Abstract
Aim
To compare the visual function in patients with short wavelength blue light blocking yellow tinted intraocular lenses (IOLs) with that in patients with non‐tinted IOLs.
Methods
74 patients scheduled for bilateral cataract surgery underwent implantation of either yellow IOLs (HOYA YA60BB) or non‐tinted IOLs (VA60BB) in both eyes. Contrast visual acuity with and without a glare source was measured under photopic (100 cd/m2) and mesopic (slightly higher luminance than typically used—5 cd/m2) conditions at 2 weeks and 3 months after surgery using the contrast sensitivity accurate tester. Visual acuity and the incidence of patients who noted cyanopsia were also examined.
Results
No significant differences between the yellow tinted and non‐tinted IOL groups were observed in mean visual acuity or in photopic or higher luminance mesopic contrast visual acuity with and without glare source at either 2 weeks or 3 months after surgery. Furthermore, there was no significant difference in contrast visual acuity loss as a result of glare. The incidence of patients who noticed cyanopsia was significantly less in the yellow tinted IOL group than in the non‐tinted IOL group at 2 weeks after surgery (p = 0.0234), but no patients reported cyanopsia at 3 months.
Conclusion
Visual function in patients with yellow tinted IOLs is virtually the same as that in patients with non‐tinted IOLs.
Keywords: yellow tinted intraocular lens, contrast sensitivity, glare disability, cyanopsia
It has long been a concern that exposure to sunlight may be a risk factor for the development of age related macular degeneration (AMD). While some epidemiological studies found a significant correlation between sunlight exposure and the development of AMD or age related maculopathy,1,2,3,4 others did not.5,6,7,8,9,10 Specifically, the Chesapeake Bay Watermen Study1 showed that long term exposure to short wavelength blue light might be related to the development of AMD. In addition, many other studies demonstrated that cataract surgery in elderly patients, with consequent aphakia or pseudophakia, is associated with an increased risk for AMD,11,12,13,14 although controversy remains.10,15,16 Since the crystalline lens becomes yellow with age, and thus blocks short wavelength light,17,18,19 this association was attributed to extraction of the yellowing crystalline lens and subsequent exposure of the retina to short wavelength light. More importantly, much biological evidence exists that short wavelength light has serious phototoxicity for the retina and for the retinal pigment epithelial cells.20,21,22,23,24,25,26
To protect the retina and retinal pigment epithelial cells from the hazards of exposure to short wavelength blue light after cataract surgery, several types of blue light blocking yellow tinted intraocular lenses (IOLs) have been developed, which absorb a great deal of the short wavelength light. However, the decrease in visible short wavelength light may lead to impairment of visual function. Specifically, controversy remains as to whether or not short wavelength light is more important for night‐time contrast sensitivity than is light of other wavelengths.27,28,29,30,31,32 Because scotopic contrast sensitivity deteriorates with age,33,34,35,36,37 there is concern that night‐time vision may be impaired in older patients who have undergone implantation of yellow tinted IOLs.28,29,30 Although previous studies reported that contrast sensitivity in eyes with a yellow tinted IOL was better than that in eyes with a non‐tinted IOL,38,39 a more recent study reported comparable sensitivity.40 However, it should be noted that contrast sensitivity under dark conditions was not examined in that study.40
The purpose of this study was to compare visual function in patients who received yellow tinted IOLs with that in patients who received non‐tinted IOLs. More specifically, in order to examine visual function under photopic and darker conditions in patients with the yellow tinted IOLs, contrast sensitivity with and without a glare source under photopic and higher than typical luminance mesopic conditions was assessed using the contrast sensitivity accurate tester (CAT‐2000; Menicon, Tokyo, Japan).
Patients and methods
Patients
All patients who were admitted sequentially to the Hayashi Eye Hospital for bilateral cataract surgery between 3 November 2004 and 20 April 2005, were screened for enrolment by a clinical research coordinator. Preoperative exclusion criteria were pathology of the cornea, retina or optic nerve, a history of ocular surgery or inflammation, or a pupillary diameter less than 6.0 mm after mydriasis; eyes scheduled for extracapsular cataract extraction, eyes of patients with diabetes, and patients who anticipated any difficulty in follow up were also excluded. Screening was continued until 80 patients who were to undergo phacoemulsification surgery and IOL implantation were recruited. The study protocol was approved by the institutional review board and informed consent was obtained from each patient.
Randomisation
All patients were randomly assigned the day before surgery to one of the two groups: those who were to undergo implantation of the yellow tinted IOLs (YA60BB; Hoya, Tokyo, Japan) and those who were to receive non‐tinted IOLs (VA60BB) in both eyes. The clinical research coordinator generated a code using a random number table, and kept concealed the assignment schedule until all data were collected. Patients and examiners were masked to the randomisation. The surgeon, who was also the data analyst, did not participate in any of the examinations or in the data collection. Both the YA60BB and the VA60BB are three piece acrylic IOLs with a 6.0 mm round optic and poly(methyl methacrylate) modified C loops.
Surgical procedures
All surgeries were performed by a single surgeon (KH) using the same technique that has been described previously.41 Firstly, a 3.5 mm scleral incision was made for IOL implantation, after which a continuous curvilinear capsulorrhexis measuring approximately 5.0 mm in diameter was accomplished using a 25 gauge bent needle. After hydrodissection, endocapsular phacoemulsification of the nucleus and aspiration of the residual cortex were carried out. Using a steel keratome, the wound was then enlarged to 4.1 mm for IOL implantation. The lens capsule was inflated with sodium hyaluronate 1% (Healon; Advanced Medical Optics, Santa Ana, CA, USA), and the IOL was inserted into the capsular bag using folding forceps. After IOL insertion, the viscoelastic material was thoroughly evacuated.
Main outcome measures
At approximately 2 weeks and 3 months after surgery, all patients underwent contrast sensitivity testing with and without a glare source, and were given a standardised questionnaire regarding glare symptoms and cyanopsia. Contrast visual acuity without glare and in the presence of a glare source (glare visual acuity) under photopic condition and under higher than typical luminance mesopic conditions were examined using the CAT‐2000. This device determines logarithm of the minimal angle of resolution (logMAR) visual acuity using five contrast visual targets under photopic and relatively higher luminance mesopic conditions.42 Measurement under the photopic lighting condition is made with chart lighting of 100 cd/m2, while chart lighting under the mesopic condition is 5 cd/m2; the glare light source of 200 lux is located in the periphery at 20° around the visual axis. Best corrected visual acuity was examined on decimal charts. The subjective refractive status and keratometric cylinder were examined using an autokeratorefractometer (KR‐7100; Topcon, Tokyo, Japan), and the pupillary diameter was measured using the Colvard pupillometer (Oasis Medical, Glendora, CA, USA). All patients also completed a written questionnaire regarding glare symptoms and cyanopsia.
Data analysis
The data obtained from both eyes were averaged and the averaged values were considered to be the representative values for each patient.43 Decimal visual acuity was converted to logMAR scale for statistical analysis. Differences between the two groups in visual acuity, contrast visual acuity with and without a glare source under bright and dark lighting conditions, manifest spherical equivalent, pupillary diameter keratometric cylinder, and other continuous variables were compared using the Mann‐Whitney U test. The non‐parametric Mann‐Whitney U test was used because the sample size was different between the two groups. The incidence of patients who noted glare symptoms or cyanopsia, and other categorical variables, were compared using the Fisher's exact test. A p value ⩽0.05 was considered to be statistically significant.
Results
Of the 405 patients screened for inclusion, 188 were not enrolled according to exclusion criteria, and 137 patients declined to be enrolled. The reasons for exclusion were other ocular pathology (70 patients, 37.2%), history of ocular surgery or inflammation (eight patients, 4.3%), eyes scheduled for extracapsular cataract extraction (13 patients, 6.9%), a poor mydriasis (12 patients, 6.4%), patients with diabetes (18 patients, 9.6%), and patients who would not be available for follow up (67 patients, 35.6%). Accordingly, 80 patients were enrolled in this study. No statistically significant difference was found in age (p = 0.3831) or sex (p = 0.5587) between the patients who were enrolled and those who were excluded. Of the 80 patients enrolled, six were excluded from the analysis; four did not appear for a follow up examination because of scheduling conflicts, one refused the examination, and one had a clinically significant epiretinal membrane in the macula. Thus, 74 patients (92.5%) completed the examinations and remained in the analysis.
The average age of the patients was 70.9 (SD 6.4) years, with a range of 58–85 years; there were 24 men and 50 women. Patient demographics are shown in table 1. No statistically significant difference was found between the groups regarding age, the ratio of men to women, manifest spherical equivalent, keratometric cylinder, or pupillary diameter.
Table 1 Patient demographics.
| Yellow IOL | Non‐tinted IOL | p Value | |
|---|---|---|---|
| No of patients | 38 | 36 | – |
| Age (SD) | 71.1 (6.7) | 70.7 (6.2) | 0.8711* |
| Sex (M/F) | 12M/26F | 12M/24F | 0.8720* |
| Refraction (D) (SD)† | −0.77 (0.42) | −0.97 (0.65) | 0.1399* |
| Astigmatism (D) (SD)‡ | 0.77 (0.47) | 0.79 (0.45) | 0.9655* |
| Pupillary diameter (mm) (SD) | 4.4 (0.7) | 4.5 (0.6) | 0.8077* |
D, dioptres; F, female; M, male.
*No statistically significant difference; †manifest spherical equivalent; ‡keratometric cylinder.
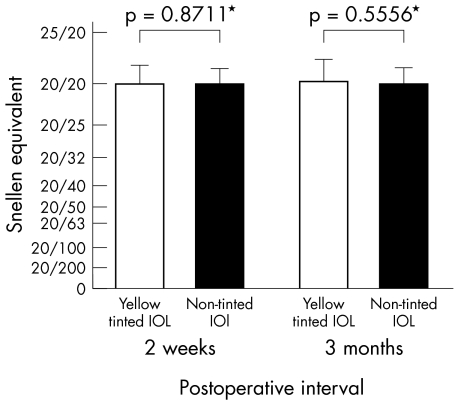
Mean visual acuity did not change significantly from 2 weeks to 3 months after surgery in either the yellow tinted or non‐tinted IOL group. When comparing the yellow tinted and non‐tinted IOL groups, no significant differences were found in mean visual acuity at either 2 weeks or 3 months after surgery (fig 1).
Figure 1 Comparison of mean (SD) visual acuity between the two groups at 2 weeks and at 3 months after surgery. No significant differences were found in mean visual acuity between the two groups at either 2 weeks or 3 months after surgery. *No significant difference.
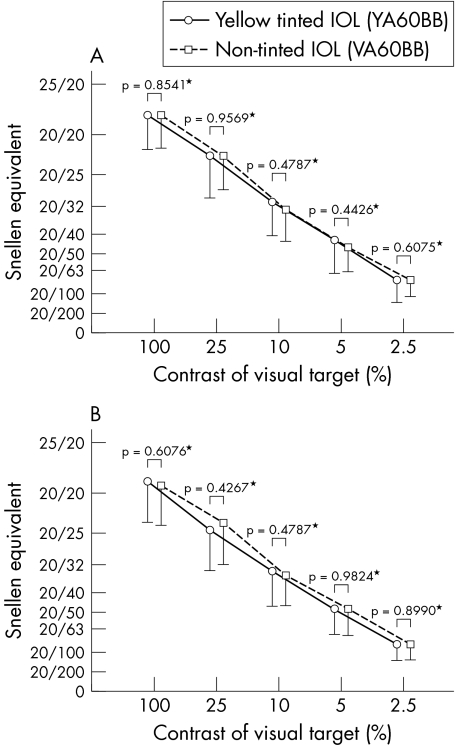
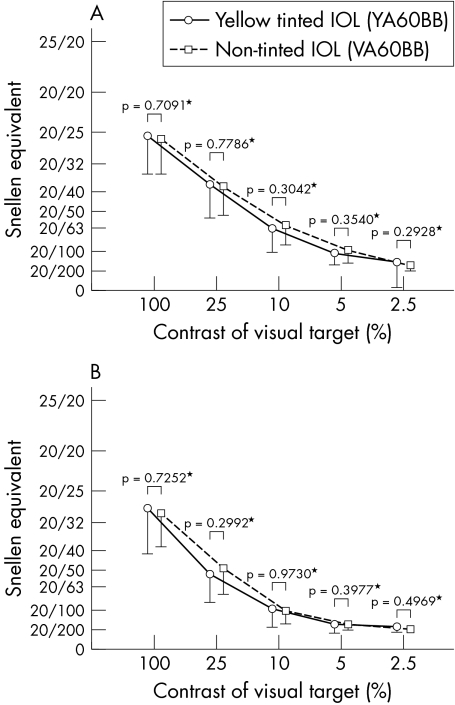
Mean contrast visual acuity and glare visual acuity under photopic and under slightly higher than typical luminance level mesopic conditions did not change significantly between 2 weeks and 3 months after surgery in either group. When comparing the yellow tinted and non‐tinted IOL groups, no significant differences were observed in mean contrast visual acuity or glare visual acuity between the two groups under either photopic (fig 2) or higher luminance mesopic (fig 3) condition at 2 weeks or 3 months after surgery.
Figure 2 Comparison of mean (SD) contrast visual acuity (A) and glare visual acuity (B) between the two groups under photopic conditions. *No significant difference was found in contrast visual acuity or in glare visual acuity under photopic conditions at 3 months after surgery. Visual acuity is expressed as Snellen equivalent.
Figure 3 Comparison between the two groups of mean (SD) contrast visual acuity (A) and glare visual acuity (B) under slightly higher than typical luminance mesopic conditions. *No significant difference was found in contrast visual acuity or in glare visual acuity under higher luminance mesopic conditions at 3 months after surgery. Visual acuity is expressed as Snellen equivalent.
Mean loss of contrast visual acuity in the presence of glare (glare disability) under either photopic or higher luminance mesopic conditions did not change significantly between 2 weeks and 3 months after surgery. When comparing the two groups, there was no significant difference in the contrast visual acuity loss as a result of glare under the two lighting conditions at 2 weeks or 3 months (table 2) after surgery.
Table 2 Loss of logMAR contrast visual acuity in the presence of a glare source in the yellow tinted and non‐tinted intraocular lens (IOL) groups at 3 months after surgery.
| Contrast (%)† | Yellow IOL | Non‐tinted IOL | p Value |
|---|---|---|---|
| Photopic | |||
| 100 | −0.024 (0.059) | −0.028 (0.066) | 0.7197* |
| 25 | −0.047 (0.064) | −0.033 (0.101) | 0.6419* |
| 10 | −0.045 (0.083) | −0.033 (0.079) | 0.7910* |
| 5 | −0.062 (0.075) | −0.028 (0.097) | 0.1036* |
| 2.5 | −0.061 (0.083) | −0.062 (0.092) | 0.9516* |
| Mesopic | |||
| 100 | −0.039 (0.072) | −0.056 (0.077) | 0.3781* |
| 25 | −0.047 (0.064) | −0.033 (0.101) | 0.6419* |
| 10 | −0.217 (0.124) | −0.236 (0.115) | 0.4305* |
| 5 | −0.219 (0.082) | −0.282 (0.118) | 0.1732* |
| 2.5 | −0.152 (0.171) | −0.143 (0.106) | 0.7341* |
*No significant difference.
†Percentage contrast of visual targets.
Table 3 shows the incidence of patients who reported glare symptoms or cyanopsia. At 2 weeks after surgery, 5.3% of patients (2/38) in the yellow tinted IOL group and 5.6% of patients (2/36) in the non‐tinted IOL group had glare symptoms; this incidence was not statistically different (p>0.9999). At 3 months after surgery, 7.9% of patients (3/38) in the yellow tinted IOL group and 5.6% of patients (2/36) in the non‐tinted IOL group still noted glare symptoms; the difference was still not significantly different (p>0.9999). At 2 weeks after surgery, five patients (13.9%) in the non‐tinted IOL group noted cyanopsia, while no patients in the yellow tinted IOL group reported cyanopsia; this difference was significantly different (p = 0.0234). However, at 3 months after surgery, no patients in either group reported symptoms of cyanopsia.
Table 3 Incidence of patients who noticed glare symptoms or cyanopsia at 2 weeks and at 3 months after surgery.
| Yellow IOL | Non‐tinted IOL | p Value | |
|---|---|---|---|
| Glare symptoms | |||
| 2 weeks | 2 (5.3%) | 2 (5.6%) | >0.9999* |
| 3 months | 3 (7.9%) | 2 (5.6%) | >0.9999* |
| Cyanopsia | |||
| 2 weeks | 0 (0%) | 5 (13.9%) | 0.0234† |
| 3 months | 0 (0%) | 0 (0%) | – |
*No significant difference, †Statistically significant difference.
Discussion
Our study has demonstrated that visual acuity, contrast sensitivity, glare sensitivity, and glare disability do not change significantly between 2 weeks and 3 months after surgery in patients who received either yellow tinted or non‐tinted IOLs. In addition, the incidence of patients who experienced glare symptoms was similar at 2 weeks and at 3 months. These results indicate that rehabilitation of visual function is immediate in patients with a yellow tinted IOL, and is similar to that in patients who received a non‐tinted IOL.
Visual acuity, contrast sensitivity, and glare sensitivity in patients with the yellow tinted and non‐tinted IOLs were similar under both photopic and mesopic conditions at both 2 weeks and 3 months after surgery. In addition, glare disability was also comparable between patients with these two types of IOL at the two postoperative times. These results suggest that visual function may not be impaired in eyes with a yellow tinted IOL compared with eyes with a non‐tinted IOL.
The incidence of patients who reported glare symptoms was almost the same in those with the yellow tinted or the non‐tinted IOL, so it seems that the yellow coloration of the IOL does not decrease glare symptoms. However, patients who received a non‐tinted IOL noticed cyanopsia at 2 weeks after surgery more commonly than did those who received the yellow IOL, but no patients in either group noted cyanopsia at 3 months. This suggests that the yellow tinted IOL can prevent cyanopsia immediately after cataract surgery, but this may not be of clinical importance, because cyanopsia disappeared by 3 months in all patients, even in those who had undergone implantation of a non‐tinted IOL.
It has been demonstrated that cataract surgery is a risk factor for the development of AMD,11,12,13,14 although other studies reported no such risk.10,15,16 The risk was attributed to the fact that extraction of the yellowing crystalline lens in older patients enhances exposure of the phototoxic short wavelength blue light to the retina, which may lead to AMD. Furthermore, there is a great deal of biological evidence that short wavelength light is hazardous to the neural retina and to the retinal pigment epithelial cells.20,21,22,23 Specifically, short wavelength light has been shown in vitro to damage the retinal pigment epithelial cells, in a way that is mediated by the accumulation of lipofuscin and its component A2E.24,25,26 Furthermore, a more recent study by Sparrow et al44 showed that yellow tinted IOL can protect against lipofuscin A2E mediated death of retinal pigment epithelial cells. Although a clinical effect has not yet been clarified, it is reasonable to assume that a yellow tinted IOL can protect the retinal pigment epithelial cells from light damage, and may subsequently help to prevent the development of AMD.44
On the other hand, it is thought that short wavelength blue light may be more essential for night‐time vision than is other wavelength light,28,29,30 although controversy remains.32 Therefore, it is of concern that short wavelength light absorbing yellow tinted IOLs may impair scotopic or mesopic contrast sensitivity. Most previous studies reported that contrast sensitivity in eyes with yellow tinted spectacles or IOLs is better than that in eyes with non‐tinted lenses.38,39,45,46 A more recent study by Rodriquez‐Galietero et al40 reported contrast sensitivity in eyes with a yellow tinted IOL to be comparable to that in eyes with a non‐tinted IOL. That study, however, examined only photopic contrast sensitivity without glare, whereas our study has clarified that visual function with and without glare under both photopic and darker conditions is similar in eyes with a yellow tinted IOL to that in eyes with a non‐tinted IOL.
To verify the clinical equivalence in contrast sensitivity and glare sensitivity between the patients with yellow tinted and non‐tinted IOLs, we calculated the statistical power to detect a clinically significant difference. When logMAR visual acuity 0.1 was assumed to be a difference of clinically meaningful magnitude, the statistical power was determined to be 83%. Thus, the statistical power was sufficient to detect differences in clinically meaningful magnitude.
Several limitations exist in this study. The first is that mesopic contrast sensitivity was examined under a higher luminance level (5 cd/m2) than that typically used for mesopic vision testing (⩽3 cd/m2). However, when we re‐examined mesopic contrast sensitivity in a portion of the patients under a luminance level of 1 cd/m2, it was similar between the patients with yellow tinted and non‐tinted IOLs. The second limitation of the study described here is that scotopic contrast sensitivity was not examined. However, the number of tasks typically performed under scotopic conditions is limited in actual life.
In conclusion, visual function under various conditions of patients in whom the short wavelength blue light absorbing yellow tinted IOLs have been implanted is virtually the same as that of patients with non‐tinted IOLs. However, because in vitro studies showed that short wavelength blue light has phototoxicity for the neural retina and retinal pigment epithelial cells,20,21,22,23,24,25,26 and that the blue light absorbing IOL could prevent damage to the retinal pigment epithelial cells,44 the yellow tinted IOL is thought to contribute to the prevention of AMD. Therefore, the use of yellow tinted IOLs is recommended for eyes at high risk to develop AMD, such as eyes with drusen or atrophy of the retinal pigment epithelium in the macula. However, further study is called for to clarify the clinical effects of yellow tinted IOLs on prevention of AMD.
Abbreviations
AMD - age related macular degeneration
IOL - intraocular lens
Footnotes
The authors have no proprietary interest in any of the materials described in this article.
References
- 1.Taylor H, West S, Mufloz B.et al The long‐term effects of visible light on the eye. Arch Ophthalmol 199211099–104. [DOI] [PubMed] [Google Scholar]
- 2.Taylor H R, Munoz B, West S.et al Visible light and risk of age‐related macular degeneration. Trans Am Ophthalmol Soc 199088163–178. [PMC free article] [PubMed] [Google Scholar]
- 3.Cruickshanks K J, Klein R, Klein B E. Sunlight and age‐related macular degeneration: the Beaver Dam eye study. Arch Ophthalmol 1993111514–518. [DOI] [PubMed] [Google Scholar]
- 4.Tomany S C, Cruickshanks K J, Klein R.et al Sunlight and the 10‐year incidence of age‐related maculopathy: the Bearver Dam Eye Study. Arch Ophthalmol 2004122750–757. [DOI] [PubMed] [Google Scholar]
- 5.Hirvela H, Luukinen H, Laara E.et al Risk factors of age‐related maculopathy in a population 70 years of age or older. Ophthalmology 1996103871–877. [DOI] [PubMed] [Google Scholar]
- 6.Darzins P, Mitchell P, Heller R F. Sun exposure and age‐related macular degeneration: an Australian case‐control study. Ophthalmology 1997104770–776. [DOI] [PubMed] [Google Scholar]
- 7.Delcourt C, Carriere I, Ponton‐Sanchez A.et al Light exposure and the risk of age‐related macular degeneration: the Pathologies Oculaires Liees a l'Age (POLA) study. Arch Ophthalmol 20011191463–1468. [DOI] [PubMed] [Google Scholar]
- 8.McCarty C A, Mukesh B N, Fu C L.et al Risk factors for age‐related maculopathy: the Visual Impairment Project. Arch Ophthalmol 20011191455–1462. [DOI] [PubMed] [Google Scholar]
- 9.Khan J C, Shahid H, Thurlby D A.et al Age related macular degeneration and sun exposure, iris colour, and skin sensitivity to sunlight. Br J Ophthalmol 20069029–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Age‐Related Eye Disease Study Research Group Risk factors for the incidence of advanced age‐related macular degeneration in the Age‐Related Eye Disease Study (AREDS): AREDS report no 19. Ophthalmology 2005112533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollack A, Marcovich A, Bukelman A.et al Age‐related macular degeneration after extracapsular cataract extraction with intraocular lens implantation. Ophthalmology 19961031546–1554. [DOI] [PubMed] [Google Scholar]
- 12.Klein R, Klein B E K, Wong T Y.et al The association of cataract and cataract surgery with the long‐term incidence of age‐related maculopathy: the Beaver Dam Eye Study. Arch Ophthalmol 20021201551–1558. [DOI] [PubMed] [Google Scholar]
- 13.Wang J J, Klein R, Smith W.et al Cataract surgery and the 5‐year incidence of late‐stage age‐related maculopathy: pooled findings from the Beaver Dam and Blue Mountain eye studies. Ophthalmology 20031101960–1967. [DOI] [PubMed] [Google Scholar]
- 14.Freeman E E, Munoz B, West S K.et al Is there association between cataract surgery and age‐related macular degeneration? : data from three population‐based studies, Am J Ophthalmol 2003135849–856. [DOI] [PubMed] [Google Scholar]
- 15.Van Der Schaft, Mooy C M, De Bruijn W C.et al Increased prevalence of disciform macular degeneration after cataract extraction with implantation of an intraocular lens. Br J Ophthalmol 199478441–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armbrecht A M, Findlay C, Aspinall P A.et al Cataract surgery in patients with age‐related macular degeneration: one‐year outcomes. J Cataract Refract Surg 200329686–693. [DOI] [PubMed] [Google Scholar]
- 17.Mellerio J. Yellowing of the human lens: nuclear and cortical contributions. Vis Res 1987271581–1587. [DOI] [PubMed] [Google Scholar]
- 18.Weale R A. Age and the transmittance of the human crystalline lens. J Physiol 1988395577–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gailard E R, Zheng L, Merriam J C.et al Age‐related changes in the absorption characteristics of the primate lens. Invest Ophthalmol Vis Sci 2000411454–1459. [PubMed] [Google Scholar]
- 20.Noell W K, Walker V S, Kang B S.et al Retinal damage by light in rats. Invest Ophthalmol 19665450–473. [PubMed] [Google Scholar]
- 21.Ham W T, Jr, Mueller H A, Sliney D H. Retinal sensitivity to damage from short wavelength light. Nature 1976260153–155. [DOI] [PubMed] [Google Scholar]
- 22.Ham W T, Jr, Ruffolo J J, Jr, Mueller H A.et al he nature of retinal radiation damage: dependence on wavelength, power level, and exposure time. Vis Res 1980201105–1111. [DOI] [PubMed] [Google Scholar]
- 23.Mainster M A, Ham W T, Jr, Delori F C. Potential retinal hazards: Instrument and environmental light sources. Ophthalmology 198390927–932. [DOI] [PubMed] [Google Scholar]
- 24.Sparrow J R, Nakanishi K, Parish C A. The lipofuscin fluorophore A2E mediates blue light‐induced damage to retinal pigmented epithelial cells. Invest Ophthalmol Vis Sci 2000411981–1989. [PubMed] [Google Scholar]
- 25.Sparrow J R, Zhou J, Ben‐Shabat S.et al Involvement of oxidative mechanisms in blue‐light‐induced damage to A2E‐laden RPE. Invest Ophthalmol Vis Sci 2002431222–1227. [PubMed] [Google Scholar]
- 26.Sparrow J R, Zhou J, Cai B. DNA is a target of the photodynamic effects elicited in A2E‐laden RPE by blue‐light illumination. Invest Ophthalmol Vis Sci 2003442245–2251. [DOI] [PubMed] [Google Scholar]
- 27.Lin K K, Lin Y C, Lee Js.et al Spectral transmission characteristics of spectacle, contact and intraocular lenses. Ann Ophthalmol 200234206–215. [Google Scholar]
- 28.Mainster M A, Sparrow J R. How much blue light should an IOL transmit? Br J Ophthalmol 2003871523–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braunstein R E, Sparrow J R. A blue‐blocking intraaocular lens should be used in cataract surgery. Arch Ophthalmol 2005123547–549. [DOI] [PubMed] [Google Scholar]
- 30.Mainster M A. Intraocular lenses should block UV radiation and violet but not blue light. Arch Ophthalmol 2005123550–555. [DOI] [PubMed] [Google Scholar]
- 31.Werner J S, Hardenbergh F E. Spectral sensitivity of the pseudophakic eye. Arch Ophthalmol 1983101758–760. [DOI] [PubMed] [Google Scholar]
- 32.Werner J S. Night vision in the elderly: consequences for seeing through a “blue filtering” intraocular lens. Br J Ophthalmol 2005891518–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gunkel R D, Gouras P. Changes in scotopic visibility thresholds with age. Arch Ophthalmol 1963694–9. [DOI] [PubMed] [Google Scholar]
- 34.Jackson G R, Owsley C, Cordle E P.et al Aging and scotopic sensitivity. Vis Res 1998383655–3662. [DOI] [PubMed] [Google Scholar]
- 35.Jackson G R, Owsley C, McGwin G., Jr Aging and dark adaptation. Vis Res 1999393975–3982. [DOI] [PubMed] [Google Scholar]
- 36.Schefrin B E, Tregear S J, Harvey L O., Jret al Senescent changes in scotopic contrast sensitivity. Vis Res 1999393728–3736. [DOI] [PubMed] [Google Scholar]
- 37.Jackson G R, Owsley C. Scotopic sensitivity during adulthood. Vis Res 2000402467–2473. [DOI] [PubMed] [Google Scholar]
- 38.Niwa Y, Yoshino Y, Okuyama F.et al Effects of tinted intraocular lens on contrast sensitivity. Ophthalmic Physiol Opt 19966297–302. [PubMed] [Google Scholar]
- 39.Yuan Z, Reinach P, Yuan J. Contrast sensitivity and color vision with a yellow intraocular lens. Am J Ophthalmol 2004138138–140. [DOI] [PubMed] [Google Scholar]
- 40.Rodoriguez‐Galietero A, Montes‐Mico R, Munoz G.et al Comparison of contrast sensitivity and color discrimination after clear and yellow intraocular lens implantation. J Cataract Refract Surg 2005311736–1740. [DOI] [PubMed] [Google Scholar]
- 41.Hayashi K, Hayashi H, Nakao F.et al Posterior capsule opacification after cataract surgery in patients with diabetes mellitus. Am J Ophthalmol 200213410–16. [DOI] [PubMed] [Google Scholar]
- 42.Hayashi K, Hayashi H. Effect of a modified optic edge design on visual function: textured‐edge versus round‐anterior, slope‐side edge. J Cataract Refract Surg 2004301668–1674. [DOI] [PubMed] [Google Scholar]
- 43.Ray W A, O'Day D M. Statistical analysis of multi‐eye data in ophthalmic research. Invest Ophthalmol Vis Sci 1985261186–1188. [PubMed] [Google Scholar]
- 44.Sparrow J R, Miller A S, Zhou J. Blue light‐absorbing intraocular lens and retinal pigment epithelium protection in vitro. J Cataract Refract Surg 200430873–878. [DOI] [PubMed] [Google Scholar]
- 45.Yap M. The effect of a yellow filter on contrast sensitivity. Ophthalmic Physiol Opt 19844227–232. [DOI] [PubMed] [Google Scholar]
- 46.De Fez M D, Luque M J, Viqueira V. Enhancement of contrast sensitivity and losses of chromatic discrimination with tinted lenses. Optom Vis Sci 200279590–597. [DOI] [PubMed] [Google Scholar]