Abstract
Background/aims
Rebound tonometry (RT) is performed without anaesthesia with a hand held device. The primary aim was to compare RT with Goldmann applanation tonometry (GAT) and to correlate with central corneal thickness (CCT). The secondary aim was to prove tolerability and practicability of RT under “study conditions” and “routine practice conditions.”
Methods
In group 1 (52 eyes/28 patients), all measurements were taken by the same physician, in the same room and order: non‐contact optical pachymetry, RT, slit lamp inspection, GAT. Patients were questioned about discomfort or pain. In group 2 (49 eyes/27 patients), tonometry was performed by three other physicians during routine examinations.
Results
RT was well tolerated and safe. Intraocular pressure (IOP) ranged between 6 mm Hg and 48 mm Hg. No different trends were found between the groups. RT tended to give slightly higher readings: n = 101, mean difference 1.0 (SD 2.17) mm Hg; 84.1% of RT readings within plus or minus 3 mm Hg of GAT; 95% confidence interval in the Bland‐Altman analysis −3.2 mm Hg to +5.2 mm Hg. Both RT and GAT showed a weak positive correlation with CCT (r2 0.028 and 0.025, respectively).
Conclusions
RT can be considered a reliable alternative for clinical screening and in cases where positioning of the head at the slit lamp is impossible or topical preparations are to be avoided.
Keywords: rebound tonometry, applanation tonometry, intraocular pressure, central corneal thickness
Tonometry is a routine ophthalmological procedure that is classically performed with the Goldmann applanation tonometer (GAT). The GAT principle is determining the force needed to flatten a predefined area of the central cornea.1 Although currently the “gold standard,” GAT has its known limitations: influence of central corneal thickness (CCT) and corneal curvature, necessity to support the upper lid during measurement, use of topical anaesthesia and fluorescein staining of the tear film. Rebound (dynamic or impact) tonometry (RT) is a new method based on a completely different principle—analysis of motion parameters of a bouncing probe after colliding with the cornea.2,3 RT has been reported to yield reliable results in rats, mice, dogs, and horses.4,5,6,7,8 The inventor of the device, A Kontiola, has also performed studies in humans.3,9 The new, commercially available device (iCare) is portable and does not require topical anaesthesia or fluorescein, what makes it an attractive measurement alternative.
The primary aim of this study was to compare RT with GAT and to correlate intraocular pressure (IOP) readings with CCT. The secondary aim was to prove tolerability of RT by the patients—that is, whether discomfort or pain were experienced, and also practicability of RT—that is, whether the RT/GAT correlation differed significantly when performed under “study conditions” and under “routine practice conditions.”
Methods
Fifty five subjects were enrolled in the study. All were regular patients of the department of ophthalmology in Bern. Patients were informed about the study and gave consent for their IOP to be checked with the two commercially available devices—the Goldmann applanation tonometer (AT900, Haag‐Streit, Köniz, Switzerland), and the iCare rebound tonometer, approved for clinical use in Switzerland (TA01, Tiolat Oy, Helsinki, Finland).
The physical and mathematical principles of RT are explained in detail in the works of Kontiola.2,3 In short, iCare has a 26.5 mg, single use metal probe with a round plastic tip (radius 0.9 mm). In a magnetic field, the probe is accelerated toward the cornea, its speed being 0.25–0.35 m/s at the time of impact. A microprocessor gauges motion parameters of the probe when it bounces back. The device is held upright in front of the eye. No topical anaesthesia is needed. After each successful measurement, an IOP reading is displayed after passing an internal quality control. Following six good quality measurements, the highest and the lowest values are discarded automatically, and the final IOP is displayed as the average of the remaining four.
Eyes with corneal pathology (for example, epithelial lesions, oedema, scarring, grafts) as well as blind eyes and eyes with ocular hypotony (IOP ⩽5 mm Hg) were excluded from the study. Another exclusion criterion was also a refractive error larger than −6.0 D myopia, +4.0 D hyperopia, or 2.0 D astigmatism.
We differentiated between two groups of patients.
Group 1
Group 1 consisted of 28 patients, 12 males and 16 females. In this group, all measurements were taken in the same room, by the same physician (KK), and in the same order. Firstly, CCT was measured with non‐contact pachymetry using an optical low coherence reflectometer (OLCR‐Pachymeter, Haag‐Streit, Köniz, Switzerland).10,11 IOP was then measured with the rebound tonometer without anaesthesia, beginning with the right eye. Next, both eyes were anaesthetised with one drop of oxybuprocaine 0.2% (Novesin, Novartis Pharma AG, Basel, Switzerland), and fluorescein (Fluorescein Paper, Haag‐Streit AG, Köniz, Switzerland) was applied. Corneas were checked at the slit lamp for epithelial erosions (possibly caused by the rebound tonometry), and then GAT was performed, beginning with the right eye. All procedures (pachymetry, RT, slit lamp inspection, GAT) were performed within 10 minutes. Patients were subsequently questioned as to whether they experienced any discomfort or pain during rebound tonometry. After sharing their experience freely, they had to make a forced choice between no, mild, moderate, or severe discomfort, and no, mild, moderate, or severe pain.
Group 2
Group 2 consisted of 27 patients, 12 males and 15 females. In this group, IOP measurements were taken once with each tonometer during a routine examination. Three other physicians working in different examination rooms with different GATs, but with the same iCare device as for group 1, contributed to data collection. The purpose of group 2 was to test the rebound tonometer in an unbiased clinical setting, corresponding to a normal working environment.
Statistical analysis
Statistical analysis was performed using SPSS for Windows, software version 11.5 (SPSS, Chicago, IL, USA). Wilcoxon rank sum test was used for the comparison of IOP readings. A Bland‐Altman plot was used to evaluate agreement and confidence interval. Significant difference was considered when p<0.05.
Results
Measurements could be performed in one or both eyes of all 55 enrolled patients. Mean age in group 1 (“study conditions”; 28 patients, 52 eyes) was 50.38 (SD 23.58) years. Mean age in group 2 (“routine practice conditions”; 27 patients, 49 eyes) was 56.18 (20.04) years. Twenty patients (40 eyes) were seen because of minor complaints or refractive problems and had no intraocular pathology. The other 35 patients (61 eyes) were included consecutively, respecting the exclusion criteria but not the diagnosis. The latter included various forms of open angle glaucoma as well as postoperative controls after glaucoma or cataract surgery.
Tolerability of rebound tonometry
RT was well tolerated. Following measurement, no fluorescein staining and no corneal epithelial lesions were observed in any of the patients. Sixty four per cent of the interviewed patients in group 1 reported no discomfort at all, 36% felt mild discomfort but no pain; none of the patients indicated moderate or severe discomfort or pain.
IOP readings
The results of the IOP measurements are presented in table 1.
Table 1 Intraocular pressure as measured by rebound tonometry (RT) and Goldmann applanation tonometry (GAT).
| Group 1 | Group 2 | All eyes | All right eyes | All left eyes | ||
|---|---|---|---|---|---|---|
| (n = 52) | (n = 49) | (n = 101) | (n = 47) | (n = 54) | ||
| RT (mm Hg) | mean (SD) | 17.95 (5.11) | 17.06 (6.40) | 17.52 (5.76) | 17.03 (4.49) | 17.98 (6.76) |
| range | 8–35 | 8–46 | 8–46 | 8–28 | 8–46 | |
| GAT (mm Hg) | mean (SD) | 16.67 (5.09) | 16.35 (6.24) | 16.51 (5.65) | 16.33 (4.0) | 16.69 (6.89) |
| range | 6–34 | 7–48 | 6–48 | 7–28 | 6–48 | |
| Difference | mean (SD) | 1.28 (2.15) | 0.71 (2.17) | 1.00 (2.17) | 0.70 (2.38) | 1.29 (1.92) |
| RT − GAT | range | −4 to +6 | −5 to +5 | −5 to +6 | −5 to +6 | −3 to +5 |
| p value | 0.0018 | 0.032 | 0.0017 | 0.048 | 0.0004 | |
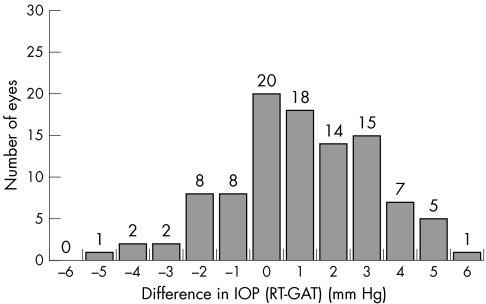
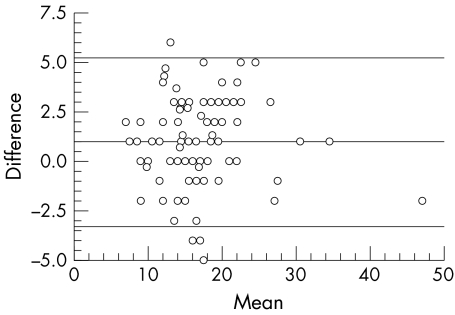
There was a small but statistically significant difference between RT and GAT that was to be traced when each group was evaluated separately, when both groups were taken together, or when only the right and only the left eyes were considered. On average, RT tended to give slightly higher readings than GAT (mean difference 1.0 (2.17) mm Hg; n = 101). The deviation of RT from GAT was skewed towards the positive values and ranged between −5 mm Hg and +6 mm Hg; 67.3% of RT readings were lying within plus or minus 2 mm Hg, and 84.1% (85/101 eyes) within plus or minus 3 mm Hg of GAT (fig 1). Measured IOP ranged between 6 mm Hg and 48 mm Hg Correlation between RT and GAT did not seem to be influenced by the IOP level. Figure 2 presents the Bland‐Altman plot for all 101 eyes. The agreement between the two methods was moderate with a systematic deviation of +1.0 mm Hg. The 95% confidence interval was between −3.2 mm Hg and +5.2 mm Hg. When the two groups of patients were compared, no significant difference was found in the way RT correlated with GAT. Scatter plots and Bland‐Altman analysis12 were performed for each group separately (not shown), but no different trends from those reported above were found.
Figure 1 Histogram: distribution of the IOP difference between rebound tonometry (RT) and Goldmann applanation tonometry (GAT); n = 101.
Figure 2 Bland‐Altman plot: mean IOP (RT+GAT/2) versus their difference (RT − GAT). The 95% confidence interval (SD 1.96) lies between −3.2 mm Hg and +5.2 mm Hg. Mean 1.0 mm Hg.
Correlation with central corneal thickness
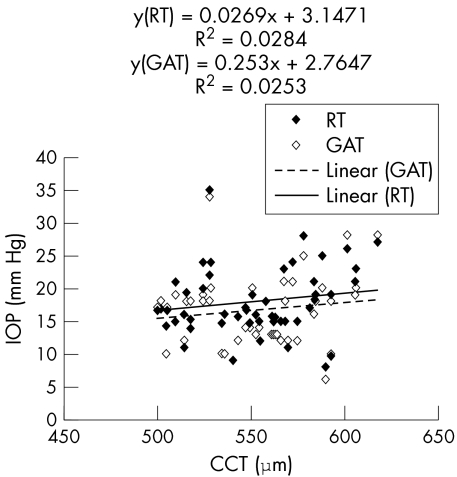
Mean CCT (n = 52, group 1) was 550.8 (32.0) μm, range 500.0–617.4 μm. The scatter plot of IOP versus CCT (fig 3) demonstrated a slight increase of RT as well as GAT readings with increasing CCT (r2 for RT = 0.0284; r2 for GAT = 0.0253), with no difference between the two tonometry methods with respect to their correlation with CCT.
Figure 3 Correlation of rebound tonometry (RT) and Goldmann applanation tonometry (GAT) with central corneal thickness (CCT); n = 52, group 1.
Discussion
The rebound tonometer is a novel, portable device that contacts the cornea but does not require topical anaesthesia or use of fluorescein and has been proved to be useful in various animal experiments.4,5,6,7,8 The clinical necessity of such a tonometry method can be seen in screening settings, in people who have difficulties in positioning their head on the slit lamp (children, wheelchair, obese), and in cases where eye drops have to be avoided.13 Non‐contact, air puff tonometers14,15 require positioning of the head on a headrest; the TonoPen XL and the Perkins tonometers are portable, but require topical anaesthesia.16,17
In this study, RT proved to be a safe and well tolerated contact procedure. We recorded no serious complaints from the patients, and were unable to find any lesions of the corneal epithelium following measurement. Further, RT results and the relation to GAT were of the same order when performed under controlled conditions and in a real life clinical setting. We conclude this to be good evidence of RT practicability. A disadvantage can be seen in the fact that the device needs to be held upright in front of the eye and therefore cannot be used in people in a supine or tilted position.
Agreement of IOP readings between RT and GAT was moderate to good. There was a systematic trend of RT to give higher readings, with 84% of the measurements falling within plus or minus 3 mm Hg from GAT. This relation was valid for a wide range of pressures. The results are thus comparable with, and even better than, those of other non‐applanation tonometers.5,14,15,16,17
Central corneal thickness seems to influence IOP readings in RT as it does in GAT, and should be considered when interpreting RT measurements in a clinical setting.
In conclusion, the iCare tonometer can be considered a reliable alternative to current tonometry devices for clinical application.
Abbreviations
CCT - central corneal thickness
GAT - Goldmann applanation tonometry
IOP - intraocular pressure: RT, rebound tonometry
Footnotes
Financial interest: none.
Competing interest: none.
References
- 1.Goldmann H, Schmidt T H. Über Applanations‐tonometrie. Ophthalmologica 1957134221–242. [DOI] [PubMed] [Google Scholar]
- 2.Kontiola A I. A new electromechanical method for measuring intraocular pressure. Doc Ophthalmol 199793265–276. [DOI] [PubMed] [Google Scholar]
- 3.Kontiola A I. A new induction‐based impact method for measuring intraocular pressure. Acta Ophthalmol Scand 200078142–145. [DOI] [PubMed] [Google Scholar]
- 4.Kontiola A I, Goldblum D, Mittag T.et al The induction/impact tonometer: a new instrument to measure intraocular pressure in the rat. Exp Eye Res 200173781–785. [DOI] [PubMed] [Google Scholar]
- 5.Goldblum D, Kontiola A I, Mittag T.et al Non‐invasive determination of intraocular pressure in the rat eye. Comparison of an electronic tonometer (TonoPen), and a rebound (impact probe) tonometer. Graefes Arch Clin Exp Ophthalmol 2002240942–946. [DOI] [PubMed] [Google Scholar]
- 6.Danias J, Kontiola A I, Filippopoulos T.et al Method for the noninvasive measurement of intraocular pressure in mice. Invest Ophthalmol Vis Sci 2003441138–1141. [DOI] [PubMed] [Google Scholar]
- 7.Knollinger A M, La Croix N C, Barrett P M.et al An evaluation of the TonoVet rebound tonometer for measuring intraocular pressure in dogs and horses. Invest Ophthalmol Vis Sci 2005464845. [DOI] [PubMed] [Google Scholar]
- 8.Filippopoulos T, Matsubara A, Danias J.et al Accuracy and sensitivity of two non‐invasive tonometers for the measurement of intraocular pressure in the mouse. Invest Ophthalmol Vis Sci 2005461257 [Google Scholar]
- 9.Kontiola A I, Puska P. Measuring intraocular pressure with the Pulsair 3000 and Rebound tonometers in elderly patients without an anesthetic. Graefes Arch Clin Exp Ophthalmol 20042423–7. [DOI] [PubMed] [Google Scholar]
- 10.Böhnke M, Wälti R, Lindgren F.et al Measuring corneal thickness in photo‐keratectomy with a reflectometry incorporated into the laser beam of the excimer laser. Klin Monatsbl Augenheilkd 1998212367–371. [DOI] [PubMed] [Google Scholar]
- 11.Ventura A C S, Böhnke M, Mojon D S. Central corneal thickness measurements in patients with normal tension glaucoma, primary open angle glaucoma, pseudoexfoliation glaucoma, or ocular hypertension. Br J Ophthalmol 200185792–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bland J M, Altman D G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 19861307–310. [PubMed] [Google Scholar]
- 13.Martinez‐de‐la‐Casa J, Garcia‐Feijoo J, Vico E.et al Comparison of dynamic contour, rebound and standard Goldmann applanation tonometry. Invest Ophthalmol Vis Sci 2005464852 [Google Scholar]
- 14.Hansen M K. Clinical comparison of the XPERT non‐contact tonometer and the conventional Goldmann applanation tonometer. Acta Ophthalmol Scand 199573176–180. [DOI] [PubMed] [Google Scholar]
- 15.Brencher H L, Kohl P, Reinke A R.et al Clinical comparison of air‐puff and Goldmann tonometers. J Am Optom Assoc 199162395–402. [PubMed] [Google Scholar]
- 16.Iester M, Mermoud A, Achache F.et al New Tonopen XL: comparison with the Goldmann tonometer. Eye 20011552–58. [DOI] [PubMed] [Google Scholar]
- 17.Garcia Resua C, Giraldez Fernandez M J, Cervino Exposito A.et al Clinical evaluation of the new TGDc‐01 “PRA” palpebral tonometer: comparison with contact and non‐contact tonometry. Optom Vis Sci 200582143–150. [DOI] [PubMed] [Google Scholar]