Abstract
Aim
To assess the effects of photodynamic therapy (PDT) with verteporfin in the treatment of subfoveal choroidal neovascularisation (CNV) secondary to Vogt‐Koyanagi‐Harada disease (VKH).
Methods
Six eyes of six patients with VKH who developed subfoveal CNV underwent standard PDT. Repeated treatments were performed at 3 month intervals for persistent leakage. Charts and angiographic data were analysed retrospectively.
Results
Age of patients ranged between 17 years and 27 years. Five CNV lesions were recent and classic (greatest lesion diameter was 1100–3100 μm). One CNV was chronic and partially scarred. Mean visual acuity (VA) at presentation was 20/200. Five patients had more than 1 year of follow up. In five eyes there was active inflammation and CNV. Of these eyes, the first three required one PDT each. The final CNV scar was smaller/stable with improvement of VA in two eyes. The third developed a larger CNV scar with loss of two lines of VA. Submacular fibrosis developed in all three. In the fourth eye, mild CNV leakage persisted after one PDT but hazy media precluded a second PDT. At 18 months the CNV scar and VA were stable. The fifth case, with mild inflammation, required three PDT. The CNV leakage became minimal, the lesion became smaller, and VA improved significantly. The sixth eye with CNV had no inflammation and needed two PDT sessions to halt the CNV leakage. The final lesion was smaller and vision was stable. There were no PDT related complications in our series.
Conclusion
Photodynamic therapy with verteporfin appears to be a safe and viable treatment option for subfoveal CNV secondary to VKH. It offers a chance for stabilisation or even improvement of vision. Further study is warranted.
Keywords: photodynamic therapy, subfoveal choroidal neovascularisation, Vogt‐Koyanagi‐Harada disease
Vogt‐Koyanagi‐Harada disease (VKH) is a bilateral, granulomatous panuveitis associated with central nervous system (CNS), auditory and cutaneous manifestations.1 It is frequently associated in its chronic stage with complications such as cataract, glaucoma, subretinal fibrosis, and choroidal neovascularisation (CNV) which limit visual outcome.2,3 CNV, particularly under the foveal centre, is a major vision threatening complication that carries a poor visual prognosis.3
Currently, there is no proved treatment for subfoveal CNV in VKH. The value of established modalities such as verteporfin photodynamic therapy (PDT) in treating CNV secondary to inflammatory etiologies in general, and to VKH in particular, has not been demonstrated. The literature includes a few series of inflammatory CNV treated with PDT4,5,6,7,8,9 with sparse information on PDT for CNV in VKH.9,10 This report describes six patients with VKH who received verteporfin PDT for subfoveal CNV.
Patients and methods
After institutional review board approval was received, the medical records and angiographic data of all consecutive patients with VKH who developed subfoveal CNV and underwent PDT between December 2000 and December 2003 were retrospectively reviewed. All patients satisfied the criteria for the diagnosis of VKH.11 In all cases, CNV was diagnosed based on the presence of a greyish or yellow subretinal lesion associated with subretinal haemorrhage and leakage on fundus fluorescein angiography (FA). Patients were included in this study if the neovascular component was either under the foveal centre or juxtafoveal with blood under the foveal centre. Photodynamic therapy was performed according to standard protocol.12 Patients were re‐examined at 3 months after each PDT. Some were evaluated sooner because of their intraocular inflammation. Examinations included Snellen visual acuity measurements, slit lamp biomicroscopy, fundus examinations, and FA. Repeated treatments were performed at 3 month intervals if leakage persisted on control FA.
Results
The patients' clinical data, CNV characteristics, and post‐PDT outcomes are summarised in table 1. Six patients (five female and one male) with CNV secondary to VKH were identified and included in the study. Patients' age ranged between 17 years and 27 years. The CNV occurred in one eye of each patient (six study eyes). In five cases (cases 1, 3–6), the CNV lesions manifested by a recent onset of decreased vision and were predominantly classic on FA (greatest lesion diameter 1100–3100 μm). In one case the CNV was chronic and partially scarred (case 2). Mean best corrected Snellen visual acuity (VA) at presentation was 20/200.
Table 1 Clinical characteristics and post‐PDT outcomes.
| Patient no/ age/sex | Duration of symptoms (weeks) | Inflammatory activity* | Initial VA | CNV at presentation | PDT sessions | VA at final follow up | CNV at final follow up | Length of follow up (months) | Other complications | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| 1/25/F | 2 | Mild | 20/200 | ActiveJuxtafoveal PC + | 1 | Pre‐cataract surgery 20/125 | InactiveSmaller | 41 | Glaucoma, cataract | Cataract surgery 16 months post‐PDTChronic recurrent VKH |
| subfoveal blood | Post‐cataract surgery 20/125 | SM fibrosis | Rx: steroids + ciclosporin A | |||||||
| 2/17/F | 105 | Mild | 20/300 | Partially scarredSubfoveal | 1 | Pre‐cataract surgery 20/200 | InactiveStable | 18 | Glaucoma, cataract | Cataract surgery 9 months post PDTChronic recurrent VKH |
| Post‐cataract surgery 20/160 | SM fibrosis | Rx: steroids + methotrexate | ||||||||
| 3/22/M | 1 | Moderate | 20/200 | ActiveSubfoveal PC | 1 | Pre‐cataract surgery 6/200 | InactiveLarger | 19 | Glaucoma, cataract | Cataract surgery 10 months post PDTChronic recurrent VKH |
| Post‐cataract surgery 20/300 | SM fibrosis | Rx: steroids + ciclosporin A | ||||||||
| 4/27/F | 10 | Severe | 20/300 | ActiveSubfoveal PC | 1† | Pre‐cataract surgery 6/200 | InactiveStable | 18 | Glaucoma, cataract | Cataract surgery 18 months post PDTChronic recurrent VKH |
| Post‐cataract surgery 20/300 | Rx: steroids + ciclosporin A | |||||||||
| 5/19/F | 2 | Mild | 20/200 | Active | 3 | 20/50 | Active | 10 | None | Chronic recurrent VKH |
| Subfoveal PC | Smaller | Rx: steroids + Imuran | ||||||||
| 6/23/F | 12 | None | 4/200 | Active | 2 | 4/200 | Inactive | 30 | None | Chronic VKH |
| Subfoveal PC | Stable | Rx: steroids + ciclosporin A | ||||||||
| RPE changes |
PC, predominantly classic; Rx, treatment; SM, submacular; RPE, retinal pigment epithelium.
*Inflammatory activity of VKH. †Inflammatory haze precluded second PDT.
Five eyes (cases 1–5) were exhibiting active chronic recurrent intraocular inflammation and were receiving systemic oral corticosteroids and immunosuppressive agents at the time of CNV diagnosis and PDT (see table 1). In three of these (cases 1–3), a single PDT session was sufficient to halt the CNV leakage. In case 1 (fig 1A, B) the CNV scar became smaller and VA improved by two lines at 7 months post‐PDT (fig 1C). Submacular fibrosis however eventually developed during the following 41 months, but without further decrease in VA (fig 1D). In case 2, PDT stopped the residual leakage of the partially scarred CNV. VA improved initially and more so after cataract surgery at 9 months. However submacular fibrosis was also present at 18 months albeit without further visual decline. Case 3 had significant pre‐existing macular post‐inflammatory pigmentary changes when the subfoveal CNV appeared (fig 2A, B). Although PDT arrested all visible angiographic leakage, a progressively enlarging submacular fibrotic scar developed and VA declined to 20/300 at 19 months (fig 2C, D). Case 4 was characterised by severe recurrent VKH. Mild CNV leakage persisted after the first PDT, but significant media haze secondary to the uveitis and cataract precluded the successful delivery of repeat PDT. Eighteen months later, after the inflammation abated and following cataract surgery, the CNV was cicatricial, stable in size and VA remained at its presenting level of 20/300. In case 5, three PDT sessions were required after which the leakage continued albeit minimally. The CNV lesion became smaller and VA improved significantly. No subfoveal fibrosis developed (fig 3A, B).
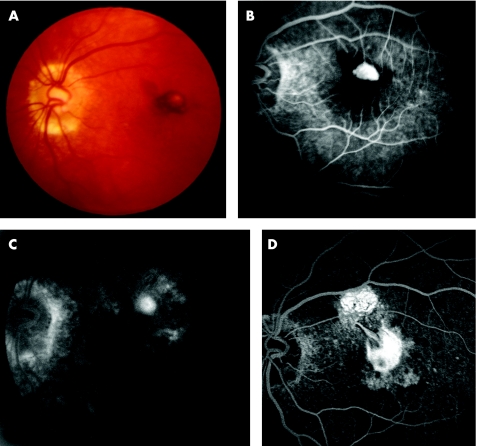
Figure 1 (A) Pre‐photodynamic therapy (PDT) fundus colour photograph showing a juxtafoveal choroidal neovascularisation (CNV) with blood under the foveal centre. (B) Pre‐PDT early phase fluorescein angiography (FA) demonstrates hyperfluorescence of a classic CNV surrounded by blocked fluorescence (subretinal blood). (C) Seven months post‐PDT late phase FA. The CNV is reduced to a smaller staining lesion. Note the RPE changes and absence of leakage from CNV. (D) 26 months post‐PDT late phase FA. Submacular fibrosis developed at the site of previous CNV and in the superior macula.
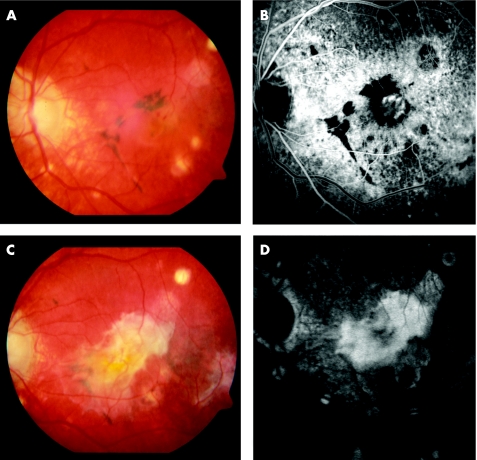
Figure 2 (A) Pre‐photodynamic therapy (PDT) fundus colour photograph showing a subfoveal choroidal neovascularisation (CNV) associated with significant pigmentary disturbances and chorioretinal atrophic scars throughout the macula. (B) Pre‐PDT early phase fluorescein angiography (FA) demonstrates the hyperfluorescent subfoveal CNV. (C) 16 months post‐PDT. Extensive submacular fibrosis developed. (D) 16 months post‐PDT. Corresponding FA.
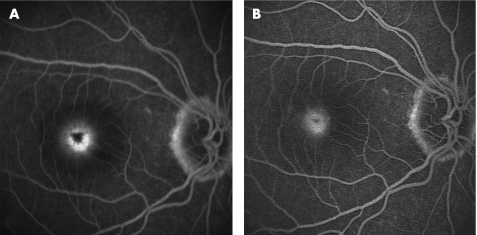
Figure 3 (A) Pre‐photodynamic therapy (PDT) late phase fluorescein angiography (FA) showing leakage from classic subfoveal choroidal neovascularisation (CNV). (B) Post third PDT late phase FA. Note the persistently leaking but smaller CNV lesion.
Case 6 was the only one that showed no concurrent intraocular inflammation when the CNV developed. The subfoveal CNV net, surrounded by a ring of subretinal haemorrhage, required two PDT sessions to halt the leakage. Six months later, the VA remained at the pretreatment level but a circle of retinal pigment epithelium changes was visible around the CNV scar in the area previously occupied by blood. There were no PDT related adverse events in our series.
Discussion
To date there is no clear standard of care for subfoveal CNV secondary to inflammatory aetiologies, VKH included.6,9 Systemic corticosteroids are generally advocated to control the inflammation, and hence decrease the inflammatory stimulus for CNV formation in order to influence CNV regression. Their use is supported by successful regressions of CNV with inflammatory control alone.4,13,14 Their efficacy, however, is inconsistent.7 Several reports exist where systemic corticosteroids with or without immunosuppressants failed to stabilise the CNV, necessitating alternative treatments such as PDT.4,6,8,10 All our patients were receiving systemic corticosteroids and immunosuppression at the time the CNV developed. Although in five cases a tighter inflammatory control may have been, in principle, necessary to achieve a successful regression of the CNV, in patient 6 the CNV persisted despite the steroid therapy and the absence of inflammation. Systemic therapy alone therefore appears insufficient to prevent or effectively treat CNV in eyes with VKH.
Although the benefit of verteporfin PDT for predominantly classic CNV in age related macular degeneration was demonstrated in randomised trials,12 the value of PDT for inflammatory CNV, particularly in VKH, is uncertain.9 Reports on series of inflammatory CNV treated with PDT suggest the possibility of improved outcomes.4,5,6,7,8,9,15,16 But PDT for CNV in VKH is reported only anecdotally.9,10 In one patient PDT was followed by marked visual improvement, but subsequent decline at 18 months because of subfoveal fibrosis.9 In another case, PDT achieved CNV regression and improvement of VA, but unexpected RPE alterations within the treatment area occurred secondarily.10
In our six patients with subfoveal CNV secondary to VKH treated with PDT, VA improved in three eyes, remained stable in two, and decreased in one eye. At final follow up, the CNV lesion size was smaller in two eyes, stable in three and larger in one eye. Many factors limited a better visual outcome in this series: the associated glaucoma in four patients (see table 1), the inflammatory media haze (case 4) which may have precluded effective photoactivation, but mostly the features of severe chronic VKH: the extensive macular pigmentary changes and retinal inflammatory damage seen in some eyes (cases 2, 3, and 4) and the submacular fibrosis, which ultimately developed in three of our cases despite cessation of the CNV leakage (cases 1, 2, and 3). Choroidal neovascularisation is known to occur primarily in severe chronic recurrent VKH, characterised by greater levels of inflammation, significant fundus pigmentary alterations, and the frequent development of subretinal fibrosis.1,2,13 CNV often exacerbates a progressively deteriorating visual course already affected by these features and generally carries a poor visual prognosis.2 Features of chronic recurrent VKH were prominent in our patients and were responsible for the limited visual recovery despite regression of the CNV.
In our series, no PDT related complications were encountered. Although submacular fibrosis appeared in three eyes, and was similarly observed in one VKH case following PDT,9 we could not conclusively ascribe it to PDT, because it occurs in 6–40% of eyes with VKH.2,17 Circular RPE alterations developed in the treatment area in case 6, but they may have resulted from reabsorption of subretinal blood rather than the laser. Similar RPE alterations were observed in the case report of PDT for CNV in VKH10 and in other aetiologies.9,15
Alternative treatments of inflammatory CNV exist but their value in VKH is uncertain. A successful submacular surgery with improvement of vision was reported in one patient with VKH.5 Improved visual outcomes with combined PDT and intravitreal triamcinolone acetonide injection compared to PDT alone were reported in series of inflammatory CNV.16,18 None of our patients received additional intravitreal steroids and it is unknown if this therapy would improve visual outcomes or alter the development of submacular fibrosis in VKH. Recent interest has focused on the anti‐angiogenic approach for treatment of CNV with vascular endothelial growth factor (VEGF) inhibitors. Encouraging CNV regressions and promising visual improvements are appearing.19,20 To date, however there are no data to assess the results of anti‐VEGF therapy in inflammatory CNV.
Despite the small number of patients in our series, the retrospective nature of the study, the lack of controls, and the concomitant use of systemic corticosteroids to control the inflammation in all our patients, which may have influenced the effect of PDT, our preliminary results indicate that PDT with verteporfin appears as a viable treatment option for CNV secondary to VKH. It offers a chance for stabilisation or even improvement of vision with a low incidence of side effects. Further investigation to elucidate the role of PDT for subfoveal CNV secondary to VKH, alone or in combination with systemic and/or local immunosuppression, is warranted.
Acknowledgements
Other members of the Photodynamic Therapy Study Group: Emad B Abboud, MD, Eman Kahtani, MD, Hassan Dhibi, MD, Essam Al‐Harthi, MD, Mark Johnston, MD, Msaad Al‐Robaie, MD, Ismail Bantan, MD.
Abbreviations
CNS - central nervous system
CNV - choroidal neovascularisation
FA - fluorescein angiography
PDT - photodynamic therapy
VA - visual acuity
VKH - Vogt‐Koyanagi‐Harada disease
Footnotes
The authors have no conflicts of interest or financial conflict with any of the instruments or topics presented in this paper.
References
- 1.Read R W, Rechodouni A, Butani N.et al Complications and prognostic factors in Vogt‐Koyanagi‐Harada disease. Am J Ophthalmol 2001131599–606. [DOI] [PubMed] [Google Scholar]
- 2.Moorthy R S, Chong L P, Smith R E.et al Subretinal neovascular membranes in Vogt‐Koyanagi‐Harada syndrome. Am J Ophthalmol 1993116164–170. [DOI] [PubMed] [Google Scholar]
- 3.Rubsamen P E, Gass J D M. Vogt Koyanagi Harada syndrome. Clinical course, therapy and long term visual outcome. Arch Ophthalmol 1991109682–687. [DOI] [PubMed] [Google Scholar]
- 4.Spaide R F, Freund K B, Slakter J.et al Treatment of subfoveal choroidal neovascularization associated with multifocal choroiditis and panuveitis with photodynamic therapy. Retina 200222545–549. [DOI] [PubMed] [Google Scholar]
- 5.Foster R E, Knight C D, Lowder C Y. Subfoveal choroidal neovascular membrane excision in Vogt‐Koyanagi‐Harada syndrome. Retina 200020547–549. [DOI] [PubMed] [Google Scholar]
- 6.Rogers A H, Duker J S, Nichols N.et al Photodynamic therapy of idiopathic and inflammatory choroidal neovascularization in young adults. Ophthalmology 20031101315–1320. [DOI] [PubMed] [Google Scholar]
- 7.Parodi M B, Di Crecchio L, Lanzetta P.et al Photodynamic therapy with verteporfin for subfoveal choroidal neovascularization associated with multifocal choroiditis. Am J Ophthalmol 2004138263–269. [DOI] [PubMed] [Google Scholar]
- 8.Leslie T, Lois N, Christopoulou D.et al Photodynamic therapy for inflammatory choroidal neovascularisation unresponsive to immunosuppression. Br J Ophthalmol 200589147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wachtlin J, Heimann H, Behme T.et al Long‐term results after photodynamic therapy with verteporfin for choroidal neovascularizations secondary to inflammatory chorioretinal diseases. Graefes Arch Clin Exp Ophthalmol 2003241899–906. [DOI] [PubMed] [Google Scholar]
- 10.Farah M E, Costa R A, Muccioli C.et al Photodynamic therapy with verteporfin for subfoveal choroidal neovascularization in Vogt‐Koyanagi‐Harada syndrome. Am J Ophthalmol 2002134137–139. [DOI] [PubMed] [Google Scholar]
- 11.Read R, Holland G, Rao N A. Revised diagnostic criteria for Vogt‐Koyanagi‐Harada disease: report of an international committee on nomenclature. Am J Ophthalmol 2001131647–652. [DOI] [PubMed] [Google Scholar]
- 12. Treatment of Age Related Macular Degeneration with Photodynamic Therapy Study Group—TAP report 2. Arch Ophthalmol 2001119198–207. [PubMed] [Google Scholar]
- 13.Lertsumitkul S, Whitcup S M, Nussenblatt R B.et al Subretinal fibrosis and choroidal neovascularization in Vogt‐Koyanagi‐Harada syndrome. Graefes Arch Clin Exp Ophthalmol 19992371039–1045. [DOI] [PubMed] [Google Scholar]
- 14.Flaxel C J, Owens S L, Mullholland B.et al The use of corticosteroids for choroidal neovascularization in young patients. Eye 199812266–272. [DOI] [PubMed] [Google Scholar]
- 15.Shanmugam M P, Shetty N, Sharma S.et al Treatment of subfoveal choroidal neovascular membrane with photodynamic therapy. Retina 200323428 discussion 428. [DOI] [PubMed] [Google Scholar]
- 16.Hogan A, Behan U, Kilmartin D J. Outcomes after combination photodynamic therapy and immunosuppression for inflammatory subfoveal choroidal neovascularisation. Br J Ophthalmol 2005891109–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuo I C, Rechdouni A, Rao N A.et al Subretinal fibrosis in patients with Vogt‐Koyanagi‐Harada disease. Ophthalmology 20001071721–1728. [DOI] [PubMed] [Google Scholar]
- 18.Rechtman E, Danis R P, Pratt L M.et al Intravitreal triamcinolone with photodynamic therapy for subfoveal choroidal neovascularisation in age related macular degeneration. Br J Ophthalmol 200488344–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michels S, Rosenfeld P J, Puliafito C A.et al Systemic bevacizumab (Avastin) therapy for neovascular age‐related macular degeneration: twelve‐week results of an uncontrolled open‐label clinical study. Ophthalmology 20051121035–1047. [DOI] [PubMed] [Google Scholar]
- 20.Avery R L, Pieramici D J, Rabena M D.et al Intravitreal bevacizumab (Avastin) for neovascular age‐related macular degeneration. Ophthalmology 2006113363–372. [DOI] [PubMed] [Google Scholar]