Abstract
Aim
To investigate depth perception in glaucoma suspects compared to glaucoma patients and controls.
Methods
Glaucoma suspects (n = 16), patients (n = 18), and normal age matched controls (n = 19) aged 40–65 years were prospectively evaluated for depth perception deficits using the Frisby test. Stereoacuity was measured by stereothreshold in seconds of arc for each group.
Results
Glaucoma suspects showed significantly increased mean stereothreshold compared to age matched normals (144.1 (SE 35.2) v 26.6 (3.7) seconds of arc; p = 0.0004). The mean stereothreshold in glaucoma patients was also increased compared to age matched normals 148.1 (33.8) v 26.6 (3.7) seconds of arc; p = 0.0004).
Conclusions
Glaucoma suspects show depth perception deficits. The impaired stereovision in glaucoma suspects suggests that binocular interactions can be disrupted in the presence of normal visual fields by standard achromatic automated perimetry.
Keywords: glaucoma, visual dysfunction, visual cortex, binocular function, stereopsis, central visual pathways
Glaucoma is a leading cause of irreversible vision loss, and its pathological correlate is the loss of retinal ganglion cells and their axons.1 Current assessment of vision loss in glaucoma is performed in a standardised way with static perimetry that tests each eye separately.2 There are also important visual functions that require interaction of inputs from both eyes; however, studies of binocular visual function in patients with glaucoma are limited in number and vary in their methodology.3,4,5,6 Among these functions, stereopsis, important for some daily activities, is a true binocular function that involves input from both eyes with the first integration at the level of the striate and extrastriate visual cortex.7
Two studies have examined stereopsis in glaucoma, and both reported stereopsis deficits in glaucoma patients. However, in glaucoma suspects, stereopsis deficits were detected in one study4 and not in the other.3 These studies used differing depth perception tests of dichoptically presented stimuli8—namely, the random dot3 and line4 stereograms. Random dot patterns eliminate monocular cues to depth; however, the test requires fusion, and this can be abnormal in some people with otherwise normal stereopsis.9 Line stereogram testing may allow depth to be inferred from monocular cues. In contrast with these “apparent” depth perception tests,8 the Frisby test of stereopsis presents real depth to the subject in the form of different plate thicknesses, such that the problems associated with dichoptically presented stimuli, including fusion, are avoided. Here we investigate depth perception in glaucoma suspects using the Frisby test, and compare the results with age matched controls and glaucoma patients.
Subjects and methods
Institutional approval was obtained from the research ethics board, St Michael's Hospital. Prospective evaluation of three subject groups was performed—namely, glaucoma suspects (n = 16), glaucoma patients (n = 18), and age matched controls (n = 19) (table 1).
Table 1 Characteristics of control, suspect, and glaucoma groups (mean (SE)).
| Control | Suspect | Glaucoma | Significance | |
|---|---|---|---|---|
| Number | 19 | 16 | 18 | – |
| Age (years) | 50.79 (1.50) | 56.50 (1.84) | 54.72 (1.98) | p>0.05 |
| Visual acuity | 0.88 (0.02) | 0.82 (0.03) | 0.81 (0.03) | p>0.05 |
| Interpupillary distance (mm) | 60.84 (0.78) | 63.00 (0.88) | 60.94 (0.85) | p>0.1 |
| Cup/disc ratio | 0.34 (0.03) | 0.49 (0.05) | 0.66 (0.04) | p<0.05 |
Patients included in the study were examined by a glaucoma specialist. Patients were required to be between the ages of 40 years and 65 years, with no history of neurological disease, orthotropic by near and far cover‐uncover tests, with a minimum best corrected visual acuity of 20/30 in each eye, differing by no greater than one Snellen acuity line, and no history of incisional surgery within the previous 4 months. Primary open angle glaucoma was defined by characteristic glaucomatous optic nerve head findings and required the presence of corresponding visual field deficits in one or both eyes. Glaucoma suspects were required to have a normal Humphrey 24‐2 visual field and intraocular pressure (IOP) at least ⩾24 mm Hg or optic nerve head findings suspicious for glaucoma. All control subjects were age matched, with no incisional surgical history, and underwent a full eye examination. Control subjects were recruited from hospital personnel or accompanying relatives of patients, and had normal eye examinations. One control subject had a history of laser peripheral iridotomy. Glaucoma suspects had no history of incisional ocular surgery, and two patients had a history of laser peripheral iridotomy. Of the patients with glaucoma, nine had no history of eye surgery, five had cataract and trabeculectomy surgery in each eye, three patients had trabeculectomy surgery in one eye, and a single patient had cataract surgery in one eye. The interpupillary distance was measured for all patients.
Stereoacuity measurement
Informed consent was obtained from patients. Stereoscopic visual acuity was assessed using the Frisby stereotest (Clement Clarke International Ltd, Essex, UK) according to the instructions of the manufacturer. The Frisby stereotest consists of three plates of Perspex glass measuring 1 mm, 3 mm, and 6 mm in thickness. This test was performed for all patients under the same conditions, and subjects had no previous experience with this type of test. Individuals performing the testing were not aware of the diagnosis. The subject's head was placed on a chin rest to prevent head movement, and plates of varying thickness were shown perpendicular to the visual axis at several viewing distances taking care to avoid plate movement. The patient was instructed to close his/her eyes before testing and between plate presentations. The subject was asked to report which of the four quadrants contained the circle in depth, and stereoacuities were recorded with a range of values from 20 seconds of arc to 340 seconds of arc. The lowest disparity which the patient can reliably discriminate was recorded and this stereothreshold was a measure of stereoacuity.
The 6 mm plate was shown at a viewing distance of 80 cm. If the answer was correct, the 3 mm plate was presented at the same distance. If the answer was incorrect for the 3 mm plate, a score of 85 seconds of arc was recorded. If the answer was correct, the 1 mm plate was presented. If the answer was incorrect, a score of 40 seconds of arc was recorded, and if the answer was correct, a score of 20 seconds of arc was recorded.
If the answer was incorrect for the 6 mm plate at 80 cm, the same plate was viewed at a distance of 60 cm. If the answer was correct, a score of 150 seconds of arc was recorded; while if the answer was incorrect, the 6 mm plate was presented at 40 cm. If the answer was correct, a score of 340 seconds of arc was recorded. If the subject could not detect the quadrant with depth effect at this distance, the highest stereothreshold value tested (340 seconds of arc) was assigned for statistical analysis.
Statistical analysis
All data were tested for normality using software (GraphPad, San Diego, CA, USA). For parameters with Gaussian distributions such as inter‐pupillary distance, visual acuity, and age, the ANOVA test was used to make comparisons among the three groups. If there was a significant difference among groups, Bonferroni's multiple comparison tests were used to compare glaucoma patients to normals and suspects to normals. For the stereovision threshold which does not show Gaussian distribution, the non‐parametric Kruskall‐Wallis test was used to compare stereothreshold values among the three groups (glaucoma patients, suspects, and normals), followed by Dunn's multiple comparison tests used to compare glaucoma patients with normals, and suspects with normals. Data are presented as mean (SE).
Results
No differences in interpupillary distance, age, or visual acuity were found among the three groups (p>0.05) (table 1). There was a significant difference between the mean cup/disc ratio between controls and suspects and controls and glaucoma (p<0.05).
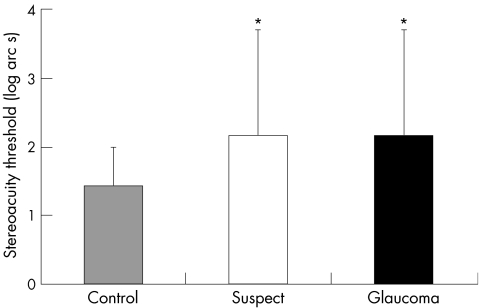
The mean stereothreshold of the glaucoma suspect and age matched control groups were 144.1 (SE 35.2) and 26.6 (3.7) seconds of arc, respectively. The mean stereothreshold in glaucoma suspects was significantly higher compared to controls (p = 0.0004) (fig 1).
Figure 1 Mean stereothreshold in seconds of arc (SE) for control (n = 19), suspect (n = 16), and glaucoma (n = 18) subjects.
The mean stereothreshold of the glaucoma and age matched control groups were 148.1 (SE 33.8) seconds of arc and 26.6 (3.7) seconds of arc, respectively. The mean stereothreshold in glaucoma patients was significantly higher compared to controls (p = 0.0004) (fig 1).
Figure 2 shows the frequency distribution of stereoacuity measurements across the three groups. Ninety five per cent of control subjects showed stereothresholds between 20–40 seconds of arc, compared to 44% of patients in both suspect and glaucoma groups. Stereothresholds of ⩾340 seconds of arc were seen only in suspect (31%) and glaucoma patients (33%).
Figure 2 Frequency distributions of stereothesholds (arc seconds) in control, suspect, and glaucoma groups.
Discussion
In this study, depth perception in control subjects using the Frisby test was similar to results obtained using other depth perception tests.10 The mean stereothreshold of 27 seconds of arc observed in control subjects was similar to mean stereothresholds of 16 and 27 seconds of arc observed in control subjects between 20 years and 50 years, and between 60 and 70 years, respectively.10
This study confirms that stereopsis is reduced in glaucoma using a different test of real depth perception.3,4 In fact, our finding of stereoacuity of 148 seconds of arc is similar to the 148 seconds of arc reported for glaucoma patients by Bassi and Galanis.3 Furthermore, reduced stereopsis in our glaucoma patients was found in a relatively younger group with mean age of 55 years compared to 62 years3 and 63 years.4 This finding supports that impaired depth perception seen in glaucoma patients is related to the disease process rather than age related visual dysfunction.10
We observed significantly reduced stereopsis in glaucoma suspects, with a mean stereothreshold of 144 seconds of arc, and this is in keeping with the 118 seconds of arc reported by Essock and co‐workers.4 This effect was seen in our study in a younger group of glaucoma suspects with mean age of 57 years compared to 61 years.4 It is not clear why stereopsis deficits were not observed in glaucoma suspects in another paper in which suspects had significantly higher intraocular pressure and cup to disc ratios compared to controls.3 It is possible that the random dot stereogram test used in that study and known to lend itself to monocular cues, may have underestimated any decreased depth perception in the glaucoma suspects.
We obtained similar results of reduced stereoacuity in both suspect and glaucoma groups, and this was not related to reduced visual acuity as the visual acuities of suspect and glaucoma were similar to that seen in normals. The Frisby test is presented to the subject in the form of different plate thicknesses with great care to avoid head and plate movement, such that the problems associated with dichoptically presented stimuli are avoided. The finding of reduced depth perception in glaucoma suspects suggests glaucomatous disease and related pre‐perimetric visual dysfunction. We cannot exclude the possibility that a non‐glaucomatous process is also involved. Further studies of a larger sample size with reduced standard deviation may help to better understand impaired depth perception in glaucoma suspects.
The neuronal basis of stereovision depends on disparity cells sensitive to binocular disparity, located in the primary visual cortex and extrastriate areas.7 Marked stereovision deficits in glaucoma suspects with normal achromatic visual fields suggest disrupted binocular vision activities. The causes of profound disruption of stereoacuity in suspects and glaucoma patients are not yet known. Spatial sampling array disorder at the retinal ganglion cell level has been previously proposed.4 There is evidence that the neuropathology of glaucoma extends beyond retinal ganglion cells to include geniculo‐cortical pathways in experimental monkey11,12,13,14,15,16,17 and human18 glaucoma. We suggest the additional interpretation that loss of stereoacuity in glaucoma suspects and glaucoma may be the result of the relative delay of input from one eye compared to input from the other eye, as seen with cortical visual evoked potentials in glaucoma suspects and patients.19,20 This may affect binocular interactions taking place at the level of the visual cortex, where neural degeneration in human glaucoma has been observed.18 Further studies of binocular functions are needed to understand visual dysfunction in glaucoma suspects and may be helpful in uncovering early disease.
Acknowledgements
The authors thank A Elefano for excellent technical assistance. This work was supported in part by the Suek Fund.
Abbreviations
IOP - intraocular pressure
SE - standard error
References
- 1.Weinreb R N, Khaw P T. Primary open‐angle glaucoma. Lancet 20043631711–1720. [DOI] [PubMed] [Google Scholar]
- 2.Sample P A, Johnson C A. Functional assessment of glaucoma. J Glaucoma 200110(Suppl 1)S49–S52. [DOI] [PubMed] [Google Scholar]
- 3.Bassi C J, Galanis J C. Binocular visual impairment in glaucoma. Ophthalmology 1991981406–1411. [DOI] [PubMed] [Google Scholar]
- 4.Essock E A, Fechtner R D, Zimmerman T J.et al Binocular function in early glaucoma. J Glaucoma 19965395–405. [PubMed] [Google Scholar]
- 5.Piltz J R, Swindale N V, Drance S M. Vernier thresholds and alignments bias in control, suspect and glaucomatous eyes. J Glaucoma 1993287–95. [PubMed] [Google Scholar]
- 6.McKendrick A M, Johnson C A, Anderson A G.et al Elevated vernier acuity thresholds in glaucoma. Investigative Ophthalmol Vis Sci 2002431393–1399. [PubMed] [Google Scholar]
- 7.Parker A J, Cumming B G. Cortical mechanisms of binocular stereoscopic vision. Prog Brain Res 2001134205–216. [DOI] [PubMed] [Google Scholar]
- 8.Lang J, Rechichi C, Sturmer J. Natural versus haploscopic stereopsis. Graefes Arch Clin Exp Ophthalmol 1991229115–118. [DOI] [PubMed] [Google Scholar]
- 9.Howard I P, Rogers B J.Binocular vision and stereopsis. New York: Oxford University Press, 1995
- 10.Brown B, Yap M K, Fan W C. Decrease in stereoacuity in the seventh decade of life. Ophthalmic Physiol Opt 199313138–142. [DOI] [PubMed] [Google Scholar]
- 11.Yücel Y H, Zhang Q, Gupta N.et al Loss of neurons in magnocellular and parvocellular layers of the lateral geniculate nucleus in glaucoma. Arch Ophthalmol 2000118378–384. [DOI] [PubMed] [Google Scholar]
- 12.Yücel Y H, Zhang Q, Weinreb R N.et al Atrophy of relay neurons in magno‐ and parvocellular layers in the lateral geniculate nucleus in experimental glaucoma. Invest Ophthalmol Vis Sci 2001423216–3222. [PubMed] [Google Scholar]
- 13.Gupta N, Yücel Y H. Glaucoma and the brain. J Glaucoma 200110(Suppl 1)S28–S29. [DOI] [PubMed] [Google Scholar]
- 14.Crawford M L, Harwerth R S, Smith E L., 3rdet al Experimental glaucoma in primates: changes in cytochrome oxidase blobs in V1 cortex. Invest Ophthalmol Vis Sci 200142358–364. [PubMed] [Google Scholar]
- 15.Yücel Y H, Zhang Q, Weinreb R N.et al Effects of retinal ganglion cell loss on magno‐, parvo‐, koniocellular pathways in the lateral geniculate nucleus and visual cortex in glaucoma. Prog Retin Eye Res 200322465–481. [DOI] [PubMed] [Google Scholar]
- 16.Lam D Y, Kaufman P L, Gabelt B T.et al Neurochemical correlates of cortical plasticity after unilateral elevated intraocular pressure in a primate model of glaucoma. Invest Ophthalmol Vis Sci 2003442573–2581. [DOI] [PubMed] [Google Scholar]
- 17.Gupta N, Yücel Y H. Brain changes in glaucoma. Eur J Ophthalmol 200313(Suppl 3)S32–S35. [DOI] [PubMed] [Google Scholar]
- 18.Gupta N, Ang L ‐ C, Noël de Tilly L.et al Human glaucoma and neural degeneration in the intra‐cranial optic nerve, lateral geniculate nucleus and visual cortex of the brain. Br J Ophthalmol 200690674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bergua A, Horn F K, Martus P.et al Stereoscopic visual evoked potentials in normal subjects and patients with open‐angle glaucomas. Graefes Arch Clin Exp Ophthalmol 2004242197–203. [DOI] [PubMed] [Google Scholar]
- 20.Parisi V. Impaired visual function in glaucoma. Clin Neurophysiol 2001112351–358. [DOI] [PubMed] [Google Scholar]