Abstract
Background/aim
It is generally assumed that similar pathways are involved in human papillomavirus (HPV) induced pathogenesis of cervical squamous intraepithelial lesions (SILs) and cancers and a subset of conjunctival intraepithelial neoplasm (CIN)—that the malignancies or pre‐cancerous lesions arise through HPV oncoproteins E6 and E7, which disrupt the pathways of p53 and the product of the retinoblastoma (Rb) gene and, in turn, increase the protein product of gene p16INK4 through the mechanism of positive feedback. Several cell cycle molecules are detected to test this hypothesis.
Methods
Nine cases of CIN and eight non‐CIN cases were analysed for the expression of Ki‐67, pRb, p53, and p16INK4 via immunohistochemistry. Nine cases of cervical high grade squamous intraepithelial lesion (HSIL), and 10 cases of cervical low grade squamous intraepithelial lesion (LSIL) were included for stain control of p16INK4a, and comparison of p16INK4a expression to CIN cases. A nested polymerase chain reaction and a genechip HPV typing were used to detect HPV infection and types in the CIN and non‐CIN samples
Results
HPV positivity was demonstrated in all of the CIN lesions but in none of the non‐CIN lesions. The Ki‐67 proliferative index (Ki‐67 PI) was statistically higher in the CIN group than the non‐CIN group; however, there were no differences of expression of pRb and p53 between the two groups and no expression of p16INK4 in all cases. All nine cases of HSIL, and seven out of 10 cases of LSIL used for stain control were immunoreactive for p16INK4a. There were statistically significant differences in overexpression of p16INK4a between the CINs and SILs
Conclusions
The Ki‐67 proliferative index may be a sensitive marker for CIN lesions and these results, with significant differences in overexpression of p16INK4a between CINs and SILs, may provide new evidence that HPV related mucosal dysplasia in different anatomical locations may lead to dissimilar molecular pathways.
Keywords: conjunctival intraepithelial neoplasm, human papillomavirus
Conjunctival intraepithelial neoplasia (CIN), the most common tumour of the ocular surface,1 is thought to be a precursor of squamous cell carcinoma (SCC), the most common conjunctival malignant neoplasm in several series.1,2,3 Invasive SCC of the conjunctiva refers to disease invasion of the basement membrane. Oncogenic associations of human papillomavirus (HPV) with uterine cervical SCC have been well established,4,5 along with the association between high risk HPV and the pre‐cancerous squamous intraepithelial lesion (SIL) of the uterine cervix.6,7,8,9,10 Previous studies have demonstrated high expression rates of p16INK4a in the cervical SILs and SCCs.11 Both lesions showed a decrease in the proportion of pRb positive cells and a generally inverse correlation with the expression of p16INK4a at the tissue level.11 Although high risk HPVs (for instance types 16 and 18) have been demonstrated in conjunctival SCC, dysplasia, and papilloma,12,13,14,15 no data are available on their results of immunohistochemical stains of pRb and p16INK4a; and the aetiological role of HPV in the development of CIN is unclear. Such data should enable us to better understand the relation between HPV and the expression of these cell cycle proteins in such cases of ocular surface neoplasms. Previous studies also showed that the E6 and E7 oncoproteins of the high risk HPVs deregulate key controls in cellular proliferation,16 promoting degradation of p53 by the cellular ubiquitin proteolysis system, thus compromising the ability of the cell to effect growth arrest when there is DNA damage.17
CIN can be divided into three groups: mild, moderate and severe dysplasia, based on the ratios of replacement of the basal epithelium by abnormal epithelial cells lacking normal maturation.18,19,20 It is very difficult to clinically differentiate histopathologically mild dysplasia from reactive epithelial changes. A similar problem was once encountered with the SIL of the cervix, but was improved using immunohistochemical staining of the Ki‐67 PI. The Ki‐67 nuclear antigen is expressed in all phases of the cell cycle, except G0,21,22,23 and has been recently found to be the most reliable indicator of cellular proliferation.21,22,23 In normal cervical squamous mucosa, Ki‐67 positive cells are found mainly in the parabasal layers and some in the basal layer.24 In cervical SILs, the number of Ki‐67 positive cells increased as the cell grading went from normal to low grade SIL (LSIL) to high grade SIL (HSIL).24
In a recent report by Ohara et al the Ki‐67 PI in conjunctival SCC and CIN was statistically higher than that in ptergium.25 In our study, we adopted a nested polymerase chain reaction (PCR) based genechip method and a subsequent DNA sequencing analysis to determine multiple HPV types in nine CIN and eight non‐CIN conjunctival mucosal samples. We also performed immunohistochemical stains of Ki‐67, p53, pRb, and p16INK4a in an endeavour to (1) understand whether Ki‐67 can serve as a diagnostic marker, and (2) detect the relation between HPV and the expression of these cell cycle proteins. A few cases of LSIL and HSIL were included for stain control of p16INK4a.
Materials and methods
Patients and specimens
Formalin fixed paraffin embedded tissue blocks were obtained from nine patients (four men and five women; mean age 51.6, range 14–75 years) with CIN and eight patients (four men and four women; mean age 40.5, range 17–51 years) without CIN. Of the nine cases of CIN, seven (77.8%) had mild dysplasia, and two (22.2%) moderate dysplasia determined via histological examination of haematoxylin and eosin stained tissue sections.18,19 We additionally graded mild dysplasia into low grade CIN and moderate and severe dysplasia into high grade CIN using similar criteria that were used in cervical SIL lesions.24 All patients underwent total surgical excision of their lesions at the Department of Ophthamology, National Taiwan University Hospital, Taipei, Taiwan, during the period from August 1994 to December 2004.
Immunohistochemistry
All specimens were formalin fixed, paraffin embedded, cut in 5 μm thick sections, and then deparaffinised, rehydrated, and underwent antigen retrieval (Trilogy, Cell Marque, Hot Springs, AR, USA, for p53, Ki‐67; Dako target retrieval solution, high pH, S3307 for pRb and p16INK4a, autoclaved, 10 minutes). The sources of the primary antibodies are as follows: p16INK4a (neomarker; JC‐8), pRb (Santa Cruz; IF8), p53 (Dako; DO‐7), Ki‐67 (Dako; MIB‐1). Staining was performed using the Ventana autostainer (DAB detection kit, iView, Ventana Medical Systems, CA, USA).
DNA extraction
Four 8 μm tissue sections were cut, mixed with proteinase digestion buffer,26 and incubated. The standard phenol‐chloroform extraction and the ethanol precipitation were used for DNA purification. In order to control the quality and quantity of the isolated DNA, a 300 base pair sequence of β actin gene was amplified by PCR as an internal control to monitor the genomic DNA extraction procedure.
HPV detection by nested PCR
Each PCR amplification reaction was carried out in a total volume of 50 μl containing a PCR master mixture (10 mM TRIS‐HCl pH 8.3, 50 mM KCl, 800 μM each of dATP, dCTP, dGTP, 600 μM dUTP, 10 pmol of each primer set, 1.25 IU/l AmpliTaq Gold DNA polymerase; Applied Biosystems, San Francisco, CA, USA). Each PCR was carried out in the Geneamp PCR System 9700 (Applied Biosystems) with the first denaturation step at 94°C for 3 minutes and final extension step at 72°C for 5 minutes. General consensus primers MY09/GP6+ were used for the first PCR round to amplify the corresponding part of the HPV L1 gene according to the original report by others,20 with some modifications.27,28,29 A nested PCR was then carried out with the primers GP5+/GP6+ according to the previously published protocols.30
HPV genotyping by genechip
A volume of 15 μl of the resultant amplified product was then hybridised with an HPV genechip (Easychip HPV Genotyping Array, King Car, Taipei, Taiwan), which offers a revert blot hybridisation to detect 39 subtypes of HPV DNA in a single reaction, as reported by Huang et al.31
Ki‐67 proliferative index
A 10×10 square grid graticule at 400× magnification was used for counting the number of Ki‐67 labelled nuclei in tumour cells. The areas for cell counting were selected from the most mitotically active parts of the CIN lesions, non‐CIN lesions, and normal conjunctival mucosa. After counting a total of 200 cells, the immunoreactive score was expressed as a percentage of the total cell count.25
Statistical analysis
The Ki‐67, p53, and pRb positive ratios of epithelial cells and p16INK4a positive rates were first compared between CIN and non‐CIN samples. Moreover, in the CIN samples, the Ki‐67 and p53 positive ratios of epithelial cells were compared between the CIN lesions and normal mucosa. p16INK4a positive rates were also compared between cases of CIN and SIL. The Mann‐Whitney U test was used to evaluate statistical differences in positive ratios of Ki‐67, p53, and pRb between the CIN lesions, non‐CIN lesions, and normal conjunctiva. The χ2 with Fisher's exact test was used to evaluate statistical differences of p16INK4a positive rates between the CIN and non‐CIN samples as well as between CIN and SIL samples. A p value less than 0.05 was considered significant.
Results
HPV detection
HPV positivity was demonstrated in all of the CIN lesions but in none of the non‐CIN lesions, as shown in table 1.
Table 1 Clinical profile, grade of CIN, and HPV typing.
| Case | No | Age (year/month) | Sex | HPV typing | Diagnosis |
|---|---|---|---|---|---|
| CIN | |||||
| 1 | 31/6 | F | 58 and 37 | Low grade CIN | |
| 2 | 14/1 | F | 11 and 18 | Low grade CIN | |
| 3 | 59/9 | M | 16 | Low grade CIN | |
| 4 | 58/9 | M | 16 | Low grade CIN | |
| 5 | 75/4 | F | 33 | High grade CIN | |
| 6 | 72 | F | 72 | Low grade CIN | |
| 7 | 59/3 | M | 16 | High grade CIN | |
| 8 | 70/1 | M | 16 | Low grade CIN | |
| 9 | 23/6 | F | 6 | Low grade CIN | |
| Non‐CIN | |||||
| 10 | 39/9 | F | negative | SLK | |
| 11 | 44/5 | F | negative | Pterygium | |
| 12 | 17/8 | M | negative | LP | |
| 13 | 47/5 | M | negative | Pterygium | |
| 14 | 51/7 | F | negative | Pterygium | |
| 15 | 46/4 | F | negative | SLK | |
| 16 | 50/7 | M | negative | Pterygium | |
| 17 | 26/6 | M | negative | LP | |
CIN, conjunctival intraepithelial neoplasm; SLK, superior limbic keratoconjunctivitis; LP, lymphoid proliferation.
Single infection by a high risk type HPV (HPV 16, 18, and 33) was detected in five of the nine CIN cases, whereas two of the nine CIN cases were infected by a single low risk HPV type (HPV 6 and 72). Two CIN cases were noted to have double infection by either two high risk types or mixed high and low risk types.
Histopathology and Ki‐67 proliferative index
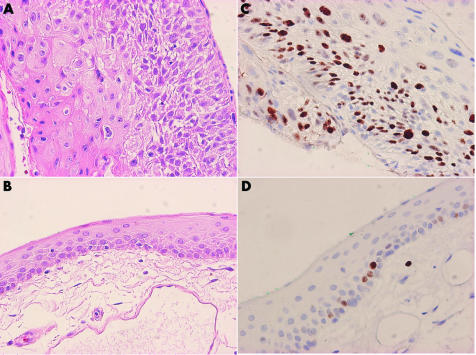
Histopathologically, two of the nine CIN cases were high grade (fig 1A), while the others were low grade CIN lesions. Two of the non‐CIN lesions were diagnosed as superior limbic keratoconjunctivitis (SLK), two were cases of lymphoid proliferation and the other four were cases of pterygium. The Ki‐67 PI of normal mucosa (fig 1B) in the CIN specimens was also counted for comparison. The Ki‐67 PI for different groups is shown in table 2.
Figure 1 (A) Haematoxylin and eosin stain of high grade CIN. (B) Haematoxylin and eosin stain of normal conjunctiva. (C) Ki‐67 stain of CIN lesion. (D) Ki‐67 stain of normal conjunctiva.
Table 2 Comparison of Ki‐67 proliferative index between different groups.
| Case | PI (%) (SD) | p Value | |
|---|---|---|---|
| CIN lesion (n = 9) | 31.56 (9.48) | <0.001 | |
| Normal conjunctival mucosa (n = 9) | 9.78 (2.11) | ||
| Non‐CIN lesion (n = 8) | 10.5 (2.07) | <0.001 | |
| CIN lesion (n = 9) | 31.56 (9.48) | ||
| Non‐CIN lesion (n = 8) | 10.5 (2.07) | 0.541 | |
| Normal conjunctival mucosa (n = 9) | 9.78 (2.11) |
The Ki‐67 PI in CIN lesions ranged from 20–48% (mean 31.56%) (SD 9.48%) (fig 1C) and were statistically higher than that of non‐CIN lesions, which ranged from 8–12% (10.5%) (2.07%), and normal mucosa of CIN samples which ranged from 8–12% (9.78%) (2.11%) (fig 1D). There was no difference between non‐CIN lesions and normal conjunctival mucosa (p = 0.541).
Immunohistochemical stains of P53, pRb, and p16INK4a
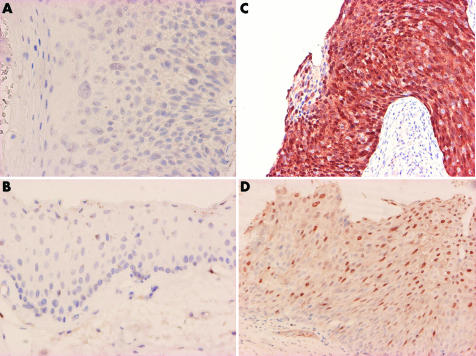
All CIN (fig 2A) and non‐CIN (fig 2B) cases were immunohistochemically non‐reactive for p16INK4a compared with the positive control models of HSIL (fig 2C) and LSIL (fig 2D). All nine cases of HSIL, and seven out of 10 cases of LSIL used for stain control were immunoreactive for p16INK4a. There were statistically significant differences in overexpression of p16INK4a between CINs and SILs (table 3).
Figure 2 (A) p16INK4a stain of high grade CIN. (B) p16INK4a stain of normal conjunctiva. (C) p16INK4a stain of HSIL. (D) p16INK4a stain of LSIL.
Table 3 Comparison of p16INK4a between CINs and SILs.
| Case | p16INK4a positive rate (%) | p Value | |
|---|---|---|---|
| CIN lesion (n = 9) | 0/9 (0) | <0.00001 | |
| HSIL (n = 9) | 9/9 (100) | ||
| CIN lesion (n = 9) | 0/9 (0) | 0.003 | |
| LSIL (n = 10) | 7/10 (70) | ||
| CIN lesion (n = 9) | 0/9 (0) | <0.00001 | |
| SIL (HSIL+LSIL) (n = 19) | 16/19(84.21) |
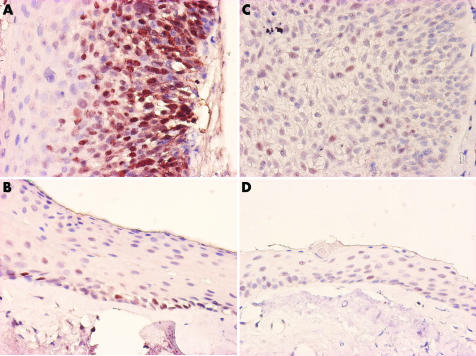
The percentage of p53 positive cells in CIN lesions (fig 3A) ranged from 8–56% (24.44%) (20.24%); this was not statistically higher (p>0.05) than that of non‐CIN lesions which ranged from 8–12% (10%) (2.14%), and normal mucosa of CIN samples (fig 3B) which ranged from 8–12% (9.78%) (2.91%). There was no difference between the p53 positivity ratios of non‐CIN lesions and normal conjunctival mucosa. (p = 0.859) (table 4).
Figure 3 (A) p53 stain of high grade CIN. (B) p53 stain of normal conjunctiva. (C) pRb stain of CIN. (D) p53 stain of non‐CIN.
Table 4 Comparison of p53 index between different groups.
| Case | % (SD) | p Value | |
|---|---|---|---|
| CIN lesion (n = 9) | 24.44 (20.24) | 0.06 | |
| Normal conjunctival mucosa (n = 9) | 9.78 (2.91) | ||
| CIN lesion (n = 9) | 24.44 (20.24) | 0.065 | |
| Non‐CIN lesion (n = 8) | 10 (2.14) | ||
| Non‐CIN lesion (n = 8) | 10 (2.14) | 0.859 | |
| Normal conjunctival mucosa (n = 9) | 9.78 (2.91) |
The percentage of pRb positive cells in CIN lesions (fig 3C) ranged from 0–12% (6.67%) (5.66%), and was not statistically higher than that of non‐CIN lesions (fig 3D), which ranged from 0–12% (4.5%) (4.99%) (table 5).
Table 5 Comparison of pRb between different groups.
| Case | PI (%) (SD) | p Value |
|---|---|---|
| CIN lesion (n = 9) | 6.67 (5.66) | 0.4144 |
| Non‐CIN lesion (n = 8) | 4.5 (4.99) |
Discussion
Previous studies have provided some evidence of an association between HPVs and the development of CINs.32,33 High risk types of HPV have been demonstrated by numerous epidemiological and molecular studies to be the aetiological agents for an overwhelming majority of cases of SILs and cervical SCC.34 When the host epithelial cell is infected with the HPV, the HPV genome integrates into the host cell's DNA. This typically disrupts the HPV E1/E2 open reading frame, resulting in the loss of function of E2, a physiological regulatory protein for the HPV E6 and E7 oncogenes.35,36 The E7 oncoprotein may contribute to cellular transformation by interfering with the p16INK4a/CDK4/cycD1/pRb cell cycle regulatory pathway and directing the catabolism of tumour suppressor protein pRb.36 p16INK4a specifically inhibits the formation of cyclin D/cdk4,6 complexes by interacting with cdk4 and cdk6,4,34,37 which in turn control the activity of pRb by phosphorylation.35 The retinoblastoma tumour suppressor protein, pRb, regulates the cell cycle at the G1/S restriction point by complexing with and inhibiting the activity of E2F, which serves as a transcription dependent promoter of cell cycle progression.38 The E2F–pRb complex dissociates in the presence of the HPV oncoprotein E7; this, in turn, activates E2F, thereby initiating the transcription of genes required for DNA replication and inappropriately forcing the cell past the G1/S restriction point into the S phase.39,40,41 Because of positive feedback,36,42,43,44,45 the functional inactivation of pRb by HPV E7 results in the reciprocal overexpression of p16INK4a.
In a previous report, strong p16INK4a staining was found in most cervical SILs and SCC with an inverse correlation between the expression of p16INK4a and the expression of pRb at the tissue level.11 No reports are available at present discussing the relation between HPV positivity and the incidence of overexpression of cell cycle proteins p16INK4a and pRb of the CIN cases.32,33 In our study, the incidences of overexpression of p16INK4a and pRb were 0% and 66.67% (6/9) in CINs, but there were no differences of overexpression of p16INK4a and pRb noted between the HPV positive cases (CINs) and HPV negative (non‐CINs) cases. Our results also failed to detect the generally inverse correlation between the expression of p16INK4a and pRb in the cases of CINs, a relation which has been demonstrated in a previous report on cervical SILs.11 These findings may represent new evidence that the molecular pathways to HPV related malignancy may be influenced by anatomical location.
In our study, the percentage of p53 positive cells in CIN lesions was not statistically higher than that of non‐CIN lesions, or of normal mucosa of CIN samples. This may imply different stabilisation of p53 protein in the CINs. The expression of p53 protein in the non‐CIN lesions and normal mucosa may have been contributed by normal p53 that is somehow abnormally stabilised or increased to the detectable level.
Ki‐67 is present in all phases of the cell cycle except the G0 phase. Ki‐67 immunohistochemical analysis has been applied in the histopathological diagnosis of malignant tumours.22,46,47 An increase of Ki‐67 positive cells was seen from the normal to LSIL, and then to HSIL.24 Similar findings have been recently reported in cases of conjunctival SCC and intraepithelial neoplasia.25 According to Ohara et al, by comparing tissue specimens obtained from patients with SCC, CINs, and non‐CIN lesions (pterygium), it was revealed that the level of Ki‐67 PI from highest to lowest was SCC > CINs > pterygium. In our case series, the Ki‐67 PI of high grade CINs (44% and 48%) seemed higher than low grade lesions (27.43%) (5.38%), but our sample size was too small. We also found that the Ki‐67 PI was statistically higher in CINs than non‐CIN lesions or normal conjunctiva. Judging from these results, we hypothesise that Ki‐67 PI may serve as a diagnostic marker for CINs and might be an important factor reflecting the malignancy grading. However, contradictory results have also been reported.48 Mahomed and Chetty investigated Ki‐67 antigen expression rates in conjunctival squamous neoplasia that ranged from mild dysplasia to invasive carcinoma and reported a very low rate of Ki‐67 antigen expression, even in HIV+ cases.48 Our explanation for this discriminative result is that different clones of antibodies and different methods of antigen retrieval procedure were used. In our experience, a proper and complete antigen retrieval procedure is necessary for successful immunohistochemical staining of Ki‐67. An inadequate antigen retrieval procedure may lead to false negative results. In our (autoclaved, 10 minutes) and Ohara's (microwave 95°C, 15 minutes) experiments, complete antigen retrieval procedures were performed, but a different procedure was accepted by Mahomed and Chetty (microwave 85°C, 10 minutes).25,48 Different related viruses may lead to different pathogenic pathways in the same kind of pre‐cancer and cancer lesions. All of our CIN and SIL cases were HPV related, but in Mahomed and Chetty's report, only HIV status was clarified. We believe that different case selection and methodology may lead to these contradictory results.
This study revealed that the Ki‐67 PI, which correlates well with the histopathological diagnosis of CINs, may be a useful marker for their clinical diagnosis. We also demonstrated new evidence that the molecular pathways leading to HPV related malignancy may be influenced by anatomical location. Further studies and more cases are needed to clarify the relation between Ki‐67 PI, histopathological grading, molecular aetiology and clinical prognosis.
Abbreviations
CIN - conjunctival intraepithelial neoplasm
HPV - human papillomavirus
HSIL - high grade squamous intraepithelial lesion
LP - lymphoid proliferation
LSIL - low grade squamous intraepithelial lesion
PCR - polymerase chain reaction
PI - proliferative index
Rb - retinoblastoma
SCC - squamous cell carcinoma
SILs - squamous intraepithelial lesions
References
- 1.Grossniklaus H E, Green W R, Luckenbach M.et al Conjunctival lesions in adults. A clinical and histopathologic review. Cornea 1987678–116. [DOI] [PubMed] [Google Scholar]
- 2.Ni C, Searl S S, Kriegstein H J.et al Epibulbar carcinoma. Int Ophthalmol Clin 1982221–33. [DOI] [PubMed] [Google Scholar]
- 3.Ash J E. Epibulbar tumors. Am J Ophthalmol 1950331203–1219. [DOI] [PubMed] [Google Scholar]
- 4.Walboomers J M M, Jacobs M V, Manos M M.et al Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 199918912–19. [DOI] [PubMed] [Google Scholar]
- 5.Zur Hausen H. Papillomavirus infections—a major cause of human cancers. Biochim Biophys Acta 1996128855–78. [DOI] [PubMed] [Google Scholar]
- 6.Koutsky L A, Holmes K K, Critchlow C W.et al A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N Engl J Med 19923271272–1278. [DOI] [PubMed] [Google Scholar]
- 7.Johnson M A, Blomfield P I, Bevan I S.et al Analysis of human papillomavirus type 16 E6–E7 transcription in cervical carcinomas and normal cervical epithelium using the polymerase chain reaction. J Gen Virol 1990711473–1479. [DOI] [PubMed] [Google Scholar]
- 8.Young L S, Bevan I S, Johnson M A.et al The polymerase chain reaction: a new epidemiological tool for investigating cervical human papillomavirus infection. Br Med J 198929814–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howley P M, Schlegel R. The human papillomaviruses. An overview. Am J Med 198885155–158. [PubMed] [Google Scholar]
- 10.Walker P G, Singer A, Dyson J L.et al The prevalence of human papillomavirus antigen in patients with cervical intra‐epithelial neoplasia. Br J Cancer 19834899–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tringler B, Gup C J, Singh M.et al Evaluation of p16INK4a and pRb expression in cervical squamous and glandular neoplasia. Human Pathol 200435689–696. [DOI] [PubMed] [Google Scholar]
- 12.Odrich M G, Jakobiec F A, Lancaster W D.et al A spectrum of bilateral squamous conjunctival tumors associated with human papillomavirus type 16. Ophthalmology 199198628–635. [DOI] [PubMed] [Google Scholar]
- 13.Karcioglu Z A, Issa T M. Human papilloma virus in neoplastic and non‐neoplastic conditions of the external eye. Br J Ophthalmol 199781595–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lauer S A, Malter J S, Meier J R. Human papillomavirus type 18 in conjunctival intraepithelial neoplasia. Am J Ophthalmol 199011023–27. [DOI] [PubMed] [Google Scholar]
- 15.McDonnell J M, Mayr A J, Martin W J. DNA of human papillomavirus type 16 in dysplastic and malignant lesions of the conjunctiva and cornea. N Engl J Med 19893201442–1446. [DOI] [PubMed] [Google Scholar]
- 16.Stanley M A. Human papillomavirus and cervical carcinogenesis. Best Pract Res Clin Obstet Gynaecol 200115663–676. [DOI] [PubMed] [Google Scholar]
- 17.Vogelstein B, Lane D, Levine A J. Surfing the p53 network. Nature 2000408307–310. [DOI] [PubMed] [Google Scholar]
- 18.Shields J A, Shields C L. Tumors of the conjunctiva and cornea. In: Smolin G, ed. The cornea:scientific foundations and clinical practice. 3rd ed. Boston: Little, Brown, 1994586–588.
- 19.Tunc M, Char D H, Crawford B.et al Intraepithelial and invasive squamous cell carcinoma of the conjunctiva: analysis of 60 cases. Br J Ophthalmol 19998398–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bauer H M, Greer C E, Manos M M. Determination of genital human papillomavirus infection by consensus PCR amplification. In: Herrington CS, ed. Diagnostic molecular pathology: a practical approach. Vol 2. Oxford: Oxford University Press, 1992
- 21.Gerdes J, Lemke H, Baisch H.et al Cell cycle analysis of a cell proliferation‐associated human nuclear antigen defined by the monoclonal antibody Ki‐67. J Immunol 19841331710–1715. [PubMed] [Google Scholar]
- 22.Lu S, Tiekso J, Hietanen S.et al Expression of cell‐cycle proteins p53, p21 (WAF‐1), PCNA and Ki67 in benign, premalignant and malignant skin lesions with implicated HPV involvement. Acta Derm Venereol 199979268–273. [DOI] [PubMed] [Google Scholar]
- 23.Rudolph P, Peters J, Lorenz D.et al Correlation between mitotic and Ki‐67 labeling indices in paraffin‐embedded carcinoma specimens. Hum Pathol 1998291216–1222. [DOI] [PubMed] [Google Scholar]
- 24.McCluggage W G, Maxwell P, Bharucha H. Immunohistochemical detection of metallothionein and MIB1 in uterine cervical squamous lesions. Int J Gynecol Pathol 19981729–35. [DOI] [PubMed] [Google Scholar]
- 25.Ohara M, Sotozono C, Tsuchihashi Y.et al Ki‐67 labeling index as a marker of malignancy in ocular surface neoplasms. Jpn J Ophthalmol 200448524–529. [DOI] [PubMed] [Google Scholar]
- 26.Zitz J C, McLachlin C M, Tate J E.et al Restriction fragment length polymorphism analysis of isotype‐labeled polymerase chain reaction‐amplified human papillomavirus DNA combines sensitivity with built‐in contaminant detection. Mod Pathol 19947407–411. [PubMed] [Google Scholar]
- 27.Feoli‐Fonseca J C, Oligny L L, Filion M.et al Direct human papillomavirus (HPV) sequencing method yields a novel HPV in a human immunodeficiency virus‐positive Quebec woman and distinguishes a new HPV clade. J Infect Dis 19981781492–1496. [DOI] [PubMed] [Google Scholar]
- 28.Feoli‐Fonseca J C, Oligny L L, Filion M.et al A two‐tier polymerase chain reaction direct sequencing method for detecting and typing human papillomaviruses in pathological specimens. Diagn Mol Pathol 19987317–323. [DOI] [PubMed] [Google Scholar]
- 29.Feoli‐Fonseca J C, Oligny L L, Filion M.et al A putative novel human papillomavirus identified by PCR‐DS. Biochem Biophys Res Commun 199825063–67. [DOI] [PubMed] [Google Scholar]
- 30.Jacobs M V, Snijders P J, van den Brule A J.et al A general primer GP5+/GP6(+)‐mediated PCR‐enzyme immunoassay method for rapid detection of 14 high‐risk and 6 low‐risk human papillomavirus genotypes in cervical scrapings. J Clin Microbiol 199735791–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang H J.et al Human papillomavirus genotyping by a polymerase chain reaction‐based genechip method in cervical carcinoma treated with neoadjuvant chemotherapy plus radical surgery. Int J Gynecol Cancer 200414639–649. [DOI] [PubMed] [Google Scholar]
- 32.Tulvatana W, Bhattarakosol P, Sansopha L.et al Risk factors for conjunctival squamous cell neoplasia:a matched case‐control study. Br J Ophthalmol 200387396–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott I U, Karp C L, Nuovo G J. Human papillomavirus 16 and 18 expression in conjunctival intraepithelial neoplasia. Ophthalmology 2002109542–547. [DOI] [PubMed] [Google Scholar]
- 34.Munoz N, Bosch F X, de Sanjose S.et al Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003348518–527. [DOI] [PubMed] [Google Scholar]
- 35.Brehm A, Kouzarides T. Retinoblastoma protein meets chromatin. Trends Biochem Sci 199924142–145. [DOI] [PubMed] [Google Scholar]
- 36.Jones D L, Munger K. Interactions of the human papillomavirus E7 protein with cell cycle regulators. Semin Cancer Biol 19967327–337. [DOI] [PubMed] [Google Scholar]
- 37.Milde‐Langosch K, Schreiber C, Becker G.et al Human papillomavirus detection in cervical adenocarcinomas by polymerase chain reaction. Hum Pathol 199324590–594. [DOI] [PubMed] [Google Scholar]
- 38.Zhang H S, Postigo A A, Dean D C. Active transcriptional repression by the Rb‐E2F complex mediates G1 arrest triggered by p16INK4a, TGFbeta, and contact inhibition. Cell 19999753–61. [DOI] [PubMed] [Google Scholar]
- 39.Stoler M H. Human papillomavirus and cervical neoplasia: a model for carcinogenesis. Int J Gynecol Pathol 20001916–28. [DOI] [PubMed] [Google Scholar]
- 40.Zerfass‐Thome K, Zwerschke W, Mannhardt B.et al Inactivation of the cdk inhibitor p27KIP1 by the human papillomavirus type 16 E7 oncoprotein. Oncogene 1996132323–2330. [PubMed] [Google Scholar]
- 41.zur Hausen H. Molecular pathogenesis of cancer of the cervix and its causation by specific human papillomavirus types. Curr Top Microbiol Immunol 1994186131–156. [DOI] [PubMed] [Google Scholar]
- 42.Dyson N, Howley P M, Munger K.et al The human papilloma virus‐16 E7 oncoprotein is able to bind the retinoblastoma gene product. Science 1989243934–940. [DOI] [PubMed] [Google Scholar]
- 43.Sherr C J. Cancer cell cycles. Science 19962741672–1677. [DOI] [PubMed] [Google Scholar]
- 44.Kamb A, Gruis N A, Weaver‐Feldhaus J.et al A cell cycle regulator potentially involved in genesis of many tumor types. Science 1994264436–440. [DOI] [PubMed] [Google Scholar]
- 45.Khleif S N, DeGregori J, Yee C L.et al Inhibition of cyclin D‐CDK4/CDK6 activity is associated with an E2F‐mediated induction of cyclin kinase inhibitor activity. Proc Natl Acad Sci USA 1996934350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ansai S, Koseki S, Hozumi Y.et al Assessment of cellular proliferation of sebaceous neoplasms by AgNOR counts and immunohistochemical demonstrations of PCNA and Ki‐67. J Dermatol 199522238–248. [DOI] [PubMed] [Google Scholar]
- 47.Hall P A, Levison D A, Woods A L.et al Proliferating cell nuclear antigen (PCNA) immunolocalization in paraffin sections: an index of cell proliferation with evidence of deregulated expression in some neoplasms. J Pathol 1990162285–294. [DOI] [PubMed] [Google Scholar]
- 48.Mahomed A, Chetty R. Human immunodeficiency virus infection, Bcl‐2, p53 protein, and Ki‐67 analysis in ocular surface squamous neoplasia. Arch Ophthalmol 2002120554–558. [DOI] [PubMed] [Google Scholar]