Abstract
Background/aims
Pseudomonas aeruginosa is a major cause of severe bacterial keratitis and remains a difficult clinical entity to treat successfully with the current arsenal of antimicrobial agents. Defensins are small cationic peptides with broad in vitro antimicrobial activity and are potential ocular therapeutic agents. The authors characterised the in vitro activity of defensins NP‐1 and NP‐3a against P aeruginosa in the presence of human tears.
Methods
A clinical Pseudomonas isolate was grown to mid‐log phase, and 1×106 colony forming units were exposed to the peptides (200 μg/ml) for up to 2 hours in the presence of varying concentrations (10–70%) of human tears.
Results
For both peptides in the presence of 10% tears, >3 log units of killing was achieved within 30 minutes. In 70% tears, NP‐1 produced >1 log unit of killing at 2 hours, indicating that, although reduced, its activity remained significant. In 20% tears, NP‐3a demonstrated 2 log units of killing at 2 hours; however, the antimicrobial activity of this defensin was completely inhibited in the presence of 70% tears.
Conclusion
These in vitro data suggest that while the microbicidal activity of some defensins may be diminished at the ocular surface in vivo, significant activity is still possible with certain peptides.
Keywords: defensin, tears, Pseudomonas
The organism Pseudomonas aeruginosa is the most common Gram negative organism associated with bacterial keratitis and is the pathogen most frequently responsible for keratitis associated with contact lens wear.1,2,3 It notoriously causes severe disease which, if untreated, leads to rapid liquefactive necrosis and perforation. As is the case with many pathogens, drug resistant strains of P aeruginosa are becoming more prevalent and may account for as much as 10% of Pseudomonas species.4,5 Thus, the need for new antibacterial agents with unique mechanisms of action is paramount.
Defensins are endogenously produced low molecular weight cationic peptides (3000–4500 Da) that possess potent antimicrobial activity against a variety of bacteria, viruses, and some fungi. These peptides are characterised by the presence of six cysteine residues, which interact to form three disulphide bonds. In mammalian tissues, based upon the position of the cysteines and their linkage, two major classes, referred to as α and β, are recognised. The defensins are major components of the granules of phagocytic cells, particularly neutrophils, and, are expressed by epithelial tissues. At the ocular surface, human β‐defensins (hBD) 1, 2, 3, and 4 are expressed by corneal and conjunctival epithelial cells and the α‐defensins human neutrophil peptides (HNP) 1–3 have been detected in tear fluid and inflamed stroma.6,7 It is believed that defensin antibacterial activity is the result of pore formation or disruption of the cell membrane of target organisms leading to disturbances in metabolism and loss of cellular contents.8,9 For more details on defensins and other cationic antimicrobial peptides, the reader is referred to several excellent reviews.6,10,11,12 We have demonstrated previously that defensins are very effective in vitro against a variety of microbial isolates from horses and humans with severe clinical ocular disease.13 With a view towards possible ocular therapeutic applications of these antimicrobial peptides, we examined the activity of two α‐defensin peptides against P aeruginosa, isolated from a severe case of human ulcerative keratitis, in the presence of human tears.
Materials and methods
Isolation and growth of P aeruginosa
The strain of P aeruginosa (designated HO‐1 by our laboratory) utilised was isolated from a case of severe ulcerative keratitis. We obtained the pathogen by scraping the base of the corneal ulcer with a sterile platinum spatula. The isolate was identified at the UC Davis Medical Center clinical microbiology laboratory using standard biochemical profiles and minimum inhibitory concentration determinations indicated that the isolate was resistant to commonly employed topical antibiotics. The isolate was subcultured twice by overnight incubation in trypticase soy broth (TSB) and re‐plating on trypticase soy agar (TSA). To generate stocks for future use, culture plates were inoculated, then after incubation at 37°C, colonies were removed from the plates using a sterile wooden stick and were then suspended in holding media (TSB with no glucose added, glycerine, and deionised water) and frozen at –20°C until passively thawed for use in these experiments.
Human tears
Tears were collected from healthy subjects at the UC Davis Eye Clinic using micropipettes after olfactory stimulation. The tears were pooled, centrifuged at 25°C for 5 minutes at 400 g, and the pellet was discarded. The tears were divided in to aliquots, then stored at –40°C for up to several days before being used in experiments.
Defensins
The rabbit α‐defensin NP‐1 was purified to homogeneity as described previously.14 Purity was assessed by polyacrylamide gel electrophoresis in acid‐urea gels and by analytical reversed phase high performance liquid chromatography (HPLC). The peptide was quantitated by amino acid analysis and was stored at −20°C, at a concentration of 1 mg/ml in 0.01% acetic acid. Rabbit α‐defensin NP‐3a was purchased from American Peptide Company (Sunnyvale, CA, USA).
Antimicrobial assay
Peptides diluted in 0.01% acetic acid were added to variable concentrations of tears diluted with 10 mM sodium phosphate buffer, pH 7.4, and allowed to incubate at room temperature for 20 minutes. The bacterial isolate was incubated at 37°C in TSB for approximately 2 hours until its optical density had increased 10‐fold to ensure mid‐log growth. The bacteria were washed and 10 μl were added to 90 μl of the defensin/tear mix to produce a suspension containing 1×106 CFU/ml, a final peptide concentration of 200 μg/ml and tear concentrations of between 10% and 70%. Controls were prepared using bacteria, 70% tears, and 0.01% acetic acid in place of the peptide. The peptide concentration was selected based on preliminary assays in which the effectiveness of the peptides over several log concentrations was examined and taking into consideration that the activity of some antimicrobial peptides is reduced at physiological salt concentrations. Because of other constituents in the assay mixture, 70% was the maximum tear concentration obtainable in these experiments. The assay mixtures were incubated at 37°C and at timed intervals, aliquots were diluted 100‐fold in phosphate buffered saline and were plated in duplicate on TSA with a spiral plater. Surviving bacteria were enumerated by counting colonies after 24 hours of incubation at 37°C. Log10 killing was determined by subtracting the log10 colony forming units (CFU)/ml after incubation with the defensins from the log10 CFU/ml at time zero for the control. The lower detection limit in counting CFU was 103 CFU/ml. The assigned value for plates in which no colonies were present was 3. Each experiment was repeated 3–6 times.
Results
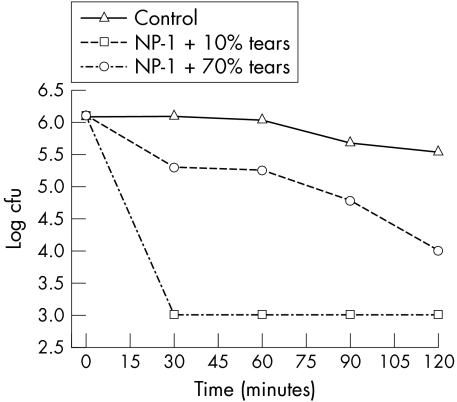
No significant killing of the P aeruginosa isolate occurred when the bacteria were incubated with 70% human tears alone (controls). As presented in figure 1, in the presence of 10% tears, NP‐1 was very effective at killing P aeruginosa, with more than 3 log units of killing within 30 minutes. In the presence of 70% tears, the effectiveness of NP‐1 was reduced, with only approximately 0.8 log units of killing within 30 minutes. However, by 2 hours, 1.54 log units of killing were achieved, and this represents more than 97% killing of the bacteria.
Figure 1 Antibacterial activity of NP‐1 against P aeruginosa in human tears. The activity of NP‐1 (200 μg/ml) against P aeruginosa was tested in the presence of 10% and 70% tears.
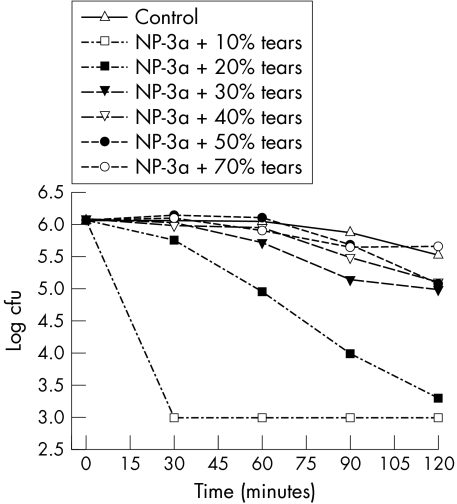
As shown in figure 2, NP‐3a was also highly effective at killing P aeruginosa in the presence of 10% tears. Again, a similar effect was observed in that at higher tear concentrations the effectiveness of NP‐3a was reduced. This effect was dependent on the concentration of tears being used. Interestingly, reduction in NP‐3a effectiveness was observed at lower tear concentrations than for NP‐1. With 20% tears, 2.3 log units of killing were achieved by 2 hours. However, with 70% tears, the killing ability of NP‐3a was eliminated at all time points.
Figure 2 Antibacterial activity of NP‐3a against P aeruginosa in human tears. The activity of NP‐3a (200 μg/ml) against P aeruginosa was tested in the presence of 10% to 70% tears.
Discussion
Defensins and related cationic peptides have potential as therapeutic agents, in part because their mode of antimicrobial action means that resistance will probably not develop easily and because they exert other non‐microbicidal effects such as promotion of wound healing.6,10,11,12 In a previous study, we documented effective microbicidal activity of defensins against ocular pathogens in a sodium phosphate buffer system.13 To gain more realistic insight into the potential activity of defensins when applied to the ocular surface, in this study we examined the effect of human tears on the antimicrobial activity of two α‐defensins.
Tears alone did not kill the strain of Pseudomonas used. This is in keeping with recent observations by Fleiszig et al,15 who showed that tears retard the growth of only some strains of P aeruginosa. We were able to document that in 10% tears, significant killing (>3 log units) by both defensin peptides employed was achieved in 30 minutes. In 70% tears (the maximum possible in our experiments), rabbit defensin NP‐1 produced greater than a full log unit of killing at 120 minutes. These data indicate that although the in vitro microbicidal activity of NP‐1 at the concentrations employed is reduced in full strength human tears, it remains significant. In 20% tears, synthetic rabbit defensin NP‐3a demonstrated 2 log units of killing after a 2 hour incubation period; however, 70% tears completely inhibited NP‐3a activity.
That NP‐1 retains activity in 70% tears and NP‐3a does not is notable and correlates with previous observations that although these peptides are of similar size (33 v 34 amino acids, respectively) and have 50% of their amino acids in common, their activity is not equivalent with NP‐1 generally being more effective than NP‐3a, and NP‐1 has antiviral activity, whereas NP‐3a does not.16,17 Our observation of differential activity of these two defensins in the presence of tears suggests that some peptides will retain significant antimicrobial activity at the ocular surface, whereas others will not. We draw this conclusion with the caveat that it is based on in vitro observations with only two peptides and one P aeruginosa isolate. Future studies with other defensin peptides and multiple P aeruginosa isolates will help clarify our findings. Our data indicate that in vitro testing to screen peptides for activity under conditions that more closely mimic those at the ocular surface rather than a simple phosphate buffer (standard for a typical antimicrobial assay) will be helpful when selecting peptides to study for their potential therapeutic value.
The nature of the reduction in defensin activity is unknown. Inactivation by salt present in the tears in not likely, since the effect of salt is minimal at high concentrations of the peptides such as those used here.18 Furthermore, since the effectiveness of NP‐1 in 70% tears and NP‐3a in 20% tears improves over time, the initial loss of activity cannot be explained by degradation of the peptides. Reversible binding of the peptides to anionic substances present in the tears is a possibility, as has been suggested for loss of β‐defensin antimicrobial activity in the presence of carboxymethylcellulose (an anionic molecule)‐containing artificial tear solutions.19 Likely candidates in tear fluid include the anionic lipocalins and mucins. However, as human tear fluid is composed of hundreds of different molecules, many yet to be definitively identified, there may be several other components that can potentially interfere with defensin antimicrobial activity.
The application of defensins as antimicrobial agents against ocular pathogens is intriguing, not only because of their broad spectrum of activity, but also because of their potential as wound healing agents. We have demonstrated the mitogenic potential of defensins for ocular cells in vitro20 and defensins have been shown to enhance fibronectin stimulated corneal epithelial cell migration.6 However, the suppression of microbicidal activity by tears demonstrated in these in vitro studies suggests that components of human tears may influence the microbicidal effectiveness of some of the peptides in vivo. Thus, the potential application of natural antibiotics such as defensins in the treatment of microbial disease on the ocular surface will depend upon the continued elucidation of their activity in the presence of tears and other solutions commonly applied to the ocular surface.
Acknowledgements
Supported in part by an unrestricted research grant from Research to Prevent Blindness, Inc, and by NIH grants EY08741 (CJM) and EY013175 (AMM).
Abbreviations
hBD - human β‐defensin
HNP - human neutrophil peptide
HPLC - high performance liquid chromatography
TSA - trypticase soy agar
TSB - trypticase soy broth
References
- 1.Schafer F, Bruttin O, Zografos L.et al Bacterial keratitis: a prospective clinical and microbiological study. Br J Ophthalmol 200185842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bourcier T, Thomas F, Borderie V.et al Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br J Ophthalmol 200387834–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mela E K, Giannelou I P, Koliopoulous J X.et al Ulcerative keratitis in contact lens wearers. Eye Contact Lens 200329207–209. [DOI] [PubMed] [Google Scholar]
- 4.Hoban D J, Biedenbach D J, Mutnick A H.et al Pathogen occurrence and susceptibility patterns associated with pneumonia in hospitalized patients in North America: results of the SENTRY Antimicrobial Surveillance Study (2000). Diagn Microbiol Infect Dis 200345279–285. [DOI] [PubMed] [Google Scholar]
- 5.Mutnick A H, Rhomberg P R, Sader H S.et al Antimicrobial usage and resistance trend relationships from the MYSTIC Program in North America (1999–2001).J Antimicrob Chemother 200453290–296. [DOI] [PubMed] [Google Scholar]
- 6.McDermott A M. Defensins and other antimicrobial peptides at the ocular surface. Ocular Surface 20042229–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McIntosh R S, Cade J E, Al‐Abed M.et al The spectrum of antimicrobial peptide expression at the ocular surface. Invest Ophthlamol Vis Sci 2005461379–1385. [DOI] [PubMed] [Google Scholar]
- 8.Van't Hof W, Veerman E C I, Helmerhorst E J.et al Antimicrobial peptides: properties and applicability. Biol Chem 2001382597–619. [DOI] [PubMed] [Google Scholar]
- 9.Shai Y. Mode of action of membrane active antimicrobial peptides. Biopolymers 200266236–248. [DOI] [PubMed] [Google Scholar]
- 10.Mannis M. The use of antimicrobial peptides in ophthalmology: an experimental study in corneal preservation and the management of bacterial keratitis. Trans Am Ophthalmol Soc 2002100243–271. [PMC free article] [PubMed] [Google Scholar]
- 11.Oppenheim J J, Biragyn A, Kwak L W.et al Role of antimicrobial peptides such as defensins in innate and adaptive immunity. Ann Rheum Dis 200362s2, ii17–s2, ii21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganz T. Defensins and other antimicrobial peptides: a historical perspective and an update. Comb Chem High Throughput Screen 20058209–217. [DOI] [PubMed] [Google Scholar]
- 13.Cullor J S, Mannis M J, Murphy C J.et al In vitro antimicrobial activity of defensins against ocular pathogens. Arch Ophthalmol 1990108861–864. [DOI] [PubMed] [Google Scholar]
- 14.Selsted M E, Szklarek D, Lehrer R I. Purification and antibacterial activity of antimicrobial peptides of rabbit granulocytes. Infect Immun 198445150–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleiszig S M, Kwong M S, Evans D J. Modification of Pseudomonas aeruginosa interactions with corneal epithelial cells by human tear fluid. Infect Immun 2003713866–3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selsted M E, Brown D M, DeLange R J.et al Primary structure of six antimicrobial peptides of rabbit peritoneal neutrophils. J Biol Chem 19852604579–4584. [PubMed] [Google Scholar]
- 17.Selsted M E, Szklarek D. Ganz T, et al. Activity of rabbit leukocyte peptides against Candida albicans. Infect Immun 198549202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagaoka I, Hirota S, Yomogida S.et al Synergistic actions of antibacterial neutrophil defensins and cathelicidins. Inflamm Res 20004973–79. [DOI] [PubMed] [Google Scholar]
- 19.Huang L C, Jean D, McDermott A M. Effect of preservative‐free artificial tears on the antimicrobial activity of human beta‐defensin‐2 and cathelicidin LL‐37 in vitro. Eye Contact Lens 20053134–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy C J, Foster B A, Mannis M J.et al Defensins are mitogenic for epithelial cells and fibroblasts. J Cell Physiol 1993155408–413. [DOI] [PubMed] [Google Scholar]