Abstract
Background
Pharmacological treatment has been successful in some forms of acquired neurological nystagmus. However, drugs are not known to be effective in idiopathic infantile nystagmus or nystagmus associated with ocular diseases.
Methods
The authors retrospectively analysed Snellen visual acuity (VA), subjective visual function, and eye movement recordings of 23 patients with nystagmus (13 secondary to multiple sclerosis, three associated with other neurological diseases, two idiopathic infantile, and five with associated ocular diseases) treated with gabapentin or memantine.
Results
With gabapentin, 10 of 13 patients with nystagmus secondary to multiple sclerosis (MS) showed some improvement. Memantine improved the VA in all three patients with MS who did not improve on gabapentin. There was no change of nystagmus in other neurological disorders. Patients with congenital nystagmus showed reduction of nystagmus and their VA changes depended on the ocular pathology.
Conclusion
Gabapentin and memantine may be effective in acquired nystagmus secondary to MS. To the authors' knowledge this is the first series of patients showing that gabapentin is effective in improving nystagmus in congenital nystagmus/nystagmus associated with ocular pathology. Memantine may be useful as an alternative drug in treating patients with nystagmus.
Keywords: acquired nystagmus, congenital nystagmus, gabapentin, memantine, pharmacological treatment
Nystagmus consists of involuntary oscillations of the eyes and can be idiopathic infantile (IIN), associated with albinism, retinal diseases or low vision, or caused by neurological diseases. In IIN, the visual acuity is reduced not only as a result of a defective visual system but also excessive image motion on the retina. The impact on vision is significant, with visual function scores worse than age related macular degeneration.1
Treatment for nystagmus remains largely empirical. A double blind study, prompted by reports that GABA (gamma‐amino butyric acid)‐ergic projections contribute to the neural integrator,2,3,4 showed that gabapentin but not baclofen (both GABAergic agents) reduced acquired nystagmus.5 Nystagmus due to MS improved in 11 out of 11 patients treated with memantine (a glutamate antagonist) and of note all these patients had pendular waveform.6 Smoking cannabis reduced nystagmus in one patient with MS.7 Anticholinergic drugs, sodium or potassium channel blockers, alcohol, clonazepan, and other antiepileptic drugs have also been administered for acquired nystagmus.6,8 Recently, potassium channel blockers were found to be effective in reducing acquired downbeat nystagmus.9
In contrast with acquired nystagmus, pharmacological treatment is not used in congenital nystagmus and there are only few case reports of nystagmus reduction.10,11 We have reported a case of congenital nystagmus associated with corneal dystrophy where the visual acuity improved with gabapentin.12
The aim of this paper is to report our experience with gabapentin and memantine in treating acquired and congenital nystagmus.
Methods
All patients with nystagmus due to neurological diseases or associated with ocular diseases or IIN, treated with gabapentin or memantine in the department of Ophthalmology at Leicester Royal Infirmary between 2000 and 2004 were included in this retrospective study. The details of the patients are given in table 1. Thirteen patients had MS; three other neurological diseases, two IIN, and five associated with ocular pathology. Patients 913 and 2212 have been reported previously.
Table 1 Patient characteristics, diagnosis, nystagmus waveform, treatment and the subjective and objective visual acuity changes after the treatment.
| Patient | Age (years) | Diagnosis | Type of nystagmus | Dosage (mg) | Duration (months) | VA before | VA after | Subjective change | % change in amplitude | Continued with treatment | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RE | LE | RE | LE | |||||||||||||
| Gabapentin treatment | ||||||||||||||||
| 1 | 50 | Multiple sclerosis | Horizontal pendular with INO | 1200 | 6 | 6/18 | 6/18 | 6/36 | 6/18 | Osci better | 50.0 | yes | ||||
| 2 | 43 | Multiple sclerosis | Horizontal jerk | 900 | 10 | 6/24 | 6/24 | 6/24 | 6/24 | Osci better | 19.4 | yes | ||||
| 3 | 42 | Multiple sclerosis | Elliptical pendular (mainly vertical) | 1200 | 6 | 6/24 | CF(3/60) | 6/24 | CF(3/120) | not better | 3.8 | no | ||||
| 4 | 62 | Multiple sclerosis | Horizontal pendular | 2400 | 6 | 6/60 | 6/60 | 6/60 | 6/60 | not better | 40.0 | no | ||||
| 5 | 23 | Multiple sclerosis | Horizontal square wave jerks | 2400 | 10 | 6/36 | 6/36 | 6/12 | 6/12 | better | 24.4 | yes | ||||
| 6 | 31 | Multiple sclerosis | Horizontal pendular | 1200 | 12 | 6/18 | 6/12 | 6/24 | 6/24 | Osci better | 3.7 | yes | ||||
| 7 | 39 | Multiple sclerosis | Elliptical pendular (mainly vertical) | 1500 | 6 | 6/24 | 6/36 | 6/60 | 6/24 | Osci better | ‐26.8 | yes | ||||
| 8 | 41 | Multiple sclerosis | Elliptical pendular and horizontal jerk | 900 | 6 | 6/36 | 6/36 | 6/36 | 6/36 | Osci better | 55.3 (P), 12.6 (J) | yes | ||||
| 9 | 45 | Multiple sclerosis | Elliptical pendular | 1200 | 5 | 6/60 | 6/60 | 6/36 | 6/60 | better* | 35.7 | yes | ||||
| 10 | 55 | Multiple sclerosis | Elliptical pendular with INO | 1200 | 4 | 6/36 | 6/24 | 6/12 | 6/12 | better | 32.6 | yes | ||||
| 11 | 61 | Multiple sclerosis | Elliptical pendular | 2400 | 18 | 6/24 | 6/60 | 6/12 | 6/60 | better | 34.4 | yes | ||||
| 12 | 59 | Multiple sclerosis | Elliptical pendular | 2400 | 18 | 6/6 | 2/60 | 6/6 | 2/60 | Osci better | 12.5 | no | ||||
| 13 | 30 | Multiple sclerosis | Elliptical pendular | 2400 | 12 | 6/60 | 4/60 | 6/60 | 4/60 | not better | 48.0 | no | ||||
| 14 | 88 | Cerebellar atrophy | Downbeat jerk | 900 | 3 | 6/9 | 6/6 | 6/9 | 6/6 | not better. | . | no | ||||
| 15 | 65 | Spino cerebellar ataxia | Downbeat jerk | 1200 | 3 | 6/5 | 6/5 | . | . | not better | . | no | ||||
| 16 | 77 | Cerebellar and occipital infarcts | Vertical, seesaw | 1800 | 2 | 6/18 | 6/18 | . | . | not better | . | no | ||||
| 17 | 25 | Idiopathic | Horizontal pendular with foveating saccades | 1200 | 30 | 6/24 | 6/18 | 6/18 | 6/12 | better subjectively | 31.4 | yes | ||||
| 18 | 34 | Idiopathic | Horizontal jerk | 1800 | 6 | 6/12 | 6/18 | 6/12 | 6/12 | no improvement | 18.1 | no | ||||
| 19 | 38 | Achromatopsia | Elliptical pendular and horizontal jerk | 2400 | 6 | 6/36 | 6/60 | 6/36 | 6/36 | better | 81.8 (P), 42.0 (J) | yes | ||||
| 20 | 41 | Achromatopsia | Horizontal jerk | 1200 | 3 | 6/60 | 6/60 | 6/36 | 6/36 | better | 60.9 | yes | ||||
| 21 | 16 | Macular coloboma | Oblique pendular | 1800 | 23 | 3/60 | 3/60 | 3/60 | 3/60 | better subjectively | 54.0 | yes | ||||
| 22 | 39 | Corneal dystrophy | Elliptical jerk | 2400 | 12 | 6/36 | 1/60 | 6/18 | 1/60 | better | 62.2 | yes | ||||
| 23 | 54 | Senior Loken syndrome | Elliptical pendular | 600 | 24 | 6/36 | 6/36 | 6/18 | 6/18 | better | 78.9 | yes | ||||
| Memantine treatment | ||||||||||||||||
| 3 | 42 | Multiple sclerosis | Elliptical pendular (mainly vertical) | 20 | 3 | 6/24 | 3/60 | 6/36 | 6/18 | better | 16.2 | yes | ||||
| 12 | 59 | Multiple sclerosis | Elliptical pendular | 20 | 4 | 6/6 | 2/60 | 6/6 | 4/60 | better | 54.6 | yes | ||||
| 13 | 30 | Multiple sclerosis | Elliptical pendular | 20 | 6 | 6/60 | 4/60 | 6/36 | 6/36 | better | 91.8 | yes | ||||
| 15 | 65 | Spinocerebellar ataxia | Downbeat jerk | 10 | 4 | 6/5 | 6/5 | 6/5 | 6/5 | not better | ‐35.8 | no | ||||
INO, internuclear ophthalmoloplegia, Osci, oscillopsia, CF, counting fingers, P, pendular, J, jerk, *(6/9 on chin elevation).
VA and subjective changes were assessed before and after treatment. Gabapentin was used in increasing doses from 900 mg/day to 2400 mg/day. Four patients who did not improve substantially with gabapentin were commenced on memantine starting with 5 mg/day increasing up to 20 mg/day in divided doses. The drugs were discontinued because of side effects if necessary.
Eye movements were recorded before and after treatment (250 Hz; EyeLink Pupil Tracker, SMI GmbH, Berlin, Germany) while viewing rear projected stimuli (screen = 1.8×1.2 m, Hitachi CP‐X958 projector). Each eye was calibrated offline by selecting foveations when fixating 0° and horizontal and vertical points at plus or minus 15° eccentricity. Visual tests in horizontal and vertical directions included saccades (targets from −20° to 20° in 10° steps, 1.5 second interval), smooth pursuit (10°/s, 20°/s and 40°/s velocities, plus or minus 20° amplitude), and steady fixation (1 minute recordings at 0° and plus or minus 15°). Peak to peak amplitudes were measured before and after drug treatment from 3–5 second sections of data during stable fixation at the primary position. The component of the nystagmus along either horizontal or vertical axes was measured depending which was the largest.
Changes in VA and amplitude were tested for significance using Wilcoxon signed rank test (SPSS v11).
Results
Gabapentin in acquired nystagmus
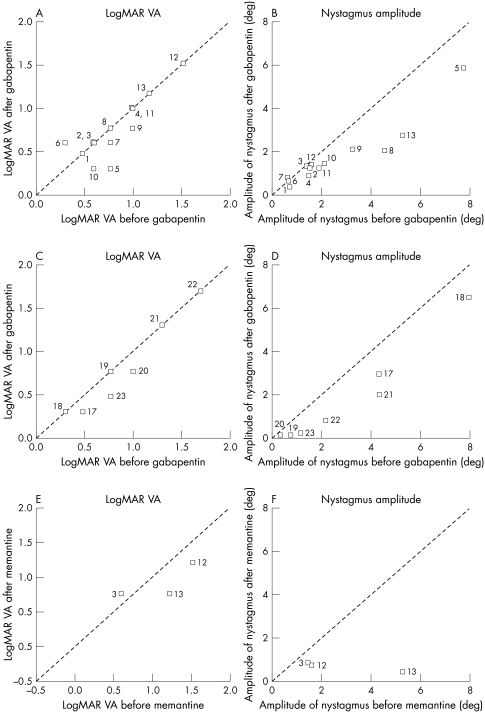
Of the 13 patients with nystagmus secondary to MS, four patients (patients 5, 9, 10, 11) showed improvement of VA (table 1). Six patients had subjective improvement of oscillopsia (patients 1, 2, 6, 7, 8, 12). The remaining three patients did not improve. The improvement in VA was not significant (Z = −1.083, p = 0.278).
The amplitude of nystagmus was reduced in 12 of 13 patients (Z = 3.04, p = 0.002), following treatment with gabapentin (fig 2B).
Figure 2 LogMAR visual acuity measured on Snellen charts and the peak to peak amplitude of nystagmus measured in the worse eye (eye with the maximum amplitude of nystagmus) before and after treatment with gabapentin, in patients with multiple sclerosis (A and B); idiopathic infantile nystagmus and nystagmus associated with ocular pathology (C and D); and patients with MS treated with memantine (E and F).
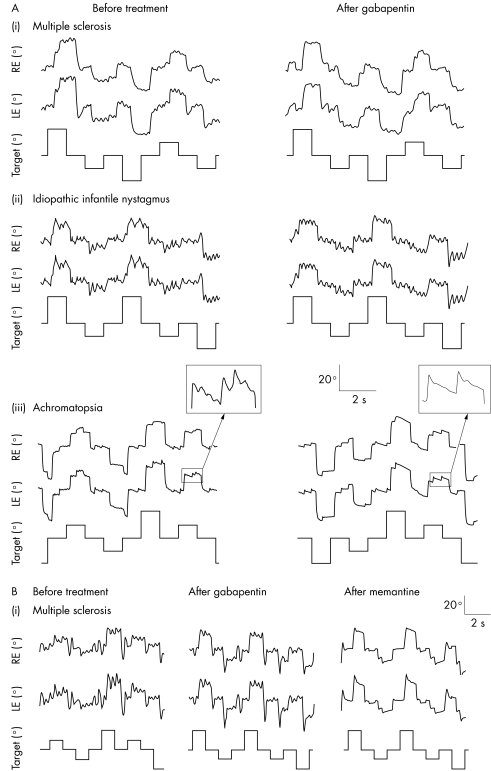
An example of a patient (patient 11) with MS with reduced amplitude of horizontal pendular nystagmus on treatment with gabapentin is shown in figure 1Ai. Nine of the 10 patients who improved choose to continue gabapentin medication. Side effects were mild, mostly consisting of transient fatigue.
Figure 1 (A) Original eye horizontal eye movement recordings of right and left eye before and after treatment with gabapentin, where (i) is a patient with multiple sclerosis before and after treatment with 2400 mg gabapentin (patient 11); (ii) a patient with idiopathic congenital nystagmus before and after treatment with 1200 mg gabapentin (patient 17); and (iii) a patient with achromatopsia before and after treatment with 2400 mg gabapentin (patient 19). (B) Shows a patient with multiple sclerosis before treatment, after treatment with 2400 mg gabapentin, and after treatment with 20 mg memantine (patient 13).
Treatment with gabapentin reduced both pendular and jerk nystagmus; however, patient 8 with jerk waveform superimposed over pendular nystagmus showed a predominant reduction in the pendular nystagmus (55.3%), while the jerk nystagmus was reduced by 12.6%.
None of the three patients with nystagmus secondary to other neurological causes (patients 14–16) had visual improvement. They discontinued gabapentin because of side effects such as fatigue, memory loss, tingling, and numbness.
Idiopathic infantile nystagmus and gabapentin
Two patients (patients 17 and 18) with IIN were treated with gabapentin. In both patients, the nystagmus amplitude was reduced following treatment. Figure 1Aii shows eye movements from patient 17. Of the two patients with IIN, one improved by one line VA in each eye, the other improved from 6/18 to 6/12 in the left eye with the right eye remaining unchanged.
Nystagmus associated with ocular pathology and gabapentin
Five patients (patients 19–23 in table 1) were treated with gabapentin. Figure 1Aiii shows the eye movements of a patient with achromatopsia (patient 19) before and after treatment with gabapentin. This patient had pendular nystagmus superimposed over jerk nystagmus. The pendular oscillations disappeared almost totally (by 81.8%) while the jerk nystagmus was reduced by 42%.
The peak to peak amplitude was reduced in all the patients with nystagmus associated with ocular pathology after gabapentin administration; however, VA changes depended on ocular pathology (fig 2C, D).
Two patients with achromatopsia improved in VA from 6/60 to 6/36 in one eye (patient 19) or both eyes (patient 20). Additionally the near vision of patient 19 improved from N14 to N8. The patient with macular pathology (patient 21) improved subjectively even though his VA remained unchanged. Patient 22, with congenital corneal dystrophy, improved by more than two lines in right VA. Left VA did not improve, probably because of amblyopia and corneal opacity. However, the amplitude of nystagmus improved in both eyes. Patient 23, with Senior Loken syndrome, took a lower dosage of gabapentin because of impaired renal function. Her VA in each eye improved from 6/36 to 6/18. None of this group of patients complained of side effects from gabapentin.
The improvement in the amplitude of nystagmus in the IIN/associated ocular pathology was statistically significant (Z = 2.366, p = 0.017). However the change in VA was not significant (Z = 1.603 p = 0.108).
Six of the seven patients with IIN/associated ocular pathology decided to continue gabapentin.
Memantine and acquired nystagmus
Of the patients who did not improve or showed only marginal improvement with gabapentin, four (patients 3, 12, 13, 15) were prescribed memantine. In figure 1B eye movement recordings of patient 13 are shown. While gabapentin had no effect, memantine abolished nystagmus almost completely. All three patients with nystagmus secondary to MS showed improvement in VA in at least one eye on treatment and the nystagmus amplitude improved in both eyes. The patient with spinocerebellar ataxia (patient 15) showed no improvement in nystagmus amplitude or in reported oscillopsia. All three patients with nystagmus secondary to MS continued using memantine.
Discussion
Gabapentin may act on nystagmus as a GABA agonist,14 a glutamate antagonist by inhibiting NMDA (N‐methyl d‐aspartate) receptors,15 or by influencing voltage sensitive sodium and calcium channels.14. Memantine is an NMDA receptor antagonist.15 The common factor between the two drugs is their anti‐glutaminergic action.
In a study,15 including eight patients, the pure GABA agonist vigabatrin did not have any effect on nystagmus, suggesting that inhibition of NMDA receptor might be the mechanism of action of gabapentin. In another study,16 visual acuity and nystagmus improved with gabapentin in three patients with acquired nystagmus. In our patients, treatment was evaluated for longer than in previous studies and MS patients had relapses, including optic neuritis and change in nystagmus. This may explain the inhomogeneous effect of gabapentin in this group. However, 10 out of 13 patients improved objectively or subjectively with gabapentin and memantine improved the VA and oscillopsia in the remaining three patients.
To our knowledge there are only single case reports published10,11,12 but no studies have included series of patients showing the effectiveness of pharmacological treatment in congenital nystagmus. In this study we describe seven patients with IIN or nystagmus associated with ocular pathology, in whom the amplitude of nystagmus improved on treatment with gabapentin. This is the first series of patients in which it has been shown that pharmacological treatment can be effective in congenital nystagmus or nystagmus due to ocular pathology. The visual acuity outcome depended on the presence of other ocular pathology. Interestingly, even patients with no cone function such as in achromatopsia showed an improvement in VA. The nystagmus foveation improved more consistently in congenital nystagmus than in acquired nystagmus.
In conclusion, we found gabapentin and memantine effective in the treatment of most patients with nystagmus included in this study. Nystagmus of patients with MS has shown good response to gabapentin. Memantine reduced nystagmus in those patients not responding to gabapentin. Memantine, therefore, is a strong candidate for treatment of nystagmus in MS. Interestingly, we found that nystagmus was reduced in all the seven patients with IIN/nystagmus associated with ocular pathology on treatment with gabapentin. An objective evaluation with a randomised double masked trial on the effectiveness of these drugs in congenital nystagmus, nystagmus associated with other ocular diseases, and acquired nystagmus is required. This would establish the types of nystagmus suitable for pharmacological treatment and which drug should be the first line of treatment.
Acknowledgements
The authors would like to thank the Nystagmus Network, UK and the Ulverscroft Foundation for financial support for this study.
Abbreviations
CF - counting fingers
GABA - gamma‐amino butyric acid
IIN - idiopathic infantile nystagmus
INO - internuclear ophthalmoloplegia
MS - multiple sclerosis
NMDA - N‐methyl d‐aspartate
Osci - oscillopsia
VA - visual acuity
Footnotes
Competing interests: none declared.
References
- 1.Pilling R F, Thomspon J R, Gottlob I. Social and visual function in nystagmus. Br J Ophthalmol 2005891278–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Straube A. Differential effects of bicuculline and muscimol microinjections into the vestibular nuclei on simian eye movements. Exp Brain Res 199186347–358. [DOI] [PubMed] [Google Scholar]
- 3.Mettens P. Effect of muscimol microinjections into the prepositus hypoglossi and the medial vestibular nuclei on cat eye movements. J Neurophysiol 199472785–802. [DOI] [PubMed] [Google Scholar]
- 4.Arnold D B. Nystagmus induced by pharmacological inactivation of the brainstem ocular motor integrator in monkeys. Vis Res 1999394286–4295. [DOI] [PubMed] [Google Scholar]
- 5.Averbuch‐Heller L. A double‐blind controlled study of gabapentin and baclofen as treatment for acquired nystagmus. Ann Neurol 199741818–825. [DOI] [PubMed] [Google Scholar]
- 6.Stark M. Drug therapy for acquired pendular nystagmus in multiple sclerosis. J Neurol 19972449–16. [DOI] [PubMed] [Google Scholar]
- 7.Schon F. Suppression of pendular nystagmus by smoking cannabis in a patient with multiple sclerosis. Neurology 1999532209–2210. [DOI] [PubMed] [Google Scholar]
- 8.Averbuch‐Heller L. Eye movements. Curr Opin Neurol 1996119626–31. [DOI] [PubMed] [Google Scholar]
- 9.Strupp M, Schüller O, Krafczyk S.et al Treatment of downbeat nystgamus with 3,4 diaminopyridine. A placebo controlled study. Neurology 200361165–170. [DOI] [PubMed] [Google Scholar]
- 10.Hertle R W, Maybodi M, Mellow S D.et al Clinical and oculographic response to Tenuate Dospan (diethylpropionate) in a patient with congenital nystagmus. Am J Ophthalmol 2002133159–160. [DOI] [PubMed] [Google Scholar]
- 11.Hertle R W, Maybodi M, Bauer R M.et al Clinical and oculographic response to Dexedrine in a patient with rod‐cone dystrophy, exotropia, and congenital aperiodic alternating nystagmus. Binocul Vis Strabismus Q 200116259–264. [PubMed] [Google Scholar]
- 12.Sarvananthan N, Gottlob I. Pharmacological treatment of congenital nystagmus. Arch Ophthalmol. (in press). [DOI] [PubMed]
- 13.Jain S, Proudlock F A, Constantinescu C S.et al Combined pharmacologic and surgical approach to acquired nystagmus due to multiple sclerosis. Am J Ophthalmol 2002134780–782. [DOI] [PubMed] [Google Scholar]
- 14.White S H. Comparative anticonvulsant and mechanistic profile of the established and newer antiepileptic drugs. Epilepsia 199940(suppl 5)S2–S10. [DOI] [PubMed] [Google Scholar]
- 15.Bandini F, Castello E, Mazzella L.et al Gabapentin but not vigabatrin is effective in the treatment of acquired nystagmus in multiple sclerosis: how valid is the GABAergic hypothesis? J Neurol Neurosurg Psychiatry 200171107–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stahl J S, Rottach K G, Averbuch‐Heller L. A pilot study of gabapentin as treatment for acquired nystagmus. Neuro‐ophthalmology 199616107–113. [DOI] [PubMed] [Google Scholar]