Abstract
Aim
To determine the rate of development of ocular disease in patients presenting with mucous membrane pemphigoid (MMP) involving their oral mucosa.
Methods
Diagnosis of oral MMP was made on the basis of clinical signs, histology, and direct and indirect immunofluorescence. Age, race, sex, age at diagnosis, progression of eye signs, duration of follow up, and time to progression of ocular disease were recorded.
Results
30 patients with established oral MMP were reviewed. The mean age at diagnosis was 65.2 years (range 46–84 years) and 16/30 (53%) were male. At initial ocular review nine (30%) patients showed ocular signs of pemphigoid, of whom two had mild (IIA IIIB), four moderate (IIB IIIC), and three severe (IIC IIID) disease. The mean interval between diagnosis of oral MMP and first ophthalmic review was 19.3 months (range 0–144). Over the period of follow up two (7%) patients developed ocular disease at 19 months and 48 months, respectively, despite having had no evidence of ocular involvement at presentation. In total, 11 (37%) patients with oral disease eventually showed ocular disease with a calculated incidence rate for the development of ocular disease of 0.03 per person year over 5 years.
Conclusions
MMP may affect different tissues at different stages, often separated by many years. Patients with MMP involving their oral mucosa are at significant risk of developing ocular disease and should remain under ophthalmic review.
Keywords: pemphigoid, mucous membrane pemphigoid, ocular disease, oral mucosa
Mucous membrane pemphigoid (MMP) is a group of autoimmune, chronic inflammatory, subepithelial blistering diseases predominantly affecting mucous membranes. It is characterised by a type II hypersensitivity reaction against bullous pemphigoid 180 (BP180),1 laminin 52 and β4 integrin3 as the predominant antigens,4 with linear deposition of IgG, IgA, or C3 along the epithelial basement membrane zone (BMZ).
Oral, ocular, genital, nasopharyngeal, anogenital, and laryngeal mucosae may all be affected, with ocular (60.1%) and oral (90.2%) involvement the most common.5 MMP is associated with subepithelial damage and scarring of the mucosal surfaces, which can be life threatening if the trachea or oesophagus is involved, or sight threatening if the eye is involved.6 The condition has been referred to by many other names including cicatricial pemphigoid, benign MMP, oral pemphigoid, desquamative gingivitis, ocular cicatricial pemphigoid. Some cases of MMP are designated linear IgA bullous dermatosis and epidermolysis bullosa acquisata based on the IgA class and target antigen (type VII collagen) of their anti‐basement membrane antibodies. The most common mimics of ocular MMP are chronic conjunctivitis, chemical injuries, drug toxicities, Sjögren syndrome and sarcoidosis.7 In MMP, direct immunofluorescence (DIF) demonstrates deposits of IgG and C3 at the dermoepidermal junction and circulating autoantibodies may be demonstrated on indirect immunofluorescence (IIF).8 In patients in whom the initial conjunctival biopsy was negative, biopsy of an extraocular site has been shown to be informative in establishing the diagnosis.9
Systemic treatment for MMP is indicated if the disease involves the eye, larynx, or oesophagus. Dapsone, prednisolone, cyclophosphamide, azathioprine, or methotrexate are agents most often used.10 Newer treatment regimens include antibiotics, nicotinamide, and intravenous immunoglobulins.11,12
The incidence of MMP in western Europe is approximately one new case per million inhabitants per year.13 In large series, up to 80% of patients with MMP have been shown to have ocular involvement.14,15 It is not clear, however, why some patients with localised disease remain stable for years even in the absence of systemic therapy, while others develop rapidly progressive ocular involvement despite immunosuppression. Previous studies have estimated the risk of developing ocular involvement in patients with isolated oral MMP at 5% per person year.9 In this study we wished to determine what proportion of our patients with oral MMP developed ocular involvement and over what period of time.
Methods
Patient population
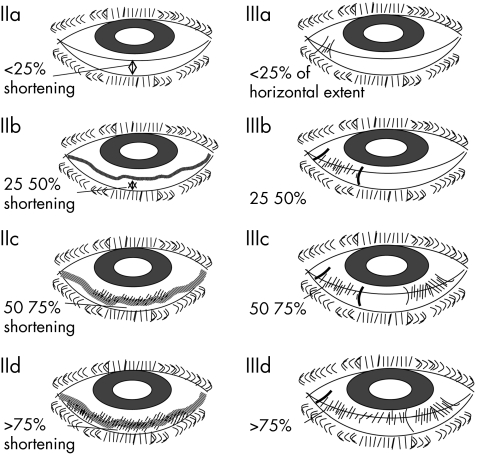
Since 1994, all patients who were diagnosed with MMP involving the oral mucosa have been referred by the department of oral medicine to the corneal and external eye disease service. All patients were examined for signs of ocular MMP and graded using the modified Foster staging described by Tauber et al16 (fig 1). Patients with oral MMP who did not have ocular involvement were seen every 6–12 months. Patients with ocular involvement were closely monitored and offered treatment.
Figure 1 Tauber/Foster grading system3 used to grade the severity of ocular involvement. Grade I not shown.
Diagnosis
A biopsy, usually of the buccal mucosa, was examined histologically and by DIF to detect complement or immunoglobulins at the BMZ. IIF was used to detect circulating autoantibodies to the BMZ using human salt‐split skin substrate. Diagnosis of MMP was based on clinical findings, histology and, in most cases, results of DIF and IIF showing linear deposition of IgG, IgA, or C3 or circulating autoantibodies. As this was a retrospective study, some of our patients with more longstanding diagnoses did not have DIF or IIF as this had not been available at the time of diagnosis. These patients had been diagnosed on the basis of clinical findings and histology.
Data collection
Information on all patients who had been seen at the department of oral medicine and diagnosed with MMP was collected by means of a retrospective case note review and entered into a database. The data collected included sex, age at diagnosis, method of diagnosis (clinical, histology, direct IF, indirect IF), results of direct and indirect IF, involvement of other sites, duration of follow up, duration from diagnosis of oral MMP to ocular review, presence and severity of ocular involvement, and progression of ocular involvement.
Results
Thirty patients were reviewed, 16 (53%) male and 14 (47%) female. The mean age at diagnosis was 65.23 years (range 46–84 years.). Of these 30 patients 29 (97%) were white and one was a Chinese male. The mean duration of follow up since initial diagnosis of oral MMP was 5.8 years (range 1–14 years). 33% (10/30) had had a delay of more than 12 months between onset of oral symptoms and diagnosis (table 1).
Table 1 Demographics of the study population, duration of disease, IF results, etc.
| Overall (n = 30) | MMP (oral only) (n = 19) | MMP (ocular and oral)(n = 11) | |
|---|---|---|---|
| Age at diagnosis (years) | |||
| Mean | 65.23 | 65.16 | 65.36 |
| Range | 46–84 | 46–84 | 51–81 |
| Sex (% men) | 16/30 (53%) | 11/19 (58%) | 5/11 (45.5%) |
| Race (% white) | 29/30 (97%) | 19/19 (100%) | 10/11 (91%) |
| Diagnostic delay >12/12 | |||
| Yes | 10 (33%) | 5 (26%) | 5 (45.5%) |
| No | 13 (43%) | 9 (48%) | 4 (36%) |
| Unknown | 7 (23%) | 5 (26%) | 2 (18%) |
| Duration of disease (years) | |||
| Mean | 5.8 | 5.4 | 6.5 |
| Range | 1–14 | 1–13 | 1–14 |
| DIF result (if known) | |||
| IgG | 2 | 2 | 0 |
| IgM | 1 | 1 | 0 |
| IgA | 3 | 0 | 3 |
| C3 | 1 | 1 | 0 |
| Mixed | 10 | 6 | 4 |
| Pattern of DIF (if known) | |||
| Granular | 1 | 0 | 1 |
| Linear | 10 | 5 | 5 |
| IIF result (if known) | |||
| Roof | 6 | 4 | 2 |
| Floor | 0 | 0 | 0 |
| Negative | 4 | 3 | 1 |
| Involvement of other sites | |||
| Anorectal/genital | 4 | 3 | 1 |
| Skin | 4 | 3 | 1 |
| Duration to first ocular review (months) | |||
| Mean | 19.3 | 21.5 | 15.5 |
| Range | 0‐144 | 0‐144 | 0‐108 |
| Eye symptoms at first ocular review (yes) | 9/30 (30%) | 0 (0%) | 9/11 (83.2%) |
DIF, direct immunofluorescence; IIF, indirect immunofluorescence; MMP, mucous membrane pemphigoid.
Diagnosis of MMP was based on both direct (DIF) and indirect (IIF) immunofluorescence in 47% (14/30), direct IF only in a further 23% (7/30) (21/30 in total had had DIF), and on histology alone in 23% (7/30) of patients. Positive DIF results were available on 17 patients. Of these 17 patients, 59% (10/17) showed a mixed immunoglobulin pattern, 12% (2/17) IgG, 18% (3/17) IgA, 6% (1/17) IgM, and 6% (1/17) C3 as the main immunoreactant type. The pattern of immunofluorescence was linear in 59% (10/17), granular in 6% (1/17), and unspecified in 35% (6/17). IIF was done in 14 patients, of whom 71% (10/14) were positive, with six localised to the roof of the split skin substrate and the remaining four unspecified. None localised to the floor of the split skin substrate (table 1).
The mean interval between diagnosis of oral MMP and first ophthalmic examination was 19.3 months (range 0–144 months). This included, however, four patients who had been lost to follow up with an interval of 60, 84, 108 and 144 months, respectively. Excluding these patients the mean interval was 7.1 months (range 0–36 months) (table 1). At first ocular review 30% (9/30) had ocular symptoms, all of whom had ocular signs of MMP (table 2); 37% (11/30) eventually showed ocular signs, with two patients developing ocular MMP despite an initially normal ocular examination. These two patients developed ocular MMP at 19 months and 48 months, respectively.
Table 2 Proportion of patients with ocular mucous membrane pemphigoid (MMP).
| Ocular involvement at referral | 30% (9/30) |
| Eventual ocular involvement | 37% (11/30) |
| No ocular MMP | 63% (19/30) |
On the basis of a system review inquiry during the consultation, 27% (8/30) of patients had involvement of mucous membranes other than mouth and eye. These included skin involvement in four patients and four patients with genitourinary and or anorectal involvement (table 1). Seventy per cent (22/30) of patients had only oral (extraocular) disease at presentation. Of the 22 patients who had only oral disease, seven (32%) had ocular disease at presentation. Two of these patients with only oral disease developed ocular disease within 5 years (19 months and 48 months) giving a calculated incidence rate for the development of ocular disease of 0.03 per person year for 5 years.
Of the 11 patients in total who had ocular MMP, five progressed over the period of follow up (including the patients who had no signs on first presentation) (table 3). Of those five who progressed, the mean duration of follow up was 6.4 years (range 2–13 years) (table 4). Fifty seven per cent (17/30) of patients received systemic treatment, 47% (9/19) of those with oral MMP alone and 73% (8/11) of those with ocular involvement. Treatment included dapsone (nine), prednisolone (nine) azathioprine (three), methotrexate (one), colchicine (one), methotrexate (one), tacrolimus (one), sulphamethopyridine (one), and thalidomide (one). Fifty three per cent (16/30) of patients with oral MMP also received local oral measures (steroid, antimicrobials, anaesthetic and ciclosporin mouthwashes).
Table 3 Severity of MMP based on the Tauber/ Foster Grading System.
| First presentation | Worst grade | |
| Minimal (I) | 0 | |
| Mild (IIA/IIIA) | 2 | |
| Moderate (IIB/IIIB) | 5 | 4 |
| Severe (IIC/IIIC) | 2 | 3 |
| Very severe (IID/IIID) | 1 | 4 |
| End stage (IV) | 0 | |
| Unknown | 1 |
Table 4 Grades for the five patients who progressed over the period of follow up.
| Patients | Grade at first ophthalmology review | Grade at latest ophthalmology review | Interval |
|---|---|---|---|
| A | |||
| Vertical | IIB | IID | 8 years |
| Horizontal | IIIB | IIIC | |
| B | |||
| Vertical | IIA | IIB | 2 years |
| Horizontal | IIIA | IIIA | |
| C | |||
| Vertical | IIA | IIC | 6 years |
| Horizontal | IIIA | IIIC | |
| D | |||
| Vertical | IIB | IIB | 3 years |
| Horizontal | IIIA | IIIC | |
| E | |||
| Vertical | IIC | IID | 13 years |
| Horizontal | IIIC | IIIC |
Discussion
Mucous membrane pemphigoid (MMP) is a multisystem disease involving mucous membranes, which may manifest in one or a number of tissues, either simultaneously or at different times. Conjunctival involvement is common in MMP, and may lead to painful sight threatening disease. The diagnosis of MMP has been facilitated by the introduction of indirect immunofluorescence on serum, which may obviate the need for a biopsy of the conjunctiva or oral mucosal.
In this study, 30% (9/30) of patients with a diagnosis of oral MMP had ocular signs and symptoms at presentation to an ophthalmologist. This differs from Thorne et al, who reported that 54% of patients had both ocular and extraocular disease at presentation, the extraocular preceding the ocular in 49.2% of cases. This may reflect a difference in referral pattern, as in this study patients were referred from oral medicine irrespective of whether there was ocular involvement.
Of the 25 patients reported by Thorne et al who only had oral involvement at presentation, four developed ocular disease within 5 years of follow up. They calculated an incidence of ocular involvement of 0.07 per person year over the first 5 years and 0.05 per person year over the 10 year follow up period. This is higher than our calculated rate of 0.03 per person year for 5 years. This difference is likely to reflect the different lag periods from the onset of oral symptoms to presentation to oral medicine, diagnosis, and subsequent ophthalmic review. Although it may be more relevant to calculate the time period from symptomatic oral disease to the development of ocular disease, it is difficult to determine the onset of mucosal involvement as, as with ocular MMP, signs may precede symptoms. As MMP is a relatively rare condition, patients may be initially misdiagnosed. The time from involvement of the oral mucosa to involvement of the eye is therefore not reliable. It would appear that the presence of ocular signs and or symptoms does not improve diagnostic accuracy among non‐ophthalmologists, as 45.5% (5/11) of those patients who had oral and ocular disease remained undiagnosed for more than 12 months compared with 26% (5/19) among patients with oral disease only.
Of the 11 patients with ocular involvement six (55%) had positive DIF, compared with 5/19 (26%) of those without ocular MMP. Two of 11 (18%) of those with ocular MMP had a positive IIF compared with four of 19 (21%) of those without ocular MMP. Based on these figures it would appear that a positive DIF result is predictive of ocular involvement; however, since not all patients had DIF/IIF done it is difficult to judge the significance of these results.
Among the patients with documented ocular involvement, 46% (5/11) progressed in severity during the period of follow up. In addition, 27% (8/30) of patients had involvement of mucous membranes other than mouth or eye, highlighting the multisystem nature of MMP and the importance of a system review. It is clear from the results of this study and others9,17 that patients presenting with MMP involving the oral mucosal are at risk for the development of ocular disease with an incidence rate of between 0.03 and 0.07 per person year for 5 years. The time interval from oral to ocular involvement is, however, variable and, importantly, many patients may be asymptomatic in the initial phases of ocular involvement. It may therefore be inappropriate to rely on the development of symptoms for ophthalmic review in patients with oral disease. Ophthalmic review is therefore indicated to detect ocular involvement. A multidisciplinary approach to treatment can then be offered.
Abbreviations
BMZ - basement membrane zone
BP - pemphigoid
DIF - direct immunofluorescence
IIF - indirect immunofluorescence
MMP - mucous membrane pemphigoid
Footnotes
Commercial relationship: none.
As this work was performed as an audit, ethical approval was not required.
References
- 1.Zillikens D. BP180 as the common autoantigen in blistering diseases with different clinical phenotypes. Keio J Med 20025121–28. [DOI] [PubMed] [Google Scholar]
- 2.Lazarova Z, Hsu R, Yee C.et al Antiepiligrin cicatricial pemphigoid represents an autoimmune response to subunits present in laminin 5 (alpha3beta3gamma2). Br J Dermatol 1998139791–797. [DOI] [PubMed] [Google Scholar]
- 3.Kumari S, Bhol K C, Simmons R K.et al Identification of ocular cicatricial pemphigoid antibody binding site(s) in human beta4 integrin. Invest Ophthalmol Vis Sci. 2001;42: 379–85, Erratum in: Invest Ophthalmol Vis Sci 2001;42:548. Ahmed AR [corrected to Razzaque Ahmed A] [PubMed]
- 4.Zillikens D. Acquired skin disease of hemidesmosomes. J Dermatol Sci 199920134–154. [DOI] [PubMed] [Google Scholar]
- 5.Thorne J E, Anhalt G J, Jabs D A. Mucous membrane pemphigoid and pseudopemphigoid. Ophthalmology 200411145–52. [DOI] [PubMed] [Google Scholar]
- 6.Foster C S. Cicatricial pemphigoid. Trans Am Ophthalmol Soc 198684527–663. [PMC free article] [PubMed] [Google Scholar]
- 7.Rowsey J J, Macias‐Rodriguez Y, Cukrowski C. A new method for measuring progression in patients with ocular cicatricial pemphigoid. Arch Ophthalmol 2004122179–184. [DOI] [PubMed] [Google Scholar]
- 8.Wojnarowska F, Eady R A J, Burge S M. Bullous eruptions. In: Champion RH, Burton JL, Burns DA, et al, eds. Textbook of dermatology. Oxford: Blackwell Science Ltd, 1998, 1873–7, 1884–7
- 9.Thorne J E, Anhalt G J, Jabs D A. Mucous membrane pemphigoid and pseudopemphigoid. Ophthalmology 200411145–52. [DOI] [PubMed] [Google Scholar]
- 10.Ciarrocca K N, Greenberg M S. A retrospective study of the management of oral mucous membrane pemphigoid with dapsone. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 199988159–163. [DOI] [PubMed] [Google Scholar]
- 11.Kirtschig G, Murrell D, Wojnarowska F.et al Interventions for mucous membrane pemphigoid and epidermolysis bullosa acquisita. Arch Dermatol 2002138380–384. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed A R, Colon J E. Comparison between intravenous immunoglobulin and conventional immunosuppressive therapy regimens in patients with severe oral pemphigoid: effects on disease progression in patients nonresponsive to dapsone therapy. Arch Dermatol 20011371181–1189. [DOI] [PubMed] [Google Scholar]
- 13.Bernard P, Vaillant L, Labeille B.et al Incidence and distribution of subepidermal autoimmune bullous skin diseases in three French regions. Bullous Diseases French Study Group. Arch Dermatol 199513148–52. [PubMed] [Google Scholar]
- 14.Foster C S. Cicatricial pemphigoid. Trans Am Ophthalmol Soc 198684527–663. [PMC free article] [PubMed] [Google Scholar]
- 15.Foster C S, Wilson L A, Ekins M B. Immunosuppressive therapy for progressive ocular cicatricial pemphigoid. Ophthalmology 198289340–353. [DOI] [PubMed] [Google Scholar]
- 16.Tauber J, Jabbur N, Foster S. Improved detection of disease progression in ocular cicatricial pemphigoid. Cornea 199211446–451. [DOI] [PubMed] [Google Scholar]
- 17.Carrozzo M, Broccoletti R, Carbone M.et al Pemphigoid of the mucous membranes. The clinical, histopathological and immunological aspects and current therapeutic concepts. Minerva Stomatol 199645455–463. [PubMed] [Google Scholar]