Abstract
Aims
Although interleukin 8 (IL‐8) is not produced in the normal cornea, it has been detected there in several pathological conditions. In this study, the direct effects of IL‐8 overexpression on the cornea was examined.
Methods
The corneal surface of severe combined immunodeficiency mice was infected by the adenovirus vector encoding human IL‐8 (IL‐8/Ad5) and clinical and pathological changes were observed at various time points.
Results
Clinically, marked angiogenesis and ulcer formation in the cornea were observed by 12 hours and 24 hours, respectively. Histologically, prominent angiogenesis was observed in the corneal stroma at 12 hours. Cleft formation between the corneal epithelium and stroma, and neutrophil infiltration into the corneal stroma were seen at 16 hours. By 24 hours after the infection with IL‐8/Ad5, a shallow ulcer was formed in the cornea. In contrast, infection with the control adenovirus carrying the β galactosidase gene (LacZ) showed neither corneal ulceration nor neutrophil infiltration. Immunohistochemical analysis showed that infection with IL‐8/Ad5 resulted in the production of IL‐8 by corneal and conjunctival stromal cells.
Conclusion
Our results indicate that IL‐8 overexpression in corneal tissue causes ulcer formation in the cornea through chemoattraction of neutrophils, suggesting the aetiological role of IL‐8 in some types of corneal ulcers.
Keywords: IL‐8, cornea, neutrophils, ulcer, adenovirus vector, mouse model
Interleukin 8 (IL‐8), a member of the chemokine family, has potent chemotactic and activating effects on neutrophils.1 In addition to these, IL‐8 can recruit T cells2 and act directly on endothelial cells to induce angiogenesis.3
Although IL‐8 is barely detectable in the normal human cornea,4,5 it has been demonstrated that IL‐8 is produced from human corneal epithelial cells or stromal keratocytes in response to a variety of stimuli.4,6,7,8 Furthermore, high levels of IL‐8 have been detected in corneas in pathological conditions.5,9 These results strongly suggest that IL‐8 has an important role in the pathogenesis of corneal diseases. However, the functional relevance of IL‐8 in corneal diseases has not been elucidated.
In this study, to elucidate the direct effects of IL‐8 overexpression on the cornea, the corneal surface of severe combined immunodeficiency (SCID) mice was infected by an adenovirus vector in which the cDNA encoding human IL‐8 is expressed under the control of the cytomegalovirus immediate early promoter (IL‐8/Ad5). Since human IL‐8 binds to the mouse receptor,10 infection of the adenovirus vector caused a strong infiltration of mouse neutrophils, resulting in dramatic clinical and pathological changes.
Materials and methods
Adenovirus vector
Generation of IL‐8/Ad5 and biological activity of IL‐8 produced by IL‐8/Ad5 were described previously.11 The adenovirus carrying the β galactosidase gene (LacZ) from Escherichia coli designated LacZ/Ad5 was used as a control virus.
SCID mice and application of IL‐8/Ad5 to the corneal surface
The eyes of 8–12 week old CB‐17 SCID mice were treated with a suspension buffer of either IL‐8/Ad5 or LacZ/Ad5 after anaesthesia. The treatment was performed by simple instillation of drops of adenovirus vectors in the centre of the cornea (2.5×105 plaque forming units (PFU) of IL‐8/Ad5 or LacZ/Ad5 in 5 μl). The eyes were kept open for 5 seconds after receiving the viral drops and then the ocular surface was rubbed with the eyelids five times. All procedures concerning animal studies were approved by the review boards of the Wistar Institute.
Histological and immunohistochemical analysis
The whole eyes were fixed overnight in 10% neutral buffered formalin, and were paraffin embedded. Replicate serial sections from each eye were stained with haematoxylin and eosin. For the identification of IL‐8 in corneal tissues, 5 μm tissue sections were incubated with either an anti‐human IL‐8 monoclonal antibody (R&D, Minneapolis, MN, USA) or an isotype matched control antibody (Dako Corp, Carpinteria, CA, USA) overnight at 4°C and were washed in PBS followed by a three step immunoperoxidase method using avidin‐biotin horseradish peroxidase (Vector Laboratories, Burlingame, CA, USA). Immunoreactivity was visualised by means of 3,3' diaminobenzidine (Sigma, St Louis, MO, USA) with a counterstain of Gill's haematoxylin No 1 (Fisher Scientific, Malvern, PA, USA).
Results
Ulcer formation in the mouse cornea
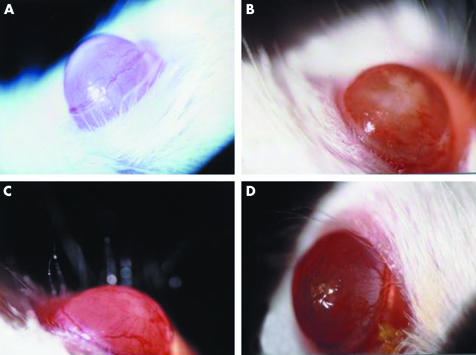
Corneas of SCID mice were infected with either IL‐8/Ad5 or LacZ/Ad5, and clinical (fig 1) and histological (fig 2) changes were observed over a 120 hour period. The initial clinical change after the application of IL‐8/Ad5 was marked angiogenesis appearing from the limbus to the cornea, which was evident by 12 hours (fig 1A). At 16 hours the corneal surface had white muddiness owing to severe cell infiltration in the corneal stroma (fig 1B, and C). By 24 hours, an ulcer was present in the central portion of the cornea (fig 1D). The corneal changes disappeared within 3–4 days, and the cornea became indistinguishable from non‐infected corneas at 120 hours (data not shown). Application of control LacZ/Ad5 caused no remarkable inflammatory response except very slight cell infiltration in the corneal stroma at 16–24 hours (data not shown).
Figure 1 Macroscopic appearance of representative SCID mouse corneas infected with IL‐8/Ad5; 5 μl of IL‐8/Ad5 or LacZ/Ad5 (2.5×107 PFU) was applied to the centre of SCID mouse corneas. (A) Cornea at 12 hours after IL‐8/Ad5 infection. (B, C) Cornea at 16 hours after IL‐8/Ad5 infection. (D) Cornea at 24 hours after IL‐8/Ad5 infection.
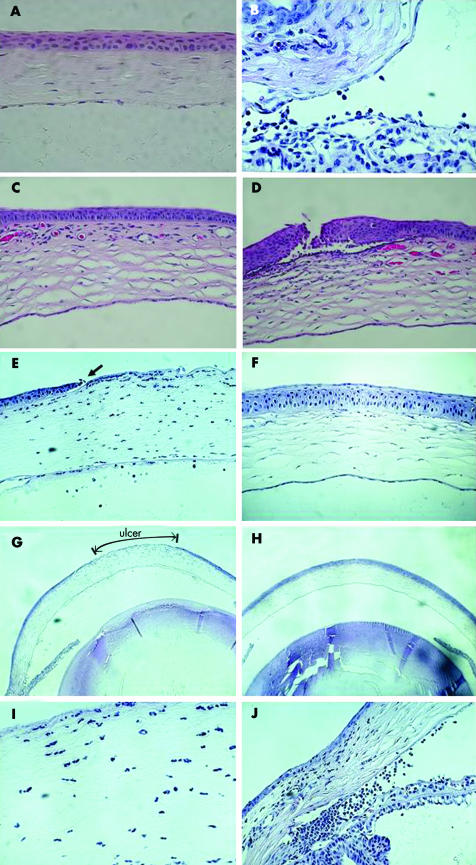
Figure 2 Histological changes in SCID mouse corneas after experimental induction with IL‐8. Eyes infected with either IL‐8/Ad5 or LacZ/Ad5 were enucleated and fixed with formalin, embedded in paraffin, and stained with haematoxylin and eosin. (A) Normal mouse cornea. (B) Anterior chamber angle at 8 hours after IL‐8/Ad5 infection. (C) Cornea at 12 hours after IL‐8/Ad5 infection. (D) Cornea at 16 hours after IL‐8/Ad5 infection. (E) Cornea at 24 hours after IL‐8/Ad5 infection. (F) Cornea at 24 hours after LacZ/Ad5 infection. (G) Whole image of the eye at 24 hours after IL‐8/Ad5 infection. (H) Whole image of the eye at 24 hours after LacZ/Ad5 infection. (I) High magnification of corneal stroma at 24 hours after IL‐8/Ad5 infection. (J) Anterior chamber angle at 24 hours after IL‐8/Ad5 infection.
Histologically, the cornea of the SCID mouse is normally covered by a corneal epithelium of 4–5 layers of epithelial cells (fig 2A). At 8 hours after infection with IL‐8/Ad5, infiltration of neutrophils was observed in the anterior chamber angle (fig 2B). A prominent formation of new blood vessels (angiogenesis), which contained red blood cells in the lumen, was detected in the upper portion of the corneal stroma as early as 12 hours after the infection (fig 2C). At 16 hours, cleft formation between the corneal epithelium and corneal stroma was present (fig 2D). The corneal epithelial tissue above the cleft became thicker and was partially destroyed, resulting in erosion of the cornea. By 24 hours, a part of the upper portion of the corneal stroma was destroyed, resulting in a shallow ulcer in the central portion of the cornea (fig 2E and G). The cleft between the corneal epithelium and stroma was also present at both sides of the ulcer (fig 2E, arrow). An extensive infiltration of neutrophils into the stroma, the ragged appearance of the stromal lamellae, and corneal endothelial cell disruption were also noted (fig 2E and I). Massive infiltration of neutrophils was also seen in the anterior chamber angle (fig 2J). The corneal ulcer was repaired by days 3–4 with re‐epithelialisation and slight scar formation (data not shown). In contrast, corneas transduced with LacZ/Ad5 at 24 hours showed neither corneal ulceration nor neutrophil infiltration (fig 2H), although a slight increase in corneal epithelial cells and keratocytes was seen in the upper portion of the corneal stroma (fig 2F and H).
IL‐8 producing cells
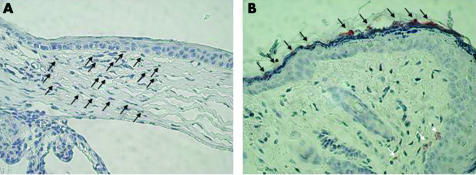
To identify the cells producing IL‐8, mouse corneal tissue was stained with anti‐IL‐8 monoclonal antibody at 8 hours after the infection with IL‐8/Ad5 (fig 3). IL‐8 protein was not expressed in the corneal epithelial cells (fig 3A), but many keratocytes were strongly IL‐8‐positive (fig 3A). Positive staining of IL‐8 was observed as a faint band of amorphous materials on some parts of the conjunctival epithelium (fig 3B). IL‐8 was also detected in several conjunctival stroma cells in the adenoid layer of the substantia propria under the conjunctival epithelium (fig 3B). IL‐8 protein was detected at 12 hours, 16 hours, and 24 hours but not at 48 hours after the infection with IL‐8/Ad5 (data not shown). IL‐8 was detected neither in the corneal tissue before infection with IL‐8/Ad5 nor after infection with LacZ/Ad5 (data not shown).
Figure 3 Immunohistological staining of IL‐8 in SCID mouse corneas after experimental induction with IL‐8. Eyes infected with IL‐8/Ad5 were enucleated at 8 hours after the infection and stained with anti‐IL‐8 monoclonal antibody. (A) Corneal epithelial cells and corneal stroma. The arrows indicate the keratocytes stained with anti‐IL‐8 monoclonal antibody. (B) Conjunctival epithelial cells and the adenoid layer of the substantia propria under the conjunctiva. The black and white arrows indicate the mucous and conjunctival stroma cells stained with anti‐IL‐8 monoclonal antibody, respectively.
Discussion
In this study we utilised a novel approach of using adenovirus gene transfer to the cornea of SCID mice to investigate the role of IL‐8 in the eye, and demonstrated that overexpression of IL‐8 in the cornea causes corneal ulcer formation. This approach has both advantages and disadvantages for the observation of the effects of IL‐8 in vivo. Since SCID mice have neutrophils, which are the main target cells for IL‐8, but not T cells, we can assess the IL‐8 induced results without taking into account of the effects of IL‐8 on T cells. Conversely, we are not able to investigate the effects of IL‐8 on T cells in this particular IL‐8 overexpressing model. In addition, the adenovirus itself is associated with significant direct (non‐T cell mediated) cytotoxicity and inflammation. Thus, we cannot exclude the possibility that the results of infection with IL‐8/Ad5 are influenced to some extent by adenovirus mediated non‐specific cytotoxicity and inflammation.
It has been reported that several kinds of corneal ulcers are associated with neutrophil infiltration and/or IL‐8 production in the cornea. Neutrophil infiltration is frequently seen in keratitis with corneal ulcer.12 Corneal ulcers induced by Pseudomonas aeruginosa infection are associated with IL‐8 expression in corneal epithelial cells13 and neutrophil infiltration.14,15 Corneal ulcers seen in acute red eye reactions are induced by lipopolysaccharide, a potent IL‐8 inducer,2 and are accompanied by prominent neutrophil infiltration.16 In Mooren's ulcer, neutrophil infiltration is seen in the upper portion of the corneal substance.17 These reports and our present results suggest that IL‐8 overexpression is involved in the pathogenesis of some of these corneal ulcers.
Our results suggest that neutrophils attracted by IL‐8 were the main players responsible for the destruction of the corneal tissue. Matsumoto et al14 indicated that superoxide dismutase prevents neutrophil induced damage to corneal tissue and suggested that reactive oxygen metabolites from activated neutrophils are the main mediators of the corneal injury caused by neutrophils. Alternatively, Nagano et al18 suggested that matrix metalloproteinases from neutrophils contribute to corneal ulceration by degrading stromal collagen.
We failed to detect IL‐8 positive corneal epithelial cells, although IL‐8/Ad5 was directly dropped onto the surface of the corneal epithelium for the infection. This is compatible with the report by Tsubota et al19 that the corneal epithelium is resistant to adenovirus infection.
IL‐8 was not detected by 48 hours after the infection with IL‐8/Ad5. This rapid decrease in IL‐8 protein may be due to rapid turnover of the infected cells as previously suggested.19 We speculate that the eventual disappearance of corneal pathology by 3–4 days after the infection with IL‐8/Ad5 is the result of the decrease in IL‐8 protein by the mechanism mentioned above.
The angiogenetic activity of IL‐8 has been demonstrated in rat3 and rabbit20 corneal pocket models. Angiogenesis in these models was evident several days after the administration of IL‐8 protein into the cornea. In the present study, however, angiogenesis was clearly observed in the cornea both clinically and histologically as early as 12 hours after infection with IL‐8/Ad5. We postulate that one of the reasons for this rapid angiogenesis is the large amount of IL‐8 produced by IL‐8/Ad5.
Acknowledgements
This study was supported in part by research grants from the Scientific Research Funds of the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Abbreviations
IL - interleukin
PFU - plaque forming units
SCID - severe combined immunodeficiency
References
- 1.Oppenheim J J, Zachariae C O, Mukaida N.et al Properties of the novel proinflammatory supergene “intercrine” cytokine family. Annu Rev Immunol 19919617–648. [DOI] [PubMed] [Google Scholar]
- 2.Larsen C G, Anderson A O, Appella E.et al The neutrophil‐activating protein (NAP‐1) is also chemotactic for T lymphocytes. Science 19892431464–1466. [DOI] [PubMed] [Google Scholar]
- 3.Koch A E, Polverini P J, Kunkel S L.et al Interleukin‐8 as a macrophage‐derived mediator of angiogenesis. Science 19922581798–1801. [DOI] [PubMed] [Google Scholar]
- 4.Elner V M, Strieter R M, Pavilack M A.et al Human corneal interleukin‐8. IL‐1 and TNF‐induced gene expression and secretion. Am J Pathol 1991139977–988. [PMC free article] [PubMed] [Google Scholar]
- 5.Spandau U H M, Toksoy A, Verhaart S.et al High expression of chemokines Gro‐α (CXCL‐1), IL‐8 (CXCL‐8), and MCP‐1 (CCL‐2) in inflamed human corneas in vivo. Arch Ophthalmol 2003121825–831. [DOI] [PubMed] [Google Scholar]
- 6.Cubitt C L, Tang Q, Monteiro C A.et al IL‐8 gene expression in cultures of human corneal epithelial cells and keratocytes. Invest Ophthalmol Vis Sci 1993343199–3206. [PubMed] [Google Scholar]
- 7.Kennedy M, Kim K H, Harten B.et al Ultraviolet irradiation induces the production of multiple cytokines by human corneal cells. Invest Ophthalmol Vis Sci 1997382483–2491. [PubMed] [Google Scholar]
- 8.Tran M T, Lausch R N, Oakes J E. Substance P differentially stimulates IL‐8 synthesis in human corneal epithelial cells. Invest Ophthalmol Vis Sci 2000413871–3877. [PubMed] [Google Scholar]
- 9.Rosenbaum J T, Planck S R, Huang X N.et al Detection of mRNA for the cytokines, interleukin‐1α and interleukin‐8, in corneas from patients with pseudophakic bullous keratopathy. Invest Ophthalmol Vis Sci 1995362151–2155. [PubMed] [Google Scholar]
- 10.Harada A, Kuno K, Nomura H.et al Cloning of a cDNA encoding a mouse homolog of the interleukin‐8 receptor. Gene 1994142297–300. [DOI] [PubMed] [Google Scholar]
- 11.Oka M, Berking C, Nesbit M.et al Interleukin‐8 overexpression is present in pyoderma gangrenosum ulcers and leads to ulcer formation in human skin xenografts. Lab Invest 200080595–604. [DOI] [PubMed] [Google Scholar]
- 12.Prause J U, Jensen O A. Pas‐positive polymorphonuclear leucocytes in corneal ulcers. Acta Ophthalmol 198058556–566. [DOI] [PubMed] [Google Scholar]
- 13.Xue M L, Zhu H, Willcox M.et al The role of IL‐1β in the regulation of IL‐8 and IL‐6 in human corneal epithelial cells during Pseudomonas aeruginosa colonization. Curr Eye Res 200123406–414. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto K, Shimmura S, Goto E.et al Lecithin‐bound superoxide dismutase in the prevention of neutrophil‐induced damage of corneal tissue. Invest Ophthalmol Vis Sci 19983930–35. [PubMed] [Google Scholar]
- 15.Rudner X L, Kernacki K A, Barrett R P.et al Prolonged elevation of IL‐1 in Pseudomonas aeruginosa ocular infection regulates macrophage‐inflammatory protein‐2 production, polymorphonuclear neutrophil persistence, and corneal perforation. J Immunol 20001646576–6582. [DOI] [PubMed] [Google Scholar]
- 16.Schultz C L, Morck D W, Mckay S G.et al Lipopolysaccharide induced acute red eye and corneal ulcers. Exp Eye Res 1997643–9. [DOI] [PubMed] [Google Scholar]
- 17.Young R G, Watson P G. Light and electron microscopy of corneal melting syndrome (Mooren's ulcer). Br J Ophthalmol 198266341–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagano T, Nakamura M, Nishida T. Differential regulation of collagen degradation by rabbit keratocytes and polyphonuclear leukocytes. Curr Eye Res 200224240–243. [DOI] [PubMed] [Google Scholar]
- 19.Tsubota K, Inoue H, Ando K.et al Adenovirus‐mediated gene transfer to the ocular surface epithelium. Exp Eye Res 199867531–538. [DOI] [PubMed] [Google Scholar]
- 20.Strieter R M, Kunkel S L, Elner V M.et al Interleukin‐8. A corneal factor that induces neovascularization. Am J Pathol 19921411279–1284. [PMC free article] [PubMed] [Google Scholar]