Abstract
Aims
To establish a keratoprosthesis (Kpro) surgical technique that maintains an intact superficial corneal layer.
Methods
A manual microkeratome (Moria LSK‐1) was used to create a 130 μm flap of approximately 10 mm diameter in the right eye of Japanese white rabbits. The stoma beneath the flap area was dissected before the removal of a 5.0 mm stromal disc. A 5.0 mm collagen I immobilised poly(vinyl alcohol) (COL‐PVA) disc was placed on the exposed posterior stroma close to Descemet's membrane. The flap was repositioned and fixed using 10‐0 nylon sutures, which were removed 2 days following surgery. The corneas were followed clinically by slit lamp microscopy and photographs. Rabbits were sacrificed after 6 months, and the transplanted corneas were examined histologically by haematoxylin and eosin staining and immunohistochemistry against vimentin and α‐smooth muscle actin (α‐SMA).
Results
The transplanted COL‐PVA discs remained transparent throughout the study, with no complications related to the flap or overlying epithelium. The interface between COL‐PVA and Descemet's membrane remained clear without signs of opacification caused by scarring or cellular deposition. Pathology revealed the intact COL‐PVA polymer in the posterior stroma, with minimal cellular infiltration along the anterior and posterior interfaces. Immunohistology shows vimentin and α‐SMA staining at levels comparable to lamellar keratoplasty control.
Conclusions
Microkeratome assisted deep lamellar keratoprosthesis may be a safe technique for the transplantation of artificial hydrogels for therapeutic purposes.
Keywords: keratoprosthesis, transplantation, microkeratome, rabbit
The search for an ideal artificial cornea has a long history, which has led to the development of several keratoprosthesis (Kpro), some of which are already in clinical use.1,2,3,4,5,6 The Dohlman‐Doane7 and AlphaCor6 Kpros are the two models currently approved by the Food and Drug Administration, and clinical studies are accumulating. The material used for Kpros should be biocompatible to the extent that excessive inflammation and scarring do not occur at the anterior and posterior interfaces of the visual axis. In order to preserve an optically clear interface, several Kpros are designed so that the corneal epithelium does not cover the anterior surface. Although satisfactory vision is achieved with a successful Kpro implant, a discontinuous epithelial layer may lead to pigment deposition or extrusion of the Kpro.6 In order to circumvent such complications, a strategy to integrate biological components to increase biocompatibility was adopted in the development of corneal equivalents.8,9 Corneal equivalents show characteristics similar to the natural cornea in vitro, however, a prototype that lasts indefinitely in vivo is yet to be designed.
The need for corneal equivalents is based on the assumption that healthy components of the epithelium, stroma, and corneal nerves are required to reconstitute the cornea. However, several studies have shown that artificial materials are stable when placed in stromal pockets, as long as stromal tissue and nerves are left intact in the remaining cornea. The presence of healthy stromal keratocytes is of importance in maintaining a healthy epithelial layer because of the many soluble factors exchanged by both tissue in an elaborate epithelial stromal “cross talk.”10 An intact epithelium with an underlying stromal layer in laser in situ keratomileusis (LASIK) flaps may explain the fewer incidences of haze observed in LASIK compared with photorefractive keratectomy (PRK).11,12,13
Opacification of the posterior stromal interface is also occasionally observed in Kpro surgery. Retrocorneal membranes are associated with Kpros that have posterior optics projecting directly into the anterior chamber,14 and retroprosthetic membranes occur in approximately 9% of patients with the AlphaCor Kpro.15 In either case, opacification can be minimised if activation of fibroblasts can be suppressed by physical or pharmacological means. From our experience with deep lamellar keratoplasty (Dro), we have found that by dissecting directly down to the Descemet's membrane (DM), interlamellar opacification following surgery is minimal. We therefore hypothesised that if Kpros can be transplanted directly onto the deep stroma, while leaving anterior stromal tissue intact beneath the epithelium, such a surgical technique may allow for a long standing artificial device. In order to achieve this architecture, we combined the use of a microkeratome with a D surgical technique already in clinical use.16,17 The technique is similar to the microkeratome assisted posterior keratoplasty described by two different groups18,19 without penetrating into the anterior chamber. In this report, we show the macroscopic and histological results of poly(vinyl alcohol) (PVA) discs transplanted in rabbits using this “microkeratome assisted deep lamellar Kpro” technique, which showed promising mid‐term results.
Material and methods
PVA implants
Collagen I was immobilised to PVA (PVA‐COL) as previously described.20 In brief, PVA powder was dissolved in a mixture of dimethyl sulfoxide (DMSO) water solvent and was allowed to stand at −20°C for 24 hours to form a gel. The surface of the gel was modified with hexamethylene dissocyantate (HMDI), which was then immersed in type I collagen solution (porcine skin, 0.5 mg/ml, Nitta Gelatin Co Ltd, Osaka, Japan). PVA‐COL was prepared as a non‐porous hydrogel, with a water content of 78% to 80%, which is similar to the host cornea. PVA‐COL discs (200 µm thickness, 5.0 mm diameter) were sterilised before surgery.
Surgical procedure
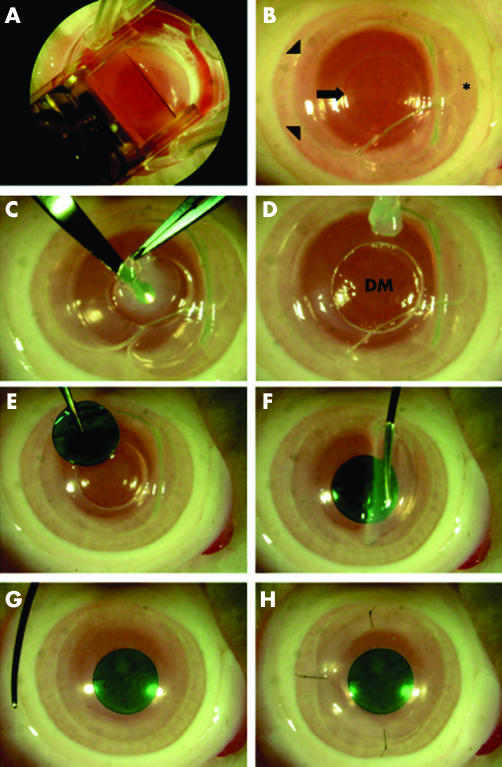
All animals were treated according to the ARVO statement for the use of animals in ophthalmology and vision research. Female Japanese white rabbits (n = 6, 3 kg body weight, Shiraishi experimental animal breeding farm, Tokyo, Japan) were anaesthetised with 4 ml intramuscular ketamine and xylazine (1:7 mixture), as well as topical xylocaine at the start of surgery. Two rabbits were used as lamellar keratoplasty () control, and four rabbits underwent the study procedure. A 130 μm flap with a nasal hinge was created using the LSK‐1 microkeratome (Moria, Antony, France). After the flap was lifted, a manual trephine (5.0 mm diameter) was used to create an incision extending into the deep stroma without perforating the DM. Air was injected into the anterior chamber, and the DM was dissociated from the posterior stromal surface using a blunt spatula as previously described for deep lamellar keratoplasty (DLKP).17 Air injection into the anterior chamber was first described by Melles et al, which allows visualisation of incision depth reducing the risk of perforating DM.16 The trephined stromal disc was then removed using micro scissors and forceps to remove any remaining stromal strands. The PVA‐COL disc was then placed into the circular space without any suture fixation. The flap was repositioned and fixed with 5 10‐0 nylon sutures. A summary of the surgical procedure is presented in figure 1.
Figure 1 Demonstration of DLKPro surgical technique using an enucleated rabbit eye. (A) A manual microkeratome is used to create a hinged flap. (B) A 5 mm incision (arrow) is trephined under the lifted flap (*). Arrowheads indicate the margin of the flap bed. Air is injected into the anterior chamber to allow visualisation of Descemet's membrane (see text for details). (C, D) Stromal tissue is removed using a lamellar knife to reveal Descemet's membrane (DM). (E–H) A 5.0 mm PVA disc (stained with trypan blue to enhance visibility) is inserted into the excised wound, and the flap is repositioned and fixed using three to five 10‐0 nylon sutures.
Flap sutures were removed 2 days following surgery after confirming that the corneal epithelium was intact. Topical antibiotics and steroids were applied twice daily for 1 week. One of the rabbits in the study group experienced flap complications (thin flaps, suture related infections), therefore, a total of three rabbits were observed and photographed for up to 6 months following surgery. One rabbit each was sacrificed after 1 month, 3 months, and 6 months with an overdose of pentobarbital, and the corneas with implants were excised and fixed for histological and immunohistochemical analysis.
Histological analysis
Corneas were fixed in 10% formalin neutral buffer solution (Mildform 10N, Wako Pure Chemical Industries, Osaka, Japan) at 4°C overnight, and then embedded in paraffin. Four μm sections were stained with haematoxylin and eosin (HE) or immunostained with primary antibodies against α‐smooth muscle actin (α‐SMA, clone 1A4, 0.2 μg/ml, Neomarkers, Lab Vision Corporation, Fremont, CA, USA) and vimentin (clone V9, 0.2 μg/ml, Neomarkers). In brief, sections were rehydrated, treated with 3% H2O2, blocked with phosphate buffered saline (PBS) containing 10% normal goat serum and 1% bovine serum albumin (BSA), and treated with primary antibody at 4°C overnight. After washing, sections were incubated with biotinised goat anti‐mouse IgG1 (0.5 μg/ml, Southern Biotechnology Associates Inc, Birmingham, AL, USA) or anti‐mouse IgG2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), treated with ABC kit (Vector Laboratories Inc, Burlingame, CA, USA), and ABC is detected by diaminobenzidine (DAB) kit (Vector Laboratories Inc).
Results
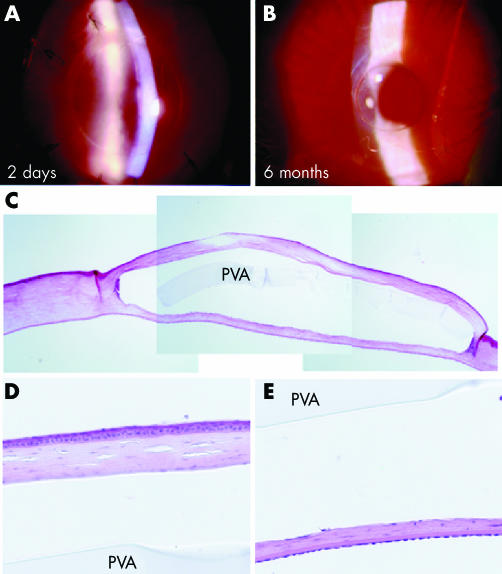
Figure 2A shows the PVA‐COL implant positioned beneath the sutured flap 2 days following surgery. The epithelium was smooth and intact, with no inflammation of the ocular surface and anterior chamber. The polymer stromal interface was smooth in both the anterior and posterior surfaces for up to 6 months (fig 2B). Minimal scarring was found along the circumference of the PVA‐COL disc, and the ocular surface was quiet without signs of inflammation or infection throughout the entire follow up period.
Figure 2 Surgical results of DLKPro. Postoperative slit photographs 2 days (A, ×22) and 6 months (B, ×16) following surgery. HE staining of the same cornea shows an intact disc within the stroma with minimal cellular infiltration (C). High magnification of the flap (D) and posterior stroma (E) shows no cellular infiltration. The space between stroma and implant is an artefact of tissue fixation. PVA = PVA implant.
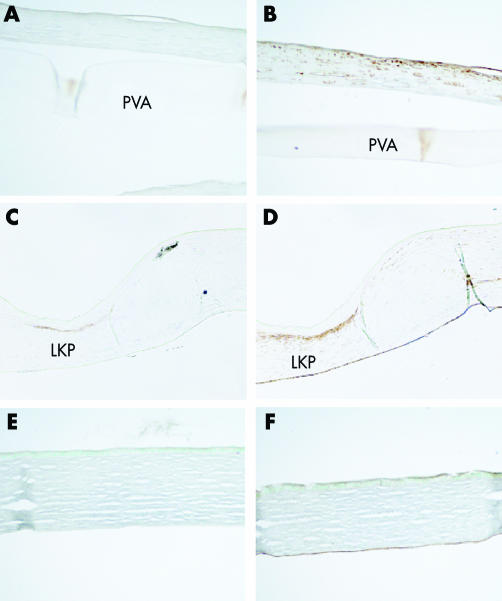
Haematoxylin and eosin stains of the cornea shows the intact polymer without signs of melting or cellular infiltration after 6 months (fig 2C). A thin layer of stroma is found beneath the disc and DM, which may represent either residual stroma at the time of surgery, or regenerated tissue during the observation period (fig 2E). The epithelium of the flap overlying the polymer is stratified without any pathological signs of thinning or erosions (fig 2D). Immunohistochemistry using an anti‐α‐SMA (fig 3A) shows that keratocyte (fibroblast) activation is minimal at the polymer‐stromal interfaces, which is consistent with the minimal scarring observed by slit lamp examination. There was a slight increase in vimentin positive cells in the flap (fig 3B) compared to normal cornea control (fig 3F), indicating that some of the keratocytes were of the fibroblast phenotype. However, the appearance of fibroblastic cells is common in corneal surgery, as shown in a rabbit with an autologous lamellar transplant (fig 3D).
Figure 3 Immunohistochemistry against α‐SMA (A) shows minimal staining compared with the lamellar keratoplasty control (C), indicating the absence of myofibroblasts. Vimentin (B) is expressed locally beneath the epithelium similar to LKP control (D) representing activated fibroblasts. (E, F) Normal control cornea stained with anti‐αSMA (E) and anti‐vimentin (F).
Discussion
Previous attempts at using artificial polymers as transplantable grafts have often encountered problems with extrusion and infection.21,22 An intact ocular surface may be a way to prevent these complications; however, long term maintenance of a stratified epithelial layer over an artificial hydrogel has proved difficult for extended periods in vivo.9 The reason why such polymers, which often support stratified epithelium in vitro,20 fail to maintain a healthy epithelium in vivo can be explained by a lack of stromal cells that are involved in the homeostasis of the epithelium.10 The surgical technique described in this report makes use of residual stroma within the flap to function both as a mechanical barrier against polymer extrusion, as well as a reservoir of keratocytes for the maintenance of the overlying epithelium. The structure of the anterior stroma is similar to the AlphaCor implant before the stage II procedure in cases without the Gunderson conjunctival flap. As shown from the histology of the implanted cornea after 6 months, keratocytes are observed between the polymer and epithelium, and the appearance of the overlying epithelium is normal without signs of mechanical stress or malnutrition. The COL‐PVA hydrogel used for the implant has already been shown to maintain a normal glycogen content in overlying epithelium demonstrated by PAS staining.20
Another vital property of keratoprosthesis is the optical clarity achieved along the visual axis after implantation. Therefore, conventional full thickness keratoprosthesis may offer better vision when successfully managed. The advantage of the material and technique used in this study is higher biocompatibility of the keratoprosthesis that is completely implanted within the stroma. Figures 2 and 3 show that opacification of the interface is minimal, with scarring observed only along the edge of the implanted disc. Such scarring does not hinder the refraction of the cornea, and may serve to secure the polymer in position within the stroma. Figure 2 also shows residual stroma between the polymer and endothelium. Since there was no apparent opacification at the posterior surface of the implant, complete exposure of DM may not be necessary. This may be beneficial since exposing DM may be difficult in clinical cases.
Clinical indications for this procedure may be limited because of the requirement of a relatively clear anterior stroma. One such indication may be keratoconus patients with relative thickness of the central cornea. Although there may be an ethical issue with using keratoprosthesis in keratoconus patients, DLKPro may be an option before PKP using donor tissue. However, we think that another broader indication may be patients with recurring bullous keratopathy. Although a healthy endothelium is required to maintain clarity in a full thickness cornea, decreased vision as a result of stromal oedema can be substantially decreased by replacing 300–400 µm of the swelled stroma with a transparent polymer. Although visual acuity may be suboptimal, functional vision may be restored in patients who otherwise may require multiple grafts because of rejection or endothelial decompensation. Animal studies to investigate this possibility are under way.
Another advantage of this technique is the simple design of the implant, which may be constructed from modified contact lens moulds. Although the disc used in this study was flat, a curved design fit for the human cornea may be more compatible for clinical use. Custom designed discs may be used modulate postoperative refraction as well, since the overall refraction of the cornea can be modified by the curvature of the disc implanted beneath the flap. The concept of refractive correction using corneal inlays has been studied, with positive clinical results.23,24 Although the results are only preliminary, improving surgical technique may compensate for some of the disadvantages of using non‐biological materials for artificial corneas. The technique that we have described combining the use of a microkeratome with DLKP procedures offers a safe and reliable method to implant Krpos into the deep stroma. An intact Descemet's membrane will act as a barrier to prevent dislocation of the Kpro into the anterior chamber, and may also minimise interface opacification because of the formation of retroprosthetic membranes.
Abbreviations
α‐SMA - α‐smooth muscle actin
BSA - bovine serum albumin
COL‐PVA - collagen I immobilised poly(vinyl alcohol)
DLKPro - deep lamellar keratoprosthesis
DM - Descemet's membrane
DMSO - dimethyl sulfoxide
HE - haematoxylin and eosin
HMDI - hexamethylene dissocyantate
Kpro - keratoprosthesis
LKP - lamellar keratoplasty
LASIK - laser in situ keratomileusis
PBS - phosphate buffered saline
PRK - photorefractive keratectomy
PVA - poly(vinyl alcohol)
Footnotes
This study was supported by The Advanced and Innovational Research Program in Life Sciences from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
References
- 1.Chirila T V. An overview of the development of artificial corneas with porous skirts and the use of PHEMA for such an application. Biomaterials 2001223311–3317. [DOI] [PubMed] [Google Scholar]
- 2.Kompa S, Redbrake C, Langefeld S.et al The Type II Aachen‐Keratoprosthesis in humans: case report of the first prolonged application. Int J Artif Organs 200124110–114. [PubMed] [Google Scholar]
- 3.Kim M K, Lee J L, Wee W R.et al Comparative experiments for in vivo fibroplasia and biological stability of four porous polymers intended for use in the Seoul‐type keratoprosthesis. Br J Ophthalmol 200286809–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoiber J, Csaky D, Schedle A.et al Histopathologic findings in explanted osteo‐odontokeratoprosthesis. Cornea 200221400–404. [DOI] [PubMed] [Google Scholar]
- 5.Dudenhoefer E J, Nouri M, Gipson I K.et al Histopathology of explanted collar button keratoprostheses: a clinicopathologic correlation. Cornea 200322424–428. [DOI] [PubMed] [Google Scholar]
- 6.Hicks C R, Crawford G J, Lou X.et al Corneal replacement using a synthetic hydrogel cornea, AlphaCor: device, preliminary outcomes and complications. Eye 200317385–392. [DOI] [PubMed] [Google Scholar]
- 7.Doane M G, Dohlman C H, Bearse G. Fabrication of a keratoprosthesis. Cornea 199615179–184. [DOI] [PubMed] [Google Scholar]
- 8.Griffith M, Osborne R, Munger R.et al Functional human corneal equivalents constructed from cell lines. Science 19992862169–2172. [DOI] [PubMed] [Google Scholar]
- 9.Shimmura S, Doillon C J, Griffith M.et al Collagen‐poly(N‐isopropylacrylamide)‐based membranes for corneal stroma scaffolds. Cornea 200322S81–S88. [DOI] [PubMed] [Google Scholar]
- 10.Wilson S E, Mohan R R, Mohan R R.et al The corneal wound healing response: cytokine‐mediated interaction of the epithelium, stroma, and inflammatory cells. Prog Retin Eye Res 200120625–637. [DOI] [PubMed] [Google Scholar]
- 11.Park C K, Kim J H. Comparison of wound healing after photorefractive keratectomy and laser in situ keratomileusis in rabbits. J Cataract Refract Surg 199925842–850. [DOI] [PubMed] [Google Scholar]
- 12.Wachtlin J, Langenbeck K, Schrunder S.et al Immunohistology of corneal wound healing after photorefractive keratectomy and laser in situ keratomileusis. J Refract Surg 199915451–458. [DOI] [PubMed] [Google Scholar]
- 13.Vesaluoma M, Perez‐Santonja J, Petroll W M.et al Corneal stromal changes induced by myopic LASIK. Invest Ophthalmol Vis Sci 200041369–376. [PubMed] [Google Scholar]
- 14.Aquavella J V, Rao G N, Brown A C.et al Keratoprosthesis. Results, complications, and management. Ophthalmology 198289655–660. [PubMed] [Google Scholar]
- 15.Hicks C R, Hamilton S. Retroprosthetic membranes in AlphaCor patients: risk factors and prevention. Cornea 200524692–698. [DOI] [PubMed] [Google Scholar]
- 16.Melles G R, Lander F, Rietveld F J.et al A new surgical technique for deep stromal, anterior lamellar keratoplasty. Br J Ophthalmol 199983327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimmura S, Shimazaki J, Omoto M.et al Deep lamellar keratoplasty (DLKP) in keratoconus patients using viscoadaptive viscoelastics. Cornea 200524178–181. [DOI] [PubMed] [Google Scholar]
- 18.Busin M, Arffa R C, Sebastiani A. Endokeratoplasty as an alternative to penetrating keratoplasty for the surgical treatment of diseased endothelium: initial results. Ophthalmology 20001072077–2082. [DOI] [PubMed] [Google Scholar]
- 19.Azar D T, Jain S, Sambursky R.et al Microkeratome‐assisted posterior keratoplasty. J Cataract Refract Surg 200127353–356. [DOI] [PubMed] [Google Scholar]
- 20.Miyashita H, Shimmura S, Kobayashi H.et al Collagen‐immobilized poly (vinyl alcohol) as an artificial cornea scaffold that supports a stratified corneal epithelium. J Biomed Mater Res B Appl Biomater 200576B56–63. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi H, Ikada Y, Moritera T.et al Collagen‐immobilized hydrogel as a material for lamellar keratoplasty. J Appl Biomater 19912261–267. [DOI] [PubMed] [Google Scholar]
- 22.Latkany R, Tsuk A, Sheu M S.et al Plasma surface modification of artificial corneas for optimal epithelialization. J Biomed Mater Res 19973629–37. [DOI] [PubMed] [Google Scholar]
- 23.Choyce D P. The correction of high myopia. Refract Corneal Surg 19928242–245. [PubMed] [Google Scholar]
- 24.Werblin T P, Peiffer R L, Binder P S.et al Eight years experience with Permalens intracorneal lenses in nonhuman primates. Refract Corneal Surg 1992812–22. [PubMed] [Google Scholar]