Two patients with haematological malignancies presented with visual symptoms during therapy with denileukin diftitox. Both experienced loss of central and colour vision, coarse pigmentary macular changes, and decreased electroretinographic amplitudes. These cases raise the possibility of a novel drug associated retinopathy.
Case 1
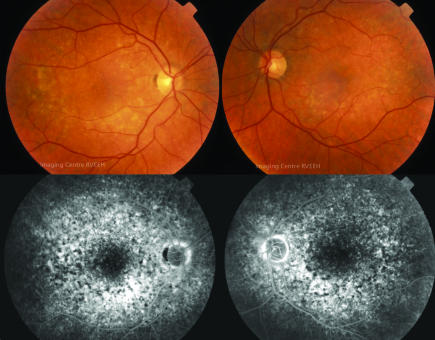
A 58 year old man with chronic lymphocytic leukaemia was enroalled in a clinical trial of denileukin diftitox (Ontak, Ligand Pharmaceuticals) receiving 10 standard cycles of therapy with good response.1 Eight months later his disease progressed and he received four further cycles. During this treatment he noted blurred vision, photopsias and difficulty adjusting to bright lights. Acuity was 6/12 bilaterally and coarse macular pigment changes were present (fig 1). Automated fields showed paracentral scotomas and electroretinograms (ERG) revealed delayed and diminished photopic wave forms with preserved scotopic responses. Brain magnetic resonance imaging (MRI) and cerebrospinal fluid (CSF) were normal.
Figure 1 Colour fundus photographs and mid‐phase fluorescein angiogram reveals marked macular pigmentary change in patient 1.
After 1 month of visual symptoms high serum levels of retinal α‐enolase antibodies were detected. Antibodies to recoverin were absent. Denileukin diftitox was stopped, he received plasmapheresis, pooled human immunoglobulin, and high dose prednisolone. There was no visual improvement though anti‐enolase testing became negative. Nine months later he was diagnosed with metastatic adenocarcinoma of unknown primary from which he died 2 months later.
Case 2
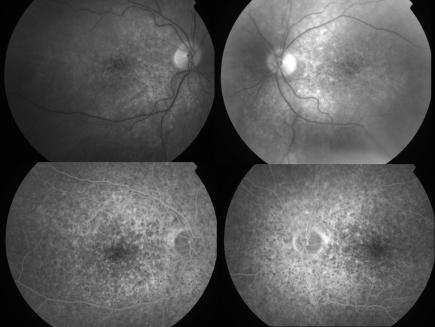
A 62 year old man with relapsed primary cutaneous T cell lymphoma (CTCL) was enrolled into a trial of denileukin diftitox and received four cycles.2 His lymphoma responded to treatment; however, he noted gradual blurring of vision. Visual acuity was 6/18 bilaterally and coarse macular pigmentary changes were present (fig 2). Brain MRI and CSF were normal. Electrophysiology revealed reduced photopic and scotopic amplitudes. Retinal antibodies against 30 kDa, 35 kDa, and 46 kDa (enolase) retinal proteins were detected. He died 2 months later from liver failure.
Figure 2 Red free photographs and mid‐phase fluorescein angiogram of patient 2.
Comment
Denileukin diftitox is a fusion protein of portions of diphtheria toxin and interleukin 2. It targets IL‐2 receptors on malignant cells and induces cell death. It is approved for the treatment of CTCL and trials in other malignancies continue. Both patients experienced visual symptoms concurrent with their denileukin diftitox therapy and were receiving no other chemotherapy agents. They had strikingly similar macular changes, ERG loss, and anti‐enolase in their sera.
There exists a theoretical link between denileukin diftitox and autoimmune retinal disease. A subset of regulatory T cells crucial in suppressing autoimmune disease also express the IL‐2 receptor subunit known as CD25.3 In a proportion of mice depleted of this regulatory subset of T cells, loss of self tolerance to retinal antigens has been reported.4
We considered other possible causes of these retinal changes including malignant infiltration and paraneoplastic retinopathy. Infiltration was excluded on the clinical fundus appearance, and the absence of progressive disease in CSF. Both fit the phenotype of cone loss that Weleber found in seven of 12 patients with presumed anti‐enolase retinopathy,5 but our patients' macular changes are distinctive. In the three reported cases of paraneoplastic retinopathy associated with haematological malignancy,6,7,8 none was associated with CLL or CTCL. Some pigmentary changes were described in these cases, but in a pattern quite different from that seen in our patients.
To date of approximately 6000 patients treated with denileukin diftitox the manufacturer has only nine reports of visual disturbance including these patients (data on file at Ligand Pharmaceuticals). Formal review of these reports has not revealed a clear pattern of ocular involvement. However, the similarity of the two cases reported here is striking, and raises the possibility of a novel toxic retinopathy.
Acknowledgements
We gratefully acknowledge the assistance of Ligand Pharmaceuticals in providing information from their database and reviewing the manuscript.
References
- 1.Morgan S J, Seymour J F, Prince H M.et al Confirmation of the activity of the interleukin‐2 fusion toxin denileukin diftitox against chemorefractory chronic lymphocytic leukemia, including cases with chromosome 17p deletions and without detectable CD25 expression. Clin Cancer Res 2004103572–3575. [PubMed] [Google Scholar]
- 2.Olsen E, Duvic M, Frankel A.et al Pivotal phase III trial of two dose levels of denileukin diftitox for the treatment of cutaneous T‐cell lymphoma. J Clin Oncol 200119376–388. [DOI] [PubMed] [Google Scholar]
- 3.Thornton A M. Signal transduction in CD4+CD25+ regulatory T cells: CD25 and IL‐2. Front Biosci 200611921–927. [DOI] [PubMed] [Google Scholar]
- 4.Takeuchi M, Keino H, Kezuka T.et al Immune responses to retinal self‐antigens in CD25(+)CD4(+) regulatory T‐cell‐depleted mice. Invest Ophthalmol Vis Sci 2004451879–1886. [DOI] [PubMed] [Google Scholar]
- 5.Weleber R G, Watzke R C, Shults W T.et al Clinical and electrophysiologic characterization of paraneoplastic and autoimmune retinopathies associated with antienolase antibodies. Am J Ophthalmol 2005139780–794. [DOI] [PubMed] [Google Scholar]
- 6.To K W, Thirkill C E, Jakobiec F A.et al Lymphoma‐associated retinopathy. Ophthalmology 20021092149–2153. [DOI] [PubMed] [Google Scholar]
- 7.Adamus G, Ren G, Weleber R G. Autoantibodies against retinal proteins in paraneoplastic and autoimmune retinopathy. BMC Ophthalmol 200445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sen H N, Chan C C, Caruso R C.et al Waldenstrom's macroglobulinemia‐associated retinopathy. Ophthalmology 2004111535–539. [DOI] [PubMed] [Google Scholar]