Abstract
Aim
To determine if recombinant tissue plasminogen activator (rtPA) injected into the vitreous cavity can penetrate the retinal vessels of porcine eyes with or without vascular occlusion.
Methods
Eight eyes (group I) of four pigs underwent clamping of the optic nerve flush with the globe for 90 minutes. One hour after reperfusion, one eye of each pig was injected with 75 μg of rtPA, and the fellow eye was injected with balanced salt solution (BSS). Eyes were processed for immunohistochemistry. Four additional eyes (group II) of two pigs were subjected to the same injections, but without optic nerve clamping.
Results
After reperfusion, the clinical picture was similar to that of a central retinal vein occlusion. Immunoperoxidase staining showed rtPA only in the retinal veins but not the retinal arteries in all eyes injected with rtPA in both groups I and II. Those eyes also showed intense rtPA staining at the level of the internal limiting membrane (ILM). No staining was seen at the level of the ILM or inside the retinal vessels in the BSS injected eyes. Immunofluorescence staining showed intense staining at the level of the ILM, but not inside the retinal vessels in the rtPA‐injected eyes.
Conclusions
rtPA may penetrate the retinal veins, but not the arteries of porcine eyes with and without vascular occlusion. The ILM may play a part in preventing rtPA penetration.
Keywords: animal model, internal limiting membrane, intravitreous injection, retinal vein occlusion, tissue plasminogen activator
Retinal vein occlusion is the second most common retinal vascular cause of visual loss after diabetic retinopathy.1 Venous obstruction elevates the retinal venous pressure leading to scattered intra‐retinal haemorrhage and macular oedema with decreased visual acuity. Associated capillary closure and subsequent retinal ischaemia can lead to intraocular neovascularisation in some eyes, which can cause further visual loss from vitreous haemorrhage or neovascular glaucoma.
Since histopathological studies have demonstrated a thrombus at the level of the lamina cribrosa in eyes with central retinal vein occlusion (CRVO),2 thrombolytic therapy has been attempted. Systemic fibrinolytic therapy can improve experimental retinal vein occlusion in rabbits.3 Several studies using intravenous recombinant tissue plasminogen activator (rtPA) and intravenous heparin have shown an improvement in visual acuity.4,5,6 However, systemic fibrinolytic therapy can have significant risks, including stroke, bleeding that requires transfusion, and even death.4,7 More localised, targeted thrombolytic treatment can improve retinal venous outflow after injection in the ophthalmic artery.8 However, this technique is associated with systemic complications. Cannulation of the retinal veins and injection of rtPA is in progress but still not without complications.9,10,11
Lahey and associates12 have reported a pilot study of intravitreal rtPA for CRVO in which 70% of patients had stable or improved vision by 6 weeks. They hypothesised that intravitreal rtPA diffuses across the retinal internal limiting membrane (ILM) and into damaged retinal capillaries, but this has not been proved. Kamei and associates have shown that rtPA does not cross a normal rabbit retina.13 However, intravitreous injection of tPA has been shown to facilitate clot lysis in a rabbit model of subretinal haemorrhage.14 Whether intravitreal rtPA is able to enter a retinal vein, with or without ischaemic injury, is unknown.
The vascular supply of the porcine retina is very similar to the human and has four major retinal arteries and veins and a capillary network in multiple layers.15 A porcine model was chosen for our study as this species has holangiotic retinal vascular pattern, whereas the rabbit eye is merangiotic, and much of the retina is avascular.
Our objective was to develop a porcine model of retinal vascular occlusion and to determine if rtPA injected into the vitreous cavity could be detected in the retinal vessels of the porcine eye with and without occlusion.
Materials and methods
All procedures were performed according to the Association of Research in Vision and Ophthalmology guidelines on use of animals in research, and the animal use protocol was approved by the institutional animal care and use committee at North Carolina State University.
Anaesthesia and pupillary dilation
Six Yorkshire cross pigs (12 eyes), aged 10 weeks and weighing 15 kg were used in the study. The animals were premedicated with intramuscular ketamine (11 mg/kg) and xylazine (2 mg/kg), and maintained on isoflurane 1–5% administered through an endotracheal tube. Mydriasis was achieved with a combination of topical tropicamide 1% and phenylephrine 2.5%.
Monitored endotracheal anaesthesia
The heart rate ranged between 80 and 100 beats per minute, with a mean arterial blood pressure of 80 mm Hg. The mean respiratory rate was 30 breaths per minute, and the oxygen saturation was maintained above 96%. Lactated Ringer's solution was used intravenously at a rate of 180–270 ml/h with a maximum volume of 900 ml per pig to maintain mean arterial blood pressure. No intraoperative vasopressors were used.
Vascular occlusion methods
Following a deep surgical plane of anaesthesia in four pigs, a temporal peritomy was performed and the lateral rectus insertion was excised. The posterior pole of the eye was exposed with blunt dissection through periocular fascia and a right angled long forceps was used to clamp the optic nerve flush with the globe. The clamping included the ciliary vessels from which originate the central retinal arcade at the optic nerve head.15 The clamp was released at 90 minutes.
Intravitreal rtPA administration
Group I
Eight eyes of four pigs underwent vascular occlusion according to the protocol described above. One hour after release of the clamp and reperfusion, one eye of each pig (n = 4) was injected in a standard sterile fashion with 75 μg (0.075 ml) of human recombinant tPA (0.1 mg/0.1 ml; Genentech, San Francisco, CA, USA) using a 30 gauge needle, and the fellow eye (n = 4) was injected with the same volume of balanced salt solution (BSS).
Group II
Four additional eyes of two pigs received the same intravitreal injections as eyes in group I but without previous vascular occlusion by clamping. Two of these four eyes were injected with rtPA and the fellow eye was injected with an equal volume of BSS. It is to be noted that eyes in group II also underwent a temporal peritomy and the lateral rectus insertion was excised. The posterior pole of the eye was exposed with blunt dissection through periocular fascia, but no vascular occlusion was attempted.
Injections in both groups were masked so that the physician injecting did not know about the contents of the syringes.
Enucleation
With the animals still under a deep plane of general anaesthesia, eyes were enucleated 2 hours after injection. Animals were euthanised using an intravenous injection of pentothal sodium after enucleation was complete.
Snap freeze technique
Preliminary studies performed in our laboratory suggested that processing of tissues with standard tissue fixatives including formaldehyde and glutaraldehyde interfered with immunohistochemical detection techniques for rtPA. We therefore devised a frozen section technique, in which the ocular tissues were snap frozen, that was associated with good immunohistochemical staining.
The globe was sectioned by making two peripheral posterior to anterior horizontal cuts and discarding the two calottes (side pieces). The remaining tissue included the central optic nerve vasculature and most of the posterior segment. The anterior segment was removed and discarded. The posterior eyecup was put in Tissue‐Tek OCT Compound (Sakura Finetek, USA, Torrance, CA, USA) in a mould. The mould was placed on an aluminium tray cooled in the vapour phase of liquid nitrogen in a Styrofoam container. The frozen specimens were wrapped in aluminium foil, transferred to dry ice, and subsequently transferred and stored in a –80°C freezer until tissue processing.
Protocol for frozen section immunohistochemistry
Frozen sections were cut at 5 μm from all tissue samples and were then air dried for 30 minutes. The sections were then fixed in 4% paraformaldehyde for 30 minutes at room temperature. The sections were then washed in phosphate buffered saline (PBS) for 3 minutes, three times each. Background Buster (Innovex Biosciences, Richmond, CA, USA) was applied to sections for 30 minutes at room temperature. The tissues were again washed in PBS as above, incubated in 3% hydrogen peroxide in PBS for 30 minutes at room temperature, and again washed in PBS. The tissue sections were then incubated in a 1:100 dilution of primary antibody (mouse IgG1 monoclonal antibody to recombinant human tissue plasminogen activator, American Diagnostica, Greenwich, CT, USA, product number ESP1) overnight for 12 hours at 4°C. The primary antibody that we employed is reported by the company to be specific for recombinant human tPA and not cross react with porcine tPA. The sections were then washed in PBS. Detection was with a stat‐Q kit (Innovex Biosciences, Richmond, CA, USA) using a secondary linking antibody applied for 10 minutes at room temperature, washed in PBS, and peroxidase‐strepavidin label applied for 10 minutes at room temperature. Sections were then washed in PBS and developed with Vector Red Substrate Kit (Vector Laboratories, Burlingame, CA, USA) for 3 minutes at room temperature. Levamisole solution (Vector Laboratories) was added to the Vector Red substrate solution to inhibit endogenous alkaline phosphatase. The slides were then washed in tap water for 10 minutes, counterstained with Harris haematoxylin (Fisher Scientific, Pittsburgh, PA, USA) for 15 seconds, and then washed again with tap water for 5 minutes. The sections were dehydrated through graded and absolute alcohol, followed by xylene, and then cover slipped with permanent mounting medium.
Negative controls, to detect non‐specific binding of the primary antibody and/or non‐specific peroxidase‐strepavidin labelling, were run in parallel in the same fashion, except that Mouse Monoclonal IgG Isotype Control (Innovex Biosciences) was applied in place of the primary antibody. Immunofluorescent staining used an indirect procedure with similar steps except that fluorescein anti‐mouse IgG (Vector Laboratories) was used for detecting primary antibody bound to injected rtPA.
Results
Clinical findings
One minute after optic nerve clamping, the retinal arteries became attenuated, followed by segmentation of the blood column within the retinal veins (fig 1B, C). After reperfusion, immediate dilation and tortuosity of the retinal veins developed followed by disc oedema (fig 1D, E, F). Dot‐blot (fig 1D), flame‐shaped (fig 1D), and preretinal haemorrhages (fig 1E) in all four quadrants developed within 2 minutes. The ophthalmoscopic picture was characteristic of a human central retinal vein occlusion (fig 1F).
Figure 1 The porcine eye and evolution of an experimental central retinal vein occlusion. (A) Normal porcine retina. Note the lack of disc oedema. (B) Ischaemic phase. Arrowhead points to attenuated vascular segment in the posterior pole, and in the periphery in (C). (D) Reperfusion phase. Dot‐blot (arrow) and flame‐shaped (arrowhead) haemorrhages. Note the oedematous optic nerve head. (E) Preretinal haemorrhage in the reperfusion phase. (F) Central retinal vein occlusion, full blown with four quadrant haemorrhage and disc oedema.
Histopathological findings
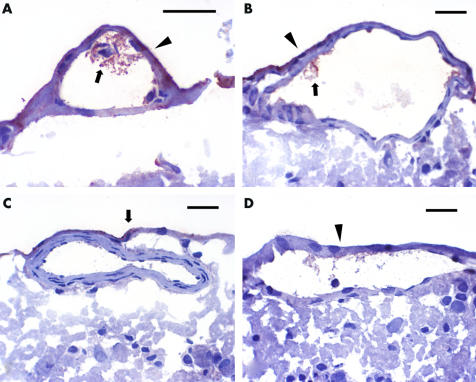
Histopathological examination of eyes in group I (occlusion) revealed intraretinal oedema and haemorrhages. Recombinant tPA was detected by immunoperoxidase in the retinal veins (fig 2A), but not arteries (fig 2C), in all eyes injected with tPA in both group I and group II. Staining within the lumen of the retinal veins was more marked in group I (occlusion) eyes (fig 2A) than group II (no occlusion) eyes (fig 2B). No labelling was detected inside the retinal vessels of eyes injected with BSS in group I, or group II (fig 2D). Diffuse labelling of the retinal internal limiting membrane (ILM) was detected in all eyes injected with rtPA (fig 2A,B, and C) but not in the eyes injected with BSS (fig 2D).
Figure 2 (A) Recombinant tissue plasminogen activator (reddish brown colour) is present inside a retinal vein (arrow) 2 hours after injection of 75 μg intravitreal rtPA in a group I porcine eye, after ischaemia reperfusion injury. Note the staining (reddish brown) of the internal limiting membrane (arrowhead). (B) Recombinant tissue plasminogen activator is present inside a retinal vein in a group II porcine eye without the ischaemic reperfusion injury, showing intravascular availability (arrow) after injection of 75 μg of intravitreal rtPA. Note the staining of the internal limiting membrane (arrowhead). (C) No rtPA is present inside a retinal artery in this group I porcine eye 2 hours after injection of 75 μg of intravitreal rtPA. Note the staining of the internal limiting membrane (arrow). (D) No staining inside the retinal vein after injection of intravitreal balanced salt solution. Note no staining of the internal limiting membrane (arrowhead). Scale = 50 μm. Immunohistochemistry using a monoclonal anti‐tPA antibody and avidin‐biotin conjugate detection method. The primary antibody is a murine monoclonal antibody that binds with human rtPA (ESP‐1). The secondary link‐horse radish peroxidase is used with both mouse and rabbit antibodies. The tertiary antibody is a peroxidase strepavidin label.
Indirect immunofluorescent technique did not show any staining within the retinal vessels of rtPA injected eyes in group I and II, but disclosed diffuse intense staining of the ILM in these eyes. No ILM staining was seen in the BSS injected eyes.
Discussion
Recombinant tissue plasminogen activator injected into the vitreous cavity of porcine eyes was detected in the retinal veins using the immunoperoxidase technique. Our findings suggest that rtPA reached the lumen of the veins in greater quantity in eyes with the occlusion (group I) (fig 2A) compared to eyes without an occlusion (group II) (fig 2B). This may have been expected because of the breakdown of the blood‐retinal barrier after vascular occlusion.
Owing to variable background staining in the immunoperoxidase stained negative control sections, we also performed indirect immunofluorescence, which did not reveal any staining inside the retinal veins of rtPA injected eyes but showed intense staining at the level of the internal limiting membrane (ILM). The cause for the different results using immunoperoxidase (IP) and immunofluorescence (IF) staining may reflect the possible lower avidity of the secondary antibody used in the IF method compared to the IP method. However, it is important to emphasise that with both methods, there was an intense staining at the level of the ILM, only in the rtPA injected eyes. This highlights the important role of the ILM as a barrier to rtPA penetration.
The rtPA dose of 75 μg was chosen for this study after review of different doses used successfully in humans without side effects.12,16 Toxicity studies in cat17 and rabbit18,19 eyes suggested rtPA was toxic at intravitreal doses above 50 μg. However, the vitreous volume of the pig is much larger than in cats and rabbits and almost equal to the human volume, suggesting that an intravitreal dose above 50 μg would be safe in the pig. In fact, subretinal rtPA in a dose less than 200 mg/l was non‐toxic in cats,20 and our selected intravitreal dose of 75 μg is equivalent to 187.5 mg/l of human vitreous and is therefore considered by us to be non‐toxic. The presence of retinal pigment epithelial (RPE) hyperplasia may indicate rtPA toxicity.17 We did not observe RPE hyperplasia in eyes injected with rtPA or BSS. However, our study was not designed to answer the toxicity profile of rtPA because of the very short duration of rtPA in the vitreous cavity before enucleation. It is to be noted that an intravitreous dose of 50 µg of rtPA with gas tamponade injected twice 3 days apart was found to be associated with RPE changes and reduced electroretinogram amplitudes in a case report.21
Given our study design, we cannot comment on the clinical efficacy of rtPA in eyes with retinal vein occlusion because the eyes were enucleated 2 hours after intravitreous injection. Recombinant tPA was not detected in the retinal arteries in our study, even in the occlusion eyes (group I). This may be due to the thicker arterial walls and perhaps less disruption of the blood‐retinal barrier in the porcine model of vascular occlusion in arteries versus veins. This finding suggests that intravitreal rtPA may be less beneficial to eyes with retinal artery occlusion compared to eyes with CRVO. A recent study in rabbit eyes has demonstrated amelioration of the clinical picture of vein occlusion after 50 μg of intravitreous rtPA, but did not demonstrate any improvement in blood flow assessed by fluorescein angiography.22
It has been postulated that rtPA injected within 21 days of acute CRVO may have a beneficial effect in some patients.12,23 If injected after more than 3 weeks of CRVO, tPA would not be effective as a result of fibrin cross linking occurring 21 days after thrombus formation.24 Some studies showed that intravitreous rtPA was beneficial in non‐ischaemic25 and ischaemic26 CRVO, if injected earlier in the course of the disease. Indeed, rtPA injected within 3 days of acute CRVO demonstrated a great effect on the course of the disease.27 Early injection of rtPA in the course of CRVO may reach the retinal veins in greater amount when the blood‐retinal barrier is maximally disrupted and exert an effective dissolution on the fresh thrombus. The effective time period for the maximum action of tPA from the beginning of the symptoms to the intravitreal injection is still to be determined. This would mainly depend on the speed of remodelling of the venous walls after an occlusion and the length of time the fresh thrombus is susceptible to rtPA. Intravenous tPA is an established treatment for acute thrombotic strokes, within 3 hours of presentation.28 In one study, only 15% of the patients presented within the 3 hour time period when tPA would be of crucial importance and would lead to a better prognosis.29 Although 15% may not be as high as one would desire, the effect of tPA on the better quality of life of these patients is of great significance. Some studies have also demonstrated that intravenous injection of tPA in patients with acute thrombotic stroke is useful within the first 6 hours, increasing the window of action of thrombolysis.28,30 A growing awareness in the ophthalmic and medical community of the possible effect that intravitreal tPA may have on prognosis, although not without complications, may allow expedient referral, at least for the trial of early injection of tPA in the vitreous cavity in CRVO, to determine the effect on prognosis and compare it to the natural history of the disease.
In conclusion, our results suggest that rtPA injected into the vitreous cavity may penetrate into retinal veins but not arteries in this non‐vitrectomised porcine model with and without vascular occlusion. The internal limiting membrane has a role as a barrier to rtPA diffusion into the retinal veins.
Abbreviations
BSS - balanced salt solution
CRVO - central retinal vein occlusion
ILM - internal limiting membrane
IF - immunofluorescence
IP - immunoperoxidase
PBS - phosphate buffered saline
RPE - retinal pigment epithelial
rtPA - recombinant tissue plasminogen activator
Footnotes
Funding support: Supported in part by Research to Prevent Blindness through the Duke University Eye Center small grant committee. The funding source has no involvement in the design, results, or interpretation of the data.
Competing interests: none.
Presented in part as a poster on 8 May 2002 at the Association of Research in Vision and Ophthalmology meeting in Fort Lauderdale, FL, USA.
References
- 1.Clarkson J. Central retinal vein occlusion. In: Ryan S, ed. Retina. St Louis: Mosby, 19941379
- 2.Green W R, Chan C C, Hutchins G M.et al Central retinal vein occlusion: a prospective histopathologic study of 29 eyes in 28 cases. Trans Am Ophthalmol Soc 198179371–422. [PMC free article] [PubMed] [Google Scholar]
- 3.Oncel M, Peyman G A, Khoobehi B. Tissue plasminogen activator in the treatment of experimental retinal vein occlusion. Retina 198991–7. [DOI] [PubMed] [Google Scholar]
- 4.Elman M J. Thrombolytic therapy for central retinal vein occlusion: results of a pilot study. Trans Am Ophthalmol Soc 199694471–504. [PMC free article] [PubMed] [Google Scholar]
- 5.Hattenbach L O, Steinkamp G, Scharrer I.et al Fibrinolytic therapy with low‐dose recombinant tissue plasminogen activator in retinal vein occlusion. Ophthalmologica 1998212394–398. [DOI] [PubMed] [Google Scholar]
- 6.Hattenbach L O, Wellermann G, Steinkamp G W.et al Visual outcome after treatment with low‐dose recombinant tissue plasminogen activator or hemodilution in ischemic central retinal vein occlusion. Ophthalmologica 1999213360–366. [DOI] [PubMed] [Google Scholar]
- 7.The GUSTO investigators An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993329673–682. [DOI] [PubMed] [Google Scholar]
- 8.Vallee J N, Aymard A, Paques M.et al Value of local ophthalmic artery fibrinolysis in severe forms of central retinal vein occlusion. J Radiol 200182137–144. [PubMed] [Google Scholar]
- 9.Weiss J N. Treatment of central retinal vein occlusion by injection of tissue plasminogen activator into a retinal vein. Am J Ophthalmol 1998126142–144. [DOI] [PubMed] [Google Scholar]
- 10.Weiss J N. Retinal surgery for treatment of central retinal vein occlusion. Ophthalmic Surg Lasers 200031162–165. [PubMed] [Google Scholar]
- 11.Weiss J N, Bynoe L A. Injection of tissue plasminogen activator into a branch retinal vein in eyes with central retinal vein occlusion. Ophthalmology 20011082249–2257. [DOI] [PubMed] [Google Scholar]
- 12.Lahey J M, Fong D S, Kearney J. Intravitreal tissue plasminogen activator for acute central retinal vein occlusion. Ophthalmic Surg Lasers 199930427–434. [PubMed] [Google Scholar]
- 13.Kamei M, Misono K, Lewis H. A study of the ability of tissue plasminogen activator to diffuse into the subretinal space after intravitreal injection in rabbits. Am J Ophthalmol 1999128739–746. [DOI] [PubMed] [Google Scholar]
- 14.Coll G E, Sparrow J R, Marinovic A.et al Effect of intravitreal tissue plasminogen activator on experimental subretinal hemorrhage. Retina 199515319–326. [DOI] [PubMed] [Google Scholar]
- 15.Bloodworth J M, Jr, Gutgesell H P, Jr, Engerman R L. Retinal vasculature of the pig. Light and electron microscope studies. Exp Eye Res 19654174–178. [DOI] [PubMed] [Google Scholar]
- 16.Elman M J, Raden R Z, Carrigan A. Intravitreal injection of tissue plasminogen activator for central retinal vein occlusion. Trans Am Ophthalmol Soc. 2001;99: 219–21; discussion 22–3, [DOI] [PMC free article] [PubMed]
- 17.Hrach C J, Johnson M W, Hassan A S.et al Retinal toxicity of commercial intravitreal tissue plasminogen activator solution in cat eyes. Arch Ophthalmol 2000118659–663. [DOI] [PubMed] [Google Scholar]
- 18.Irvine W D, Johnson M W, Hernandez E.et al Retinal toxicity of human tissue plasminogen activator in vitrectomized rabbit eyes. Arch Ophthalmol 1991109718–722. [DOI] [PubMed] [Google Scholar]
- 19.Johnson M W, Olsen K R, Hernandez E.et al Retinal toxicity of recombinant tissue plasminogen activator in the rabbit. Arch Ophthalmol 1990108259–263. [DOI] [PubMed] [Google Scholar]
- 20.Benner J D, Morse L S, Toth C A.et al Evaluation of a commercial recombinant tissue‐type plasminogen activator preparation in the subretinal space of the cat. Arch Ophthalmol 19911091731–1736. [DOI] [PubMed] [Google Scholar]
- 21.Chen S N, Yang T C, Ho C L.et al Retinal toxicity of intravitreal tissue plasminogen activator: case report and literature review. Ophthalmology 2003110704. [DOI] [PubMed] [Google Scholar]
- 22.Nehemy M B, Passos E. Intravitreal recombinant tissue plasminogen activator (rt‐PA) in experimental retinal vein occlusion in rabbits. Invest Ophthalmol Vis Sci ARVO E‐Abstract 20023524
- 23.Glacet‐Bernard A, Kuhn D, Vine A K.et al Treatment of recent onset central retinal vein occlusion with intravitreal tissue plasminogen activator: a pilot study. Br J Ophthalmol 200084609–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Folk J C, Hershey J M, Rivers M B. Lack of effectiveness of tissue plasminogen activator 20 or more days after vitrectomy. Arch Ophthalmol 1991109614. [DOI] [PubMed] [Google Scholar]
- 25.Weizer J S, Fekrat S. Intravitreal tissue plasminogen activator for the treatment of central retinal vein occlusion. Ophthalmic Surg Lasers Imaging 200334350–352. [PubMed] [Google Scholar]
- 26.Mahmoud T, Fekrat S. Ischemic central retinal vein occlusion in a patient with anticardiolipid antibodies on coumadin responds to intravitreal tPA [ARVO abstract]. Invest Ophthalmol Vis Sci 200153S1270 [Google Scholar]
- 27.Ghazi N G, Noureddine B N, Haddad R S.et al Intravitreal tissue plasminogen activator in the management of central retinal vein occlusion. Retina 200323780–784. [DOI] [PubMed] [Google Scholar]
- 28.Ringleb P A, Schellinger P D, Schranz C.et al Thrombolytic therapy within 3 to 6 hours after onset of ischemic stroke: useful or harmful? Stroke 2002331437–1441. [DOI] [PubMed] [Google Scholar]
- 29.Katzan I L, Hammer M D, Hixson E D.et al Utilization of intravenous tissue plasminogen activator for acute ischemic stroke. Arch Neurol 200461346–350. [DOI] [PubMed] [Google Scholar]
- 30.Hacke W, Donnan G, Fieschi C.et al Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt‐PA stroke trials. Lancet 2004363768–777. [DOI] [PubMed] [Google Scholar]