Abstract
Aim
To examine the immunolocalisation of stromal cell derived factor 1 (SDF‐1) and its receptor CXCR4 in aged control human donor eyes and eyes with age related macular degeneration (AMD).
Methods
Postmortem eyes from eight aged control donors (mean age 79.8 years) and from 12 donors with AMD (mean age 83.9 years) were cryopreserved and sectioned through the macular region. SDF‐1 and CXCR4 were localised using streptavidin alkaline phosphatase immunohistochemistry and then sections were bleached. Three independent masked observers scored the immunohistochemical reaction product.
Results
In aged control retinas, SDF‐1 immunoreactivity was most intense in inner photoreceptor matrix (IPM). CXCR4 showed a similar pattern of immunostaining, but was more prominent in inner segments of photoreceptors. In aged control and AMD choroid, SDF‐1 and CXCR4 localisations were most prominent in retinal pigment epithelial (RPE) cells and choroidal stroma. However, the intensity for SDF‐1 was significantly reduced in RPE (p<0.0001) and choroidal stroma (p<0.05) in late AMD eyes. SDF‐1 and CXCR4 immunoreactivities were weak or nearly absent in disciform scars with choroidal neovascularisation (CNV). Circulating cells, presumably leucocytes, were most intensely positive for CXCR4.
Conclusions
These results show that changes in distribution and relative levels of SDF‐1/CXCR4 were not evident in early AMD. This suggests that SDF‐1/CXCR4 may not contribute to the formation of CNV in AMD, in that CXCR4+ cells were not incorporated into neovascularisation. However, the examples of CNV studied were within disciform scars, so the authors cannot comment on the role of SDF‐1/CXCR4 in the early stages of CNV formation.
Keywords: stromal cell derived factor, choroid, age related macular degeneration, choroidal neovascularisation
Angiogenesis in the form of choroidal neovascularisation (CNV) is an important pathobiological event encountered in a variety of chorioretinal diseases.1 CNV is a major cause of vision loss in patients with age related macular degeneration (AMD). The pathogenesis of CNV is clearly multifactorial, but generally considered to be driven by growth factors promoting angiogenesis. The historical paradigm was that the cellular components of the new vessel complex (endothelial cells, smooth muscle cells, and pericytes) were derived from cells resident within adjacent pre‐existing blood vessels—that is, by angiogenesis. However, recent studies in animal models suggest that circulating endothelial precursor cells (EPCs) and haematopoietic stem cells (HSCs) participate in this process and other pathophysiological angiogenesis, including retinal neovascularisation.2,3,4
Stromal cell derived factor 1 (SDF‐1) is a member of the CXC chemokine subfamily that was originally isolated from murine bone marrow stromal cells and functions as a chemoattractant for leucocytes.5 SDF‐1 is involved in HSC and EPC cell homing6,7 and recruitment of HSCs from the bone marrow to target tissue.8,9 Recently, it has been suggested that members of the CXC chemokine family can either promote or inhibit vasculogenesis/angiogenesis depending on the presence or absence of a structural functional domain ELF.10 SDF‐1 lacks an ELF domain and therefore should be antiangiogenic. However, SDF‐1 has been reported to be angiogenic in dermis11 yet have no effect in the corneal micropocket assay.10 SDF‐1 also acts as an angiogenic agent in several in vivo and in vitro model systems7,12 and may have a role in proliferative retinopathy by providing the directional cues necessary for EPCs to reach sites of ischaemia.13
CXCR4, a seven transmembrane spanning G protein coupled receptor, is the only known receptor for SDF‐1 and is expressed on various endothelial cells, HSCs, and EPCs.8,14,15 CXCR4 is also expressed on lymphocytes, monocytes, neutrophils, microglia, and ganglion cell precursors in retina.16,17 Its expression is increased after treatment with VEGF or bFGF.7 In brain tumours, CXCR4 is immunohistochemical expressed in regions of angiogenesis, degeneration, and necrotic changes.18 Mice deficient in either SDF‐1 or CXCR4 had defective formation of large vessels supplying the gastrointestinal tract, severely reduced B lymphopoiesis, reduced myelopoiesis in fetal liver, and a virtual absence of myelopoiesis in bone marrow.19,20
Considering the importance of the chemokine SDF‐1 and its receptor, CXCR4, in regulation of leucocyte trafficking, modulation of haematopoietic cell proliferation, adhesion to extracellular matrix molecules, and regulation of angiogenesis like CNV, we investigated SDF‐1 and CXCR4 expression in the retina and choroid of aged human control donor eyes and in eyes with AMD. To our knowledge, this is the first study describing the distribution of SDF‐1 and CXCR4 in the adult human eye.
Materials and methods
Donor eyes
Human donor eyes were obtained with the assistance of Janet Sunness, MD (Great Baltimore Medical Center, Baltimore, MD, USA) and Carol Applegate and the National Disease Research Interchange (NDRI; Philadelphia, PA, USA) within 11–36 hours after death. Eyes of the following donors were used in the study: 12 subjects with AMD (age range 61–105 years; mean age 83.9 years); eight aged control donors (age range 75–86 years; mean age 79.8 years) with no history of chorioretinal disease. All donors were white. Table 1 has the characteristics of each subject. The protocol of the study adhered to the tenets of the Declaration for Helsinki regarding research involving human tissue and was approved by the Johns Hopkins JCCI. The diagnosis of AMD was made by reviewing ocular medical history on the eye bank transmittal sheet and the postmortem gross examination of posterior eyecup, using transmitted and reflected illumination with a dissecting microscope (Stemi 2000; Carl Zeiss Meditec, Inc, Thornwood, NY, USA).
Table 1 Characteristics of human donor cryopreserved eyes.
| Case | Time (hours) | Age/race/sex | Primary cause of death | Medical history | Ocular diagnosis | |
|---|---|---|---|---|---|---|
| DET | PMT | |||||
| Aged | ||||||
| 1 | 2.5 | 33 | 75/W/F | Heart disease | Normal | |
| 2 | 7 | 27 | 76/W/F | Lung cancer | HTN, CNF | Normal |
| 3 | 1 | 26 | 77/W/M | COPD | HTN | Normal |
| 4 | 2.5 | 28 | 80/W/M | COPD | Normal | |
| 5 | 7.15 | 28 | 80/W/M | Intracranial haemorrhage | HTN, angioplasty | Normal |
| 6 | 3 | 15 | 82/W/M | Metastasis brain cancer | Normal | |
| 7 | 3 | 16 | 83/W/M | Cardiac respiratory arrest | Normal | |
| 8 | 5 | 26 | 86/W/F | Respiratory failure | Normal | |
| AMD | ||||||
| 9 | 3.5 | 34 | 61/W/M | Metastasis oesophageal cancer | AMD, early | |
| 10 | 3.5 | 42 | 69/W/F | Subarachnoid haemorrhage | Pulmonary fibrosis | AMD (GA), late |
| 11 | 4 | 33 | 74/W/M | Prostate cancer | AMD, early | |
| 12 | 7 | 30 | 75/W/M | Aspiration pneumonia | AMD (GA), late | |
| 13 | 3 | 33 | 79/W/M | Pneumonia | HTN, asthma | AMD, early |
| 14 | 5 | 29 | 81/W/F | Myocardial infarction | HTN | AMD, early |
| 15 | 3 | 12 | 83/W/M | Prostate cancer | DM, HTN | AMD, early |
| 16 | 4 | 20 | 93/W/F | Multisystem failure | DM, HTN | AMD (disciform scar), late |
| 17 | 3 | 36 | 94/W/M | Cardiac failure | AMD (disciform scar), late | |
| 18 | 3.5 | ? | 95/W/M | Cardiomyopathy | AMD (disciform scar), late | |
| 19 | 2 | 33 | 98/W/F | Old age | AMD, early | |
| 20 | 4.5–5 | 11 | 105/W/M | COPD | AMD (disciform scar, GA), late | |
DET, death to enucleation time; PMT, postmortem time (death to fixation); W, white; AMD, age related macular degeneration; DM, diabetes mellitus; HTN, hypertension; COPD, chronic obstructive pulmonary disease; GA, geographic atrophy.
Tissue preparation
After the anterior segment of the eye was removed, the posterior eye cup was fixed in 2% paraformaldehyde in 0.1 M sodium phosphate buffer (pH 7.4) with 5% sucrose at room temperature for 1 hour. The tissue was cut into coulottes of vitreous/retina/choroid complex and cryopreserved as previously described.21 Serial 8 μm sections were cut from the inferior macula.
Immunohistochemistry
Streptavidin alkaline phosphatase (APase) immunohistochemistry was performed on cryosections using a nitroblue tetrazolium (NBT) development system as previously described.22 After permeabilising with absolute methanol, sections were blocked with 2% normal goat or rabbit serum in TRIS buffered saline (TBS; pH 7.4 with 1% bovine serum albumin, BSA) and with an avidin‐biotin complex (ABC) blocking kit (Vector Laboratories, Inc, Burlingame, CA, USA). Sections were then incubated at 4°C overnight with 1:100 goat anti‐human SDF‐1 antibody (sc‐6193, Santa Cruz, CA, USA), 1:5000 rabbit anti‐human CXCR4 antibody (NLS1380, Novus Biologicals, Littleton, CO, USA), or mouse anti‐human CD‐34 (1:800; Signet Laboratory, Dedham, MA, USA) antibody in adjacent tissue section to label blood vessels. To demonstrate the validity of antibody binding, anti‐SDF‐1 antibody was pre‐incubated with a 200‐fold excess of peptide or human serum albumin overnight at 4°C before use. As a negative control, the primary antibodies were omitted and no staining was observed. The pigment was bleached from retinal pigment epithelium (RPE) and choroidal melanocytes as described previously.22 Finally, cover slips were mounted with Kaiser's glycerogel.
Three independent masked observers blindly graded the relative intensity of the immunoreactivity for both antibodies in retinal and choroidal structures, using a previously described eight point grading system.23
Statistical analysis
Mean score (SD) from the graders was calculated for each retinal and choroidal structure. The Kolmogorov and Smirnov method was used to determine the Gaussian distribution of these scores. The p values were determined by comparing mean scores from the aged control eyes with scores from eyes with AMD using the Student's t test and assuming unequal variance and two tails. The p value <0.05 was considered significant. Statistical analysis was done with InStat software (version 2.0, GraphPad Software, San Diego, CA, USA).
Results
Immunolocalisation of SDF‐1 and CXCR4 in aged control human retina/choroid
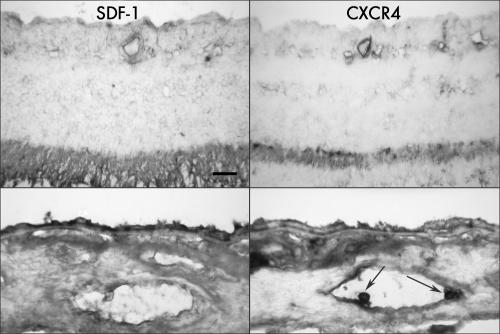
In retina, the immunostaining of SDF‐1 was most intense in inner photoreceptor matrix (IPM) and present to a lesser extent in internal limiting membrane (ILM) and large retinal blood vessels. CXCR4 showed a similar pattern of immunostaining, but was more prominent in inner segments of photoreceptors and associated with the endothelial cells of some retinal blood vessels (fig 1). There was no apparent difference in immunoreactivity level of either SDF‐1 and CXCR4 antibodies in ILM and large retinal blood vessels. Immunostaining of both antibodies was very weak or absent in the inner neural retina.
Figure 1 SDF‐1 and CXCR4 immunoreactivity in retina (top) and choroid (bottom) from an aged control eye (case 3). Intense SDF‐1 immunoreactivity is present in the inner photoreceptor matrix with significantly less present in some retinal vessels. CXCR4 has a similar pattern of immunostaining, but is more prominent in the inner segment area of photoreceptors and associated with the endothelial cells of blood vessels. In choroid, immunostaining of SDF‐1 is most prominent in retinal RPE cells and less intensively positive in choroidal blood vessels and choroidal stroma, where it appeared diffuse. CXCR4 is less prominent in RPE than SDF‐1 but comparable in choroid. Note the leucocytes within the choroidal blood vessel lumen are intensively labelled with CXCR4 (arrows). Pigment in the choroidal sections was bleached from RPE and choroidal melanocytes. Bar = 20 μm.
In choroid/RPE complex, immunostaining of SDF‐1 was most prominent in both apical and basal portions of RPE cells (fig 1). The immunostaining for both SDF‐1 and CXCR4 in large choroidal vessels, the choriocapillaris, and intercapillary septa was less intense and distinct and diffuse throughout choroidal stroma (fig 1). Immunoreactivity for SDF‐1 and CXCR4 was not uniform but rather heterogeneous. So the scores of immunoreactivity represent the graders' overall impression of the immunoreactivity in a particular structure throughout the whole tissue section. In some choroidal tissue sections, circulating cells, presumably leucocytes, within choroidal vascular lumens as well as in choroidal stroma were also intensely immunostained for CXCR4 (figs 1 and 2). SDF‐1 immunoreactivity was blocked by preincubation of the antibody with 200‐fold excess peptide used as antigen (data not shown).
Figure 2 Immunolocalisation of SDF‐1 and CXCR4 in retina (top) and choroid (bottom) in an early AMD eye (case 15). The pattern and intensity of immunostaining of both antibodies appeared identical to the aged control eye in figure 1, the CXCR4 immunoreactivity is more weakly labelled in RPE than the SDF‐1 immunoreactivity. Bar = 20 μm.
Immunolocalisation of SDF‐1 and CXCR4 in AMD retina/choroid
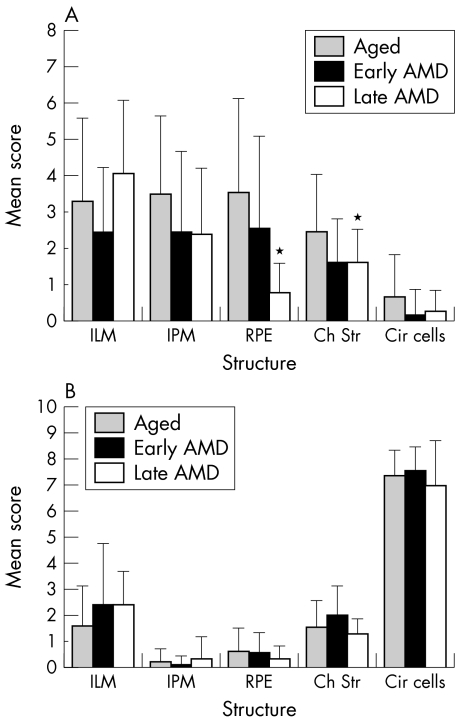
Retina and choroid of early AMD eyes had a pattern and intensity of SDF‐1 and CXCR4 staining similar to aged control eyes (fig 2). CXCR4 immunostaining appeared lower in RPE cells of early AMD eyes (fig 2) compared to aged control eyes. However, there was no significant difference in immunoreactivity scores for SDF‐1 and CXCR4 in retinal and choroidal structures between aged control and early AMD eyes (fig 3).
Figure 3 Mean immunoreactivity scores (SD) for retinal and choroidal structures of aged control (shaded), early AMD (solid), and late AMD (open) eyes. The immunoreactivity scores for SDF‐1 (A) were significantly decreased in RPE (p<0.0001; p<0.0075) and choroidal stroma (both p<0.05) in late AMD eyes compared with the aged control and early AMD eyes, respectively. There was no significant difference in the immunoreactivity scores for the CXCR4 in all the structures graded between AMD and aged control eyes (B).Ch str, choroidal stroma; Cir cells, circulating cells; ILM, internal limiting membrane; IPM, inner photoreceptor matrix; RPE, retinal pigment epithelium; *p<0.05.
Haematoxylin and eosin staining was used for the morphology of the choroid in aged control and AMD eyes. Basal laminar deposits (BLD) or drusen were negative for SDF‐1 and CXCR4 immunoreactivity (data not shown). The identity of BLDs was confirmed with periodic acid Schiff staining on serial sections.
In late AMD eyes, expression of SDF‐1 was variable in retina. As shown in figure 4 (case 18), intense immunoreactivity for SDF‐1 was associated with ILM, nerve fibre layer, and retinal blood vessels in some eyes, whereas CXCR4 immunoreactivity was weak in nerve fibre layer and retinal blood vessels. This was not a constant finding in late AMD eyes; only two late AMD eyes had intense SDF‐1 immunoreactivity in the nerve fibre layer. However, the immunoreactivity score for SDF‐1 was significantly lower in RPE cells (p<0.0001) and choroidal stroma (p<0.05) in late AMD eyes than in aged control eyes (fig 3). There was significantly lower SDF‐1 in RPE in late AMD eyes than in early AMD eyes (p = 0.0075). There was no significant difference in immunoreactivity scores for SDF‐1 in choriocapillaris, intercapillary septa, and large choroidal vessels in AMD eyes compared with aged control eyes. All mean immunoreactivity scores for the retinal and choroidal structures of the aged control versus AMD eyes are shown in figure 3.
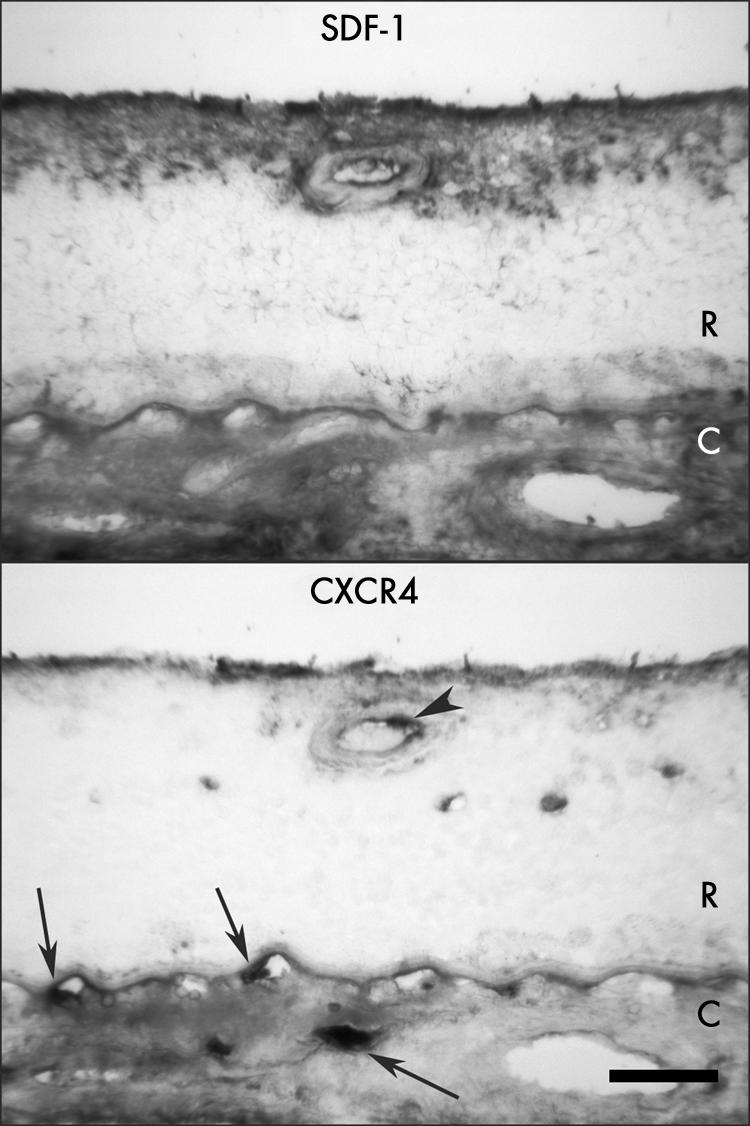
Figure 4 Immunolocalisation of SDF‐1 and CXCR4 in retina and choroid from a late AMD eye (case 18) shows intense SDF‐1 immunoreactivity associated with ILM, nerve fibre layer, and retinal blood vessels, whereas CXCR4 immunoreactivity is weakly labelled in nerve fibre layer and retinal blood vessels. Retinal endothelial cells and presumably an adherent leucocyte (arrowhead) are positive for CXCR4. In choroid, immunostaining of SDF‐1 and CXCR4 is very weak or negative in RPE, but the reaction product appeared more diffuse in choroidal stroma. Leucocytes are positive for CXCR4 (arrows). R, retina; C, choroid. Bar = 50 μm.
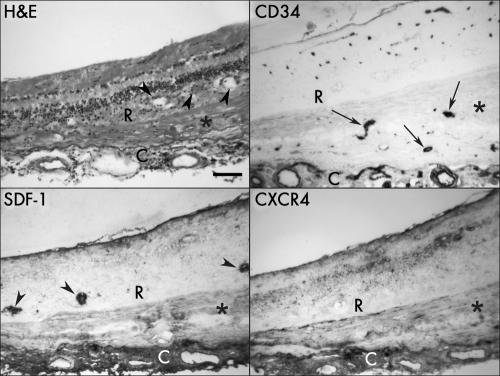
In late AMD eyes (cases 16–18 and 20), disciform scars were present and had small CNV formations. The specimen from a late AMD eye (case 16) having a disciform scar with small CNV formation within the scar showed no SDF‐1 and CXCR4 immunoreactivity in CNV, whereas both antibodies weakly labelled the disciform scar (fig 5). The immunostaining for both antibodies in choroidal vessels, including the choriocapillaris and choroidal stroma, was diffuse. Some pigmented cells in outer retina, perhaps “trans‐differentiated RPE cells,”24 were intensively labelled with SDF‐1, whereas no CXCR4 was associated with migrating cells (fig 5).
Figure 5 Sections of retina and choroid from late AMD eye (case 16) with a disciform scar and small CNV formation (CD34+, arrows) within the scar showing negative SDF‐1 and CXCR4 immunoreactivity associated with CNV, whereas both antibodies weakly label the disciform scar itself (asterisk). Note the apparent trans‐differentiated RPE cells in retina that are intensively labelled with SDF‐1 (arrowheads). The immunoreaction product from both antibodies appeared more diffuse in choroid. Haematoxylin and eosin (H&E) staining demonstrate the morphological changes in retina and choroid. R, retina; C, choroid. Bar = 100 μm.
Discussion
This study demonstrates for the first time the immunolocalisation of SDF‐1 and its receptor CXCR4 in normal aged human retina and choroid. The most prominent localisation of SDF‐1 was in RPE cells and IPM, while the most prominent localisation of CXCR4 was in cells within the lumen of blood vessels. The localisation of SDF‐1 and CXCR4 was similar in early AMD and aged control eyes, suggesting that SDF‐1/CXCR4 may not have a prominent role in the early stages of CNV formation in AMD.
The finding of SDF‐1 prominently localised in RPE was not surprising in that Crane et al found that RPE cells in culture can constitutively produce SDF‐1.25 Furthermore, they found by flow cytometry that RPE express CXCR4, which we also observed in RPE in tissue sections. The high levels of SDF‐1 in photoreceptor outer segments or IPM and CXCR4 in photoreceptor inner segments were unexpected. Photoreceptors are not considered to signal leucocyte or EPC homing and inner segments are not mobile so the rationale for CXCR4 in these structures is unclear. Perhaps RPE produced SDF‐1 diffuses and binds to matrix components of the IPM and choroidal stroma, another site where SDF‐1 and CXCR4 were prominent in choroid. Why exactly CXCR4 is associated with inner segments is unknown at this time unless there are soluble forms of CXCR4 that are shed and diffuse to bind to other proteins or matrix components.
Some reports have shown that resident RPE cells directly produced various angiogenic factors and thus contribute to the pathogenesis of AMD.26,27 Since SDF‐1 appears to be constitutively produced by RPE and is present in choroidal stroma of all subjects, perhaps an increase in circulating CXCR4+ cells during inflammatory processes results in accumulation of these cells in RPE/Bruch's membrane (BM)/choriocapillaris (CC) complex and further activation of them occurs because RPE produce cytokines. These ocular infiltrated inflammatory cells may then produce angiogenic factors that have a role in formation of CNV. Our recent immunohistochemical study of VEGF demonstrated that leucocytes had the greatest levels of vascular endothelial growth factor (VEGF) in RPE/BM/CC complex.28 Several laboratories have suggested the importance of inflammation and recruitment of inflammatory cells in the initial phase of CNV.29,30
Recruitment of endothelial precursor cells to sites of injury is a well established phenomenon in several different animal models of angiogenesis and vascular repair.31,32 This has been clearly demonstrated in experimental retinal and choroidal neovascularisation.2,3,4,33 The study by Sengupta et al suggests that SDF‐1 is the attractant for these precursors.34
In human AMD, the cellular composition of CNV has not been well defined. Clearly, endothelial cells are essential in the formation of the lumens of perfused new capillaries. However, CNV are fibrovascular lesions that also consist of vascular smooth muscle cells, poorly differentiated myofibroblastoid cells, proliferating RPE cells, and inflammatory cells.35,36,37 These cells are typical of a wound healing response. Although the laser induced CNV is not identical to CNV in AMD, this model shares enough biological similarity that preclinical studies of pathogenesis and drug efficacy are said to predict human response. Like human AMD, it is a wound healing event in some ways. Monocytes38 and neutrophils39 are recruited to the site of laser injury and preventing this infiltration lessens the angiogenic response. Like EPCs, both of these cell types can express CXCR4,40,41 so recruitment to laser induced CNV may involve SDF‐1. Blocking SDF‐1 prevents EPCs from homing to laser induced CNV.34 Therefore, it is surprising that we did not observe a significant increase in SDF‐1 in early or advanced AMD or human CNV. However, a shortcoming of our study is the lack of early CNV in our specimens. All of our CNV formations were within disciform scars and, therefore, were end stage.
This study suggests that the chemokine SDF‐1 and its receptor, CXCR4, are not related to early AMD and, therefore, may not contribute to the formation of CNV in AMD. However, the examples of CNV studied were within disciform scars, so we cannot comment on SDF‐1/CXCR4 in developing CNV. Furthermore, the data suggest that SDF‐1 levels may be less in advanced AMD than in normal aged choroidal stroma and RPE. This suggestion is probably the result of the decrease in RPE cell number and presence in advanced AMD and the significant thinning of choroidal stroma with age. This study presents the interesting scenario of constitutive production of a potent chemokine for homing of leucocytes and EPCs. Why this does not result in accumulation of CXCR4+ cells in RPE/BM/CC normally remains to be elaborated.
Acknowledgements
This work was supported by NIH grant EY‐01765 (Wilmer), the RPB (Research to Prevent Blindness, Wilmer), and the Foundation Fighting Blindness (GL). The authors are grateful to the eye donors and their relatives for their generosity and Janet Sunness, MD (Great Baltimore Medical Center, Baltimore, MD) and Carol Applegate for their assistance in acquiring human donor eyes.
Abbreviations
ABC - avidin‐biotin complex
AMD - age related macular degeneration
BLD - basal laminar deposits
BM - Bruch's membrane
BSA - bovine serum albumin
CC - choriocapillaris
CNV - choroidal neovascularisation
COPD - chronic obstructive pulmonary disease
DET - death to enucleation time
DM - diabetes mellitus
EPCs - endothelial precursor cells
GA - geographic atrophy
HSCs - haematopoitic stem cells
HTN - hypertension
ILM - internal limiting membrane
IPM - inner photoreceptor matrix
NBT - nitroblue tetrazolium
PMT - postmortem time
RPE - retinal pigment epithelium
SDF‐1 - stromal cell derived factor 1
TBS - TRIS buffered saline
VEGF - vascular endothelial growth factor
Footnotes
Competing interests: There are no competing interests.
References
- 1.Green W R, Wilson D J. Choroidal neovascularization. Ophthalmology 1986931169–1176. [DOI] [PubMed] [Google Scholar]
- 2.Espinosa‐Heidmann D G, Caicedo A, Hernandez E P.et al Bone marrow‐derived progenitor cells contribute to experimental choroidal neovascularization. Invest Ophthalmol Vis Sci 2003444914–4919. [DOI] [PubMed] [Google Scholar]
- 3.Grant M B, May W S, Caballero S.et al Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med 20028607–612. [DOI] [PubMed] [Google Scholar]
- 4.Sengupta N, Caballero S, Mames R N.et al The role of adult bone marrow‐derived stem cells in choroidal neovascularization. Invest Ophthalmol Vis Sci 2003444908–4913. [DOI] [PubMed] [Google Scholar]
- 5.Shirozu M, Nakano T, Inazawa J.et al Structure and chromosomal localization of the human stromal cell‐derived factor 1 (SDF1) gene. Genomics 199528495–500. [DOI] [PubMed] [Google Scholar]
- 6.Mohle R, Bautz F, Rafii S.et al The chemokine receptor CXCR‐4 is expressed on CD34+ hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell‐derived factor‐1. Blood 1998914523–4530. [PubMed] [Google Scholar]
- 7.Salcedo R, Oppenheim J J. Role of chemokines in angiogenesis: CXCL12/SDF‐1 and CXCR4 interaction, a key regulator of endothelial cell responses. Microcirculation 200310359–370. [DOI] [PubMed] [Google Scholar]
- 8.Gupta S K, Lysko P G, Pillarisetti K.et al Chemokine receptors in human endothelial cells. Functional expression of CXCR4 and its transcriptional regulation by inflammatory cytokines. J Biol Chem 19982734282–4287. [DOI] [PubMed] [Google Scholar]
- 9.Peled A, Grabovsky V, Habler L.et al The chemokine SDF‐1 stimulates integrin‐mediated arrest of CD34(+) cells on vascular endothelium under shear flow. J Clin Invest 19991041199–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arenberg D A, Polverini P J, Kunkel S L.et al In vitro and in vivo systems to assess role of C–X–C chemokines in regulation of angiogenesis. Methods Enzymol 1997288190–220. [DOI] [PubMed] [Google Scholar]
- 11.Salcedo R, Wasserman K, Young H A.et al Vascular endothelial growth factor and basic fibroblast growth factor induce expression of CXCR4 on human endothelial cells: In vivo neovascularization induced by stromal‐derived factor‐1alpha. Am J Pathol 19991541125–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirshahi F, Pourtau J, Li H.et al SDF‐1 activity on microvascular endothelial cells: consequences on angiogenesis in in vitro and in vivo models. Thromb Res 200099587–594. [DOI] [PubMed] [Google Scholar]
- 13.Butler J M, Guthrie S M, Koc M.et al SDF‐1 is both necessary and sufficient to promote proliferative retinopathy. J Clin Invest 200511586–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feil C, Augustin H G. Endothelial cells differentially express functional CXC‐chemokine receptor‐4 (CXCR‐4/fusin) under the control of autocrine activity and exogenous cytokines. Biochem Biophys Res Commun 199824738–45. [DOI] [PubMed] [Google Scholar]
- 15.Volin M V, Joseph L, Shockley M S.et al Chemokine receptor CXCR4 expression in endothelium. Biochem Biophys Res Commun 199824246–53. [DOI] [PubMed] [Google Scholar]
- 16.Chalasani S H, Baribaud F, Coughlan C M.et al The chemokine stromal cell‐derived factor‐1 promotes the survival of embryonic retinal ganglion cells. J Neurosci 2003234601–4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forster R, Kremmer E, Schubel A.et al Intracellular and surface expression of the HIV‐1 coreceptor CXCR4/fusin on various leukocyte subsets: rapid internalization and recycling upon activation. J Immunol 19981601522–1531. [PubMed] [Google Scholar]
- 18.Rempel S A, Dudas S, Ge S.et al Identification and localization of the cytokine SDF1 and its receptor, CXC chemokine receptor 4, to regions of necrosis and angiogenesis in human glioblastoma. Clin Cancer Res 20006102–111. [PubMed] [Google Scholar]
- 19.Ma Q, Jones D, Borghesani P R.et al Impaired B‐lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4‐ and SDF‐1‐deficient mice. Proc Natl Acad Sci USA 1998959448–9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tachibana K, Hirota S, Iizasa H.et al The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature 1998393591–594. [DOI] [PubMed] [Google Scholar]
- 21.Lutty G A, Merges C, Threlkeld A B.et al Heterogeneity in localization of isoforms of TGF‐β in human retina, vitreous, and choroid. Invest Ophthalmol Vis Sci 199334477–487. [PubMed] [Google Scholar]
- 22.Bhutto I A, Kim S Y, McLeod D S.et al Retinal and choroidal localization of collagen XVIII and the endostatin portion of collagen XVIII in aged human control and in age‐related macular degeneration subjects. Invest Ophthalmol Vis Sci 2004451544–1552. [DOI] [PubMed] [Google Scholar]
- 23.McLeod D S, Lefer D J, Merges C.et al Enhanced expression of intracellular adhesion molecule‐1 and P‐selectin in the diabetic human retina and choroid. Am J Pathol 1995147642–653. [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Stone J. Role of astrocytes in the control of developing retinal vessels. Invest Ophthalmol Vis Sci 1997381653–1666. [PubMed] [Google Scholar]
- 25.Crane I J, Wallace C A, McKillop‐Smith S.et al CXCR4 receptor expression on human retinal pigment epithelial cells from the blood‐retina barrier leads to chemokine secretion and migration in response to stromal cell‐derived factor 1 alpha. J Immunol 20001654372–4378. [DOI] [PubMed] [Google Scholar]
- 26.Grossniklaus H E, Ling J X, Wallace T M.et al Macrophage and retinal pigment epithelium expression of angiogenic cytokines in choroidal neovascularization. Mol Vis 20028119–126. [PubMed] [Google Scholar]
- 27.Yu M J, Shen W Y, Lai M C.et al The role of vascular endothelial growth factor (VEGF) in abnormal vascular changes in the adult rat eye. Growth Factors 200017301–312. [DOI] [PubMed] [Google Scholar]
- 28.Bhutto I A, McLeod D S, Hasegawa T.et al Pigment epithelial‐derived factor (PEDF) and vascular endothelial growth factor (VEGF) in aged choroid and eyes with age‐related macular degeneration. Exp Eye Res 20068397–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cousins S, Csaky K. Immunology of age‐related macular degeneration. In: Lim J, ed. Age‐related macular degeneration. New York: Marcel Dekker, 200227–65.
- 30.Hageman G, Luthert P, Chong N.et al An integrated hypothesis that considers drusen as biomarkers of immune‐mediated processes at the RPE‐Bruch's membrane interface in aging and age‐related macular degeneration. Prog Ret Eye Res 200120705–732. [DOI] [PubMed] [Google Scholar]
- 31.Asahara T, Masuda H, Takahashi T.et al Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 199985221–228. [DOI] [PubMed] [Google Scholar]
- 32.Bailey A S, Jiang S, Afentoulis M.et al Transplanted adult hematopoietic stems cells differentiate into functional endothelial cells. Blood 200410313–19. [DOI] [PubMed] [Google Scholar]
- 33.Grant M B, Caballero S, Brown G A.et al The contribution of adult hematopoietic stem cells to retinal neovascularization. Adv Exp Med Biol 200352237–45. [DOI] [PubMed] [Google Scholar]
- 34.Sengupta N, Caballero S, Mames R N.et al Preventing stem cell incorporation into choroidal neovascularization by targeting homing and attachment factors. Invest Ophthalmol Vis Sci 200546343–348. [DOI] [PubMed] [Google Scholar]
- 35.Green W R. Histopathology of age‐related macular degeneration. Mol Vis 1999527. [PubMed] [Google Scholar]
- 36.Lopez P F, Sippy B D, Lambert H M.et al Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age‐related macular degeneration‐related choroidal neovascular membranes. Invest Ophthalmol Vis Sci 199637855–868. [PubMed] [Google Scholar]
- 37.Sarks S H, Sarks J P. Age‐related maculopathy: nonneovascular age‐related macular degeneration and the evolution of geographic atrophy. In: Schachat AP, ed. Retina. 3rd ed. Philadelphia: Mosby, 20011064–1099.
- 38.Sakurai E, Anand A, Ambati B K.et al Macrophage depletion inhibits experimental choroidal neovascularization. Invest Ophthalmol Vis Sci 2003443578–3585. [DOI] [PubMed] [Google Scholar]
- 39.Zhou J, Pham L, Zhang N.et al Neutrophils promote experimental choroidal neovascularization. Mol Vis 200511414–424. [PubMed] [Google Scholar]
- 40.Link D C. Neutrophil homeostasis: a new role for stromal cell‐derived factor‐1. Immunol Res 200532169–178. [DOI] [PubMed] [Google Scholar]
- 41.Nagase H, Miyamasu M, Yamaguchi M.et al Cytokine‐mediated regulation of CXCR4 expression in human neutrophils. J Leukoc Biol 200271711–717. [PubMed] [Google Scholar]