Abstract
Aim
To investigate possible changes in relative mitochondrial DNA (mtDNA) content in patients with non‐arteritic anterior ischaemic optic neuropathy (NAION).
Methods
19 patients with NAION were compared to 32 controls matched for age, sex distribution, and ethnicity. DNA was extracted from leucocytes and competitive multiplex polymerase chain reaction was carried out with two primer pairs (one pair for mtDNA ND1 gene and the other pair for β actin nuclear gene) in the presence of a fluorescent dye.
Results
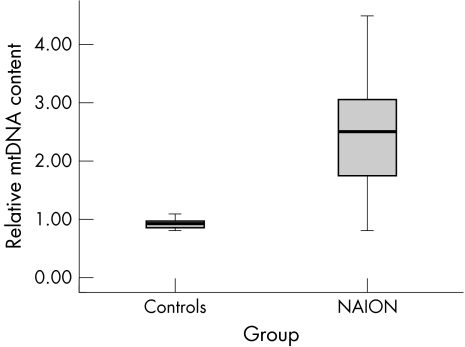
The mean relative mtDNA content in controls (0.93 (SD 0.11); 95% CI 0.89 to 0.97) was significantly less than in NAION patients (2.40 (1.05); 95% CI 1.90 to 2.91; p<0.001). Relative mtDNA content was negatively correlated with Snellen visual acuity (Spearman's rho; r = −0.37; p = 0.022).
Conclusion
Increased relative mtDNA content in NAION patients may imply a response to oxidative stress, possibly in part because of mitochondrial respiratory chain defects. Significantly more non‐synonymous mtDNA nucleotide changes, significantly increased relative mtDNA content, and a significant association between relative mtDNA content and visual acuity all imply that mitochondrial abnormalities may be a risk factor for NAION.
Keywords: non‐arteritic ischaemic optic neuropathy, mitochondrial DNA, Leber's hereditary optic neuropathy
The number of mitochondria in a cell and the amount of mitochondrial DNA (mtDNA) per mitochondrion together constitute the mtDNA content of the cell. Cells carefully regulate the mtDNA content,1 but they also seem able to adjust relative (to nuclear DNA) mtDNA content upward by duplication of mitochondria or proliferation of mtDNA as compensation for reduced ATP synthesis.2,3 For example, relative mtDNA content increases in certain tissues as respiratory function declines with age4 and oxidative stress.5
Leber's hereditary optic neuropathy (LHON) is a maternally inherited hereditary visual problem that is commonly associated with certain mtDNA mutations.6 The mitochondrial changes in LHON cause a metabolic defect,7 and relative mtDNA content is increased in leucocytes of some patients,8,9 in unaffected carriers of LHON mutations,8,9 and in other mitochondrial disorders.2
We reported previously that a group of 19 patients with NAION had a significantly increased prevalence of non‐synonymous mtDNA nucleotide changes,10,11 implying that mitochondrial abnormalities might be a risk factor for this optic neuropathy. We decided to investigate whether NAION patients also have changes in relative mtDNA content.
Patients and methods
The 19 NAION patients in this report are the same patients described previously.10,11 They met standard clinical criteria for NAION with sudden onset of subjective symptoms, best corrected visual acuity (VA) 20/64 or worse, a relative afferent pupillary defect (bilateral disease excepted), optic disc oedema documented on initial funduscopic exam, a visual field defect consistent with optic neuropathy, and age 50 years or older and/or substantial atherosclerotic risk factors. Patients were excluded if they had a family history of LHON; evidence of temporal arteritis; a history typical of optic neuritis, multiple sclerosis, or another inflammatory disease; substantial recovery of VA or visual field more typical of an inflammatory process; malignancy or a tumour near the optic nerve; more than one optic neuropathic process; or another possible cause of visual loss.
DNA was extracted from whole blood samples of NAION patients (14 males and five females; mean age 58.8 (SD 1.95) years) and 32 controls (21 males and 11 females; mean age 59.3 (1.75) years) of similar age, sex distribution, and ethnicity. All controls were blood donors at the King Faisal Specialist Hospital and Research Centre who represented the spectrum of Saudi Arabs and who reported no symptomatic metabolic, genetic, or ocular disorders on an extensive questionnaire regarding family history, past medical problems, and current health. These controls were unrelated to one another, were unrelated to these NAION patients, and were different from the 100 controls recruited previously for the purpose of mtDNA sequence comparison.10 For each control, the entire mtDNA genome was sequenced and established to be free from pathogenic or potentially pathogenic mtDNA sequence changes.
Competitive multiplex polymerase chain reaction (PCR) was carried out with two simultaneous primer sets as described previously.12 One pair was designed to amplify a 450 bp fragment of the ND1 mitochondrial gene and the other pair to amplify a 315 bp fragment of the β actin nuclear gene, which served as an internal control. PCR products were separated on 1% agarose gel at 100 V for 1 hour, and the intensity of the two bands was quantified by the use of gel imager (Typhoon 9410; GE Amersham Biosciences, Schenectady, NY, USA). Relative mtDNA content was determined for each patient and control by dividing the fluorescence intensity of the ND1 (mitochondrial gene) band by the intensity of the β actin (nuclear gene) band. Controls were run simultaneously with patients to avoid experimental variability.
Results
Table 1 presents demographic features for each NAION patient. Fourteen patients had non‐synonymous mtDNA sequence changes not previously reported as polymorphisms,10 while 13 were diabetic and 10 were hypertensive. The average visual acuity was slightly better than 20/100, and all had either central or nerve fibre bundle visual field loss in at least one eye. Table 1 also reports relative mtDNA content for each patient.
Table 1 NAION patients clinical information, sequence changes and relative mtDNA level.
| Patient | Age (years) | Sex | DM | HBP | VA | NS mtDNA sequence change(s) | Relative mtDNA content | |
|---|---|---|---|---|---|---|---|---|
| Right eye | Left eye | |||||||
| 1 | 71 | M | Yes | Yes | 20/100 | CF 1 foot | 7308, 14480, 14870 | 2.20 |
| 2 | 70 | M | No | No | HM | HM | 9957 | 2.10 |
| 3 | 69 | F | Yes | No | CF 3 feet | 20/100 | 5308 | 4.50 |
| 4 | 69 | M | No | No | CF 5 feet | 20/60 | 11337 | 2.50 |
| 5 | 62 | F | Yes | Yes | 20/40 | 20/200 | 4216, 13708, 15257 | 3.20 |
| 6 | 67 | M | No | Yes | 20/400 | CF 3 feet | None detected | 2.80 |
| 7 | 56 | M | Yes | Yes | HM | 20/30 | None detected | 3.20 |
| 8 | 63 | M | Yes | Yes | 20/400 | 20/80 | 4216, 13708 | 1.70 |
| 9 | 53 | M | Yes | No | 20/200 | 20/200 | 6335 | 2.70 |
| 10 | 55 | M | Yes | Yes | 20/200 | 20/20 | None detected | 1.80 |
| 11 | 54 | M | Yes | Yes | 20/25 | 20/200 | 4762 | 2.60 |
| 12 | 52 | M | No | No | 20/200 | 20/25 | 4216, 13708, 15257 | 3.30 |
| 13 | 50 | F | Yes | No | 20/100 | CF 5 feet | None detected | 4.30 |
| 14 | 61 | F | No | No | 20/200 | 20/40 | 12340 | 2.90 |
| 15 | 44 | M | Yes | Yes | 20/25 | 20/100 | 9961 | 1.90 |
| 16 | 55 | M | Yes | Yes | 20/50 | 20/20 | 15674 | 0.85 |
| 17 | 46 | M | No | No | 20/20 | CF 3 feet | 4216, 4917, 13834 | 0.81 |
| 18 | 53 | M | Yes | No | 20/20 | 20/50 | 4216, 13708, 15257 | 0.93 |
| 19 | 68 | F | Yes | Yes | 20/60 | 20/40 | None detected | 1.39 |
DM, diabetes mellitus; HBP, high blood pressure; VA, Snellen visual acuity; NS, non‐synonymous, sequence change that results in an amino acid alteration; Relative mtDNA content, see Methods.
Figure 1 shows that mean relative mtDNA content for controls was 0.93 (0.11) (95% CI 0.89 to 0.97), which was significantly less than for NAION patients (2.40 (1.05); 95% CI 1.90 to 2.91; p<0.001). The optimal relative mtDNA content value to distinguish between controls and patients was 1.04 as calculated by receiver operator characteristic (ROC) curve (ROC curve not shown; area under ROC curve = 0.882; 95% CI 0.75 to 1.01). Sixteen NAION patients (84%) had relative mtDNA content greater than this value. Relative mtDNA content had a weak negative correlation with Snellen visual acuity bilaterally (higher levels of relative mtDNA content predicting lower visual acuity; Spearman's rho; r = −0.37; p = 0.022) but not with age, sex, or the presence of non‐synonymous mtDNA nucleotide changes.
Figure 1 Box plot comparing relative mtDNA content in NAION patients and controls. Box plot comparing relative mtDNA content in 32 controls with 19 NAION patients.10 The difference of the means was statistically significant (see text; p<0.0001).
Discussion
NAION and LHON are clinically distinct types of optic neuropathy, but they share certain clinical characteristics, including simultaneous or sequential subacute optic nerve injury with swelling of the optic disc, most often without pain or recovery of vision. We reported previously that primary LHON mutations were not present in these 19 NAION patients but that 14 patients had other non‐synonymous mtDNA sequence changes not thought to be polymorphisms,10 a prevalence significantly greater than controls.11 We proposed that mtDNA nucleotide changes might be a previously unrecognised risk factor for NAION and that maternal inheritance might be obscured by relatively less pathogenicity and penetrance of these mtDNA sequence changes compared to primary LHON mutations and by interaction with other risk factors such as age, hypertension, and diabetes.10
Prothrombotic polymorphisms reported in association with NAION (including factor II G20210A prothrombin variant, factor V Leiden G1691A variant, MTHFR C677T and A1298C variants, platelet glycoprotein receptor IIIa allele, and apolipoprotein E allele)13,14 were not present in this group, and atherosclerotic risk factors were also not significantly different from controls matched for age, sex, and ethnicity.11 The fact that the occurrence of NAION was not easily explained by the presence of prothrombotic or atherosclerotic risk factors in this group again focused attention on the possibility that mitochondrial abnormalities might be important in the development of NAION.
These NAION patients have significantly increased relative mtDNA content when compared to controls. Many of them also have hypertension, diabetes, and potentially pathogenic mtDNA mutations, all of which have been associated with respiratory chain defects.2,15 Increased relative mtDNA content in NAION patients may be a compensatory response similar to that which has now been proposed in ageing,4 LHON patients with the 11778 mutation,8 unaffected LHON mutation carriers,8,9 and certain mitochondrial diseases.16 Interestingly, relative mtDNA content was not increased in LHON patients harbouring the 14484 primary LHON mutation, but it was increased in asymptomatic carriers from the same families,9 implying that this compensatory response might at times be protective. Conversely, patients with dominant optic atrophy had reduced relative mtDNA content.17 Relative mtDNA content is quite possibly an epiphenomenon of other mitochondrial and/or metabolic disturbances, just as elevated blood glucose levels in diabetes are an epiphenomenon of other hormonal and metabolic abnormalities. As with diabetes, it is also possible that relative mtDNA content could be considered a risk factor for NAION that is partially independent of other factors.
Surprisingly, higher relative mtDNA content was weakly predictive of lower visual acuity. No other risk factor for NAION has been reported to be quantitatively correlated with an estimate of visual functioning.18 Elevated levels of mtDNA content may reflect a response to oxidative stress, and increased oxidative stress may increase risk of more profound optic nerve injury. At the very least, it is unique that a measure possibly reflecting certain aspects of mitochondrial function is correlated with a measure reflecting certain aspects of visual function in a spontaneous optic neuropathy.
NAION patients have more non‐synonymous mtDNA nucleotide alterations than controls. They have increased relative mtDNA content, and relative mtDNA content is correlated with visual acuity. These observations suggest that one component of optic nerve injury in NAION might be metabolic in addition to, or instead of, ischaemic. Empirical treatment of NAION has been ineffective,19,20 and a possible mitochondrial connection opens up additional potential avenues for investigation and treatment. Assessing relative mtDNA content and, although laborious, sequencing the entire mtDNA coding region may be warranted in NAION patients.
Abbreviations
LHON - Leber's hereditary optic neuropathy
mtDNA - mitochondrial DNA
NAION - non‐arteritic anterior ischaemic optic neuropathy
PCR - polymerase chain reaction
ROC - receiver operator characteristic
VA - visual acuity
References
- 1.Robin E D, Wong R. Mitochondrial DNA molecules and virtual number of mitochondria per cell in mammalian cells. J Cell Physiol 1988136507–513. [DOI] [PubMed] [Google Scholar]
- 2.Wei Y H. Mitochondrial DNA mutations and oxidative damage in aging and diseases: an emerging paradigm of gerontology and medicine. Proc Natl Sci Counc Repub China B 19982255–67. [PubMed] [Google Scholar]
- 3.Wei Y H, Lee H C. Oxidative stress, mitochondrial DNA mutation, and impairment of antioxidant enzymes in aging. Exp Biol Med (Maywood) 2002227671–682. [DOI] [PubMed] [Google Scholar]
- 4.Lee H C, Lu C Y, Fahn H J.et al Aging‐ and smoking‐associated alteration in the relative content of mitochondrial DNA in human lung. FEBS Lett 1998441292–296. [DOI] [PubMed] [Google Scholar]
- 5.Lee H C, Wei Y H. Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. Int J Biochem Cell Biol 200537822–834. [DOI] [PubMed] [Google Scholar]
- 6.Newman N J. Hereditary optic neuropathies: from the mitochondria to the optic nerve. Am J Ophthalmol 2005140517–523. [DOI] [PubMed] [Google Scholar]
- 7.Brown M D, Trounce I A, Jun A S.et al Functional analysis of lymphoblast and cybrid mitochondria containing the 3460, 11778, or 14484 Leber's hereditary optic neuropathy mitochondrial DNA mutation. J Biol Chem 200027539831–39836. [DOI] [PubMed] [Google Scholar]
- 8.Yen M Y, Chen C S, Wang A G.et al Increase of mitochondrial DNA in blood cells of patients with Leber's hereditary optic neuropathy with 11778 mutation. Br J Ophthalmol 2002861027–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishioka T, Soemantri A, Ishida T. mtDNA/nDNA ratio in 14484 LHON mitochondrial mutation carriers. J Hum Genet 200449701–705. [DOI] [PubMed] [Google Scholar]
- 10.Bosley T M, Abu‐Amero K K, Ozand P T. Mitochondrial DNA nucleotide changes in non‐arteritic ischemic optic neuropathy. Neurology 2004631305–1308. [DOI] [PubMed] [Google Scholar]
- 11.Abu‐Amero K K, Bosley T M. Prothrombotic and atherosclerotic risk factors lack significance in NAION patients harbouring mitochondrial DNA mutations. Br J Ophthalmol 200690119–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kao S H, Chao H T, Liu H W.et al Sperm mitochondrial DNA depletion in men with asthenospermia. Fertil Steril 20048266–73. [DOI] [PubMed] [Google Scholar]
- 13.Nagy V, Facsko A, Takacs L.et al Activated protein C resistance in anterior ischaemic optic neuropathy. Acta Ophthalmol Scand 200482140–143. [DOI] [PubMed] [Google Scholar]
- 14.Glueck C J, Wang P, Bell H.et al Nonarteritic anterior ischemic optic neuropathy: associations with homozygosity for the C677T methylenetetrahydrofolate reductase mutation. J Lab Clin Med 2004143184–192. [DOI] [PubMed] [Google Scholar]
- 15.Poulton J, Holt I J. Mitochondrial DNA: does more lead to less? Nat Genet 19948313–315. [DOI] [PubMed] [Google Scholar]
- 16.Poulton J, Morten K J, Weber K.et al Are duplications of mitochondrial DNA characteristic of Kearns‐Sayre syndrome? Hum Mol Genet 19943947–951. [DOI] [PubMed] [Google Scholar]
- 17.Kim J Y, Hwang J M, Ko H S.et al Mitochondrial DNA content is decreased in autosomal dominant optic atrophy. Neurology 200564966–972. [DOI] [PubMed] [Google Scholar]
- 18.Ischemic Optic Neuropathy Decompression Trial Research Group Characteristics of patients with nonarteritic anterior ischemic optic neuropathy eligible for the Ischemic Optic Neuropathy Decompression Trial. Arch Ophthalmol 19961141366–1374. [DOI] [PubMed] [Google Scholar]
- 19.Fazzone H E, Kupersmith M J, Leibmann J. Does topical brimonidine tartrate help NAION? Br J Ophthalmol 2003871193–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ischemic Optic Neuropathy Decompression Trial Research Group Optic nerve decompression surgery for nonarteritic anterior ischemic optic neuropathy (NAION) is not effective and may be harmful. JAMA 1995273625–632. [PubMed] [Google Scholar]