid observed in this study may result from morphological changes known to occur with age. Histopathological studies have demonstrated thickening,2 reduction in hyaluronic acid,13 as well as accumulation of advanced glycation end products,14 proteoglycans,15 collagen,16 elastin,13 and lipids17,18,19,20 in Bruch's membrane as we get older. This deposition of debris in Bruch's membrane is accompanied by accumulation of electron dense beaded material on the elastic zone microfibrils.6 The chemical nature of this electron dense beaded material is unknown but everything suggests it is related to the ageing processes. The association of this material with microfibrils could interfere with the normal flexibility and extensibility of the elastic fibres making Bruch's membrane less capable of stretching.
The fact that the reduction in elasticity occurs from an early age before the significant increase in debris in Bruch's membrane occurs, indicates it might be the consequence of the biochemical alteration in the structure and function of elastic fibres. This could be the consequence of an abnormal homeostatic turnover of Bruch's membrane components. Previous studies carried out in our laboratory and elsewhere have demonstrated the presence of proteolytic enzymes involved in degradative processes of elastic fibres (matrix metalloproteases and serine proteases),21,22 as well as their inhibitors23,24 in Bruch's membrane. Identification of the biosynthetic and degradative pathways for elastic fibres in Bruch's membrane would allow in the future to manipulate them and influence the changes occurring with age.
In summary, this study has demonstrated an age dependent decrease in the elasticity of Bruch's membrane‐choroid and no difference between normal and AMD samples from donors of similar age. This reduced elasticity may be associated with a diminished choroidal blood flow and transport capacity across Bruch's membrane. The decrease in elasticity may be the consequence of age dependent metabolic degradation of elastic fibres with a possible contribution from thickening and deposition in Bruch's membrane as part of the ageing process.
Abbreviations
AMD - aged related macular degeneration
ECM - extracellular matrix
PBS - phosphate buffered saline
OCT - optical coherence tomography
RPE - retinal pigment epithelium
References
- 1.Chen L, Miyamura N, Ninomiya Y.et al Distribution of the collagen IV isoforms in human Bruch's membrane. Br J Ophthalmol 20032212–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramrattan R S, van der Schaft T L, Mooy C M.et al Morphometric analysis of Bruch's membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci 1994352857–2864. [PubMed] [Google Scholar]
- 3.Moore D J, Hussain A A, Marshall J. Age‐related variation in the hydraulic conductivity of Bruch's membrane. Invest Ophthalmol Vis Sci 1995361290–1297. [PubMed] [Google Scholar]
- 4.Starita C, Hussain A A, Pagliarini S.et al Hydrodynamics of ageing Bruch's membrane: implications for macular disease. Exp Eye Res 199662565–572. [DOI] [PubMed] [Google Scholar]
- 5.Hussain A A, Rowe L, Marshall J. Age‐related alterations in the diffusional transport of amino acids across the human Bruch's‐choroid complex. J Opt Soc Am A Opt Image Sci Vis 200219166–172. [DOI] [PubMed] [Google Scholar]
- 6.Korte G E, D'Aversa G. The elastic tissue of Bruch's membrane. Connections to choroidal elastic tissue and the ciliary epithelium of the rabbit and human eyes. Arch Ophthalmol 19891071654–1658. [DOI] [PubMed] [Google Scholar]
- 7.Chauhan D S, Marshall J. The interpretation of optical coherence tomography images of the retina. Invest Ophthalmol Vis Sci 1999402332–2342. [PubMed] [Google Scholar]
- 8.Kielty C M, Sherratt M J, Marson A.et al Fibrillin microfibrils. Adv Protein Chem 200570405–436. [DOI] [PubMed] [Google Scholar]
- 9.Wheatley H M, Traboulsi E I, Flowers B E.et al Immunohistochemical localization of fibrillin in human ocular tissues. Relevance to the Marfan syndrome. Arch Ophthalmol 1995113103–109. [DOI] [PubMed] [Google Scholar]
- 10.Silver D M, Farrell R A, Langham M E.et al Estimation of pulsatile ocular blood flow from intraocular pressure. Acta Ophthalmol Suppl 198919125–29. [DOI] [PubMed] [Google Scholar]
- 11.Langham M E, Kramer T. Decreased choroidal blood flow associated with retinitis pigmentosa. Eye 19904(Pt 2)374–381. [DOI] [PubMed] [Google Scholar]
- 12.Ravalico G, Toffoli G, Pastori G.et al Age‐related ocular blood flow changes. Invest Ophthalmol Vis Sci 1996372645–2650. [PubMed] [Google Scholar]
- 13.Green W R, Enger C. Age‐related macular degeneration histopathologic studies. The 1992 Lorenz E. Zimmerman Lecture. Ophthalmology 19931001519–1535. [DOI] [PubMed] [Google Scholar]
- 14.Handa J T, Verzijl N, Matsunaga H.et al Increase in the advanced glycation end product pentosidine in Bruch's membrane with age. Invest Ophthalmol Vis Sci 199940775–779. [PubMed] [Google Scholar]
- 15.Pauleikhoff D, Harper C A, Marshall J.et al Aging changes in Bruch's membrane. A histochemical and morphologic study. Ophthalmology 199097171–178. [PubMed] [Google Scholar]
- 16.Marshall G E, Konstas A G, Lee W R. Collagens in ocular tissues. Br J Ophthalmol 199377515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holz F G, Sheraidah G, Pauleikhoff D.et al Analysis of lipid deposits extracted from human macular and peripheral Bruch's membrane. Arch Ophthalmol 1994112402–406. [DOI] [PubMed] [Google Scholar]
- 18.Bird A C. The Bowman lecture. Towards an understanding of age‐related macular disease. Eye 200317457–466. [DOI] [PubMed] [Google Scholar]
- 19.Ruberti J W, Curcio C A, Millican C L.et al Quick‐freeze/deep‐etch visualization of age‐related lipid accumulation in Bruch's membrane. Invest Ophthalmol Vis Sci 200341753–1759. [DOI] [PubMed] [Google Scholar]
- 20.Curcio C A, Millican C L, Bailey T.et al Accumulation of cholesterol with age in human Bruch's membrane. Invest Ophthalmol Vis Sci 200142265–274. [PubMed] [Google Scholar]
- 21.Alexander J P, Bradley J M, Gabourel J D.et al Expression of matrix metalloproteinases and inhibitor by human retinal pigment epithelium. Invest Ophthalmol Vis Sci 1990122520–2528. [PubMed] [Google Scholar]
- 22.Guo L, Hussain A A, Limb G A.et al Age‐dependent variation in metalloproteinase activity of isolated human Bruch's membrane and choroid. Invest Ophthalmol Vis Sci 1999402676–2682. [PubMed] [Google Scholar]
- 23.Kamei M, Hollyfield J G. TIMP‐3 in Bruch's membrane: changes during aging and in age‐related macular degeneration. Invest Ophthalmol Vis Sci 1999402367–2375. [PubMed] [Google Scholar]
- 24.Vranka J A, Johnson E, Zhu X.et al Discrete expression and distribution pattern of TIMP‐3 in the human retina and choroid. Curr Eye Res 199716102–110. [DOI] [PubMed] [Google Scholar]