Abstract
Aim
To define the clinical and histopathological characteristics of primary lacrimal sac lymphoma in a predominantly white population.
Methods
Specimens of lacrimal sac lymphoma and follow up data were solicited from members of the Ophthalmic Oncology Task Force of the European Organization for Research and Treatment of Cancer (EORTC) and the European Ophthalmic Pathology Society (EOPS). Specimens were stained with haematoxylin and eosin and an immunohistochemical panel against leucocyte antigens was applied. Diagnosis was reached by consensus of five experienced pathologists according to the World Health Organization classification system. The histopathological findings were correlated with the clinical data.
Results
Of 15 primary lacrimal sac lymphomas, five (33%) were diffuse large B cell lymphoma (DLBCL), five (33%) were extranodal marginal zone B cell lymphoma of mucosa associated lymphoid tissue (MALT lymphoma), three were classified as “transitional MALT lymphoma,” being in transition from MALT lymphoma to DLBCL, and two were unclassified B cell lymphomas. Nine of the patients were female, and the median age at the time of diagnosis was 71 years (range 45–95 years). The most frequent presenting symptoms were epiphora (85%), swelling in the region of the lacrimal sac (79%), and dacryocystitis (21%). All but one patient presented in stage I. Systemic spread occurred in three of nine patients (33%). The 5 year overall survival was 65%.
Conclusions
DLBCL and MALT lymphoma are equally common in the lacrimal sac in contrast with the remaining periorbital and/or orbital region where MALT lymphoma predominates.
Keywords: ocular lymphoma, lacrimal sac, MALT lymphoma
Lymphoid neoplasms constitute 10–15% of all tumours in the ocular region.1 Malignant lymphoid tumours, lymphomas, may either originate in the periorbital and/or orbital region (primary lymphoma) or arise in this region as a result of systemic spread (secondary lymphoma). Depending on the location of the tumour the patients may present with asymptomatic conjunctival salmon patches, ptosis or lid swelling because of eyelid involvement, painless proptosis and diplopia caused by an orbital mass, and with epiphora caused by infiltration of the lacrimal sac.2 Distinction between more than 40 different subtypes of lymphoma requires extensive histomorphological and immunophenotypical analyses. The relevance of these investigations is evident because the risk of tumour spread, treatment, and prognosis vary depending on the specific lymphoma type.3
In the ocular region, the majority of lymphomas are extranodal marginal zone B cell lymphomas of mucosa associated lymphoid tissue type (MALT lymphomas).4,5,6 MALT lymphoma is a low grade lymphoma. Systemic spread occurs typically late and localised disease is treated successfully with radiation therapy and a number of other treatments.7
Lacrimal sac lymphoma is infrequent,8,9 and most of the published cases classified according to the World Health Organization (WHO) classification are surprisingly diffuse large B cell lymphomas (DLBCL). DLBCL is a high grade non‐Hodgkin's lymphoma that runs an aggressive course but is potentially curable.10
This study was performed in order to assemble a large, unselected data set of primary lacrimal sac lymphoma to investigate the distribution of tumour subtypes and the clinical characteristics of these infrequent tumours in a predominantly white population.
Material and methods
Inclusion criteria
The study was designed as a retrospective study. Primary lacrimal sac lymphoma was diagnosed when the patient fulfilled the following criteria: (1) histopathology showed an infiltrate of malignant lymphoid cells in the lacrimal sac, and (2) no previous diagnosis of malignant lymphoma elsewhere has been known. All patients were anonymised with regard to the investigators.
Histopathological specimens
The specimens were collected from pathology institutes affiliated with the Ophthalmic Oncology Task Force of the European Organization for Research and Treatment of Cancer (EORTC) and European Ophthalmic Pathology Society (EOPS). A total of 18 tumours were retrieved and the clinical records of each patient were reviewed. Three patients were excluded because the review of the clinical records showed that lacrimal sac involvement was secondary and represented dissemination from a lymphoma localised elsewhere. Thus, 15 patients who fulfilled the inclusion criteria were enrolled in the study.
Clinical data
Clinical data recorded included year of diagnosis, sex, age at diagnosis, symptoms and clinical findings for 14 patients, evidence of systemic involvement including the stage of the disease at the time of diagnosis according to the Ann Arbor Staging Classification11 for nine patients, the type of therapy for 10 patients, the duration of clinical follow up and the stage of disease at last follow up for seven patients, and the date and cause of eventual death for six patients. Attempts to retrieve clinical data for the remaining patients were unsuccessful.
Histopathological examination
All specimens were stained with haematoxylin and eosin and an immunohistochemical panel with antibodies directed against bcl‐2, bcl‐6, CD3, CD5, CD10, CD20, CD23, CD43, CD79α, cyclin D‐1, MUM‐1, and MIB‐1 was applied. Five experienced pathologists examined the sections. Difficult cases were discussed in a plenum of all five pathologists, and a consensus diagnosis was reached on the basis of morphology and the immunophenotype according to the World Health Organization (WHO) lymphoma classification system.3
Results
Patients with primary lacrimal sac lymphoma were elderly (table 1). Their median age was 71 years (range 45–95) and 80% of the patients were older than 60 years. Nine of the patients were female and five were male.
Table 1 Clinical data of 15 primary lacrimal sac lymphomas in a predominantly white population.
| Case | Diagnosis | Year of diagnosis | Sex | Age (years) | Symptoms | Clinical findings | Systemic spread (months after diagnosis) | |
|---|---|---|---|---|---|---|---|---|
| A | DLBCL | 1989 | M | 95 | Dacryocystitis, epiphora, swelling | Obstruction of LS, ulcerating tumour | Not investigated because of old age | |
| B | DLBCL | 1995 | F | 59 | Epiphora, swelling | Tumour | None | |
| C | DLBCL | 1948 | M | 45 | Epiphora, swelling | Tumour | NA | |
| D | DLBCL | 1969 | F | 69 | Dacryocystitis, epiphora, swelling | Tumour | NA | |
| E | DLBCL | 1999 | M | 75 | Swelling | Tumour | None | |
| F | MALT | 2003 | M | 93 | Epiphora, swelling | Tumour | None | |
| G | MALT | 1985 | M | 71 | NA | NA | LN: regional + above diaphragm + BM (8) | |
| H | MALT | 1966 | F | 69 | Epiphora, swelling | Tumour, obstruction of LS | NA | |
| I | MALT | 1976 | F | 86 | Dacryocystitis, epiphora, swelling | Tumour, obstruction of LS | Not investigated because of old age | |
| J | MALT | 1991 | F | 71 | Epiphora, swelling | Tumour, obstruction of LS | None | |
| K | Transitional MALT | 1998 | F | 62 | Epiphora | Tumour, obstruction of LS | LN: neck (6) | |
| L | Transitional MALT | 1999 | F | 80 | Epiphora | Tumour, obstruction of LS | LN: orbit + above diaphragm (0)* | |
| M | Transitional MALT | 1970 | F | 85 | Swelling | Tumour | NA | |
| N | B cell, unclassified | 1990 | M | 57 | Epiphora, swelling | Tumour involving lid/conjunctiva | None | |
| O | B cell, unclassified | 2002 | F | 64 | Epiphora | Tumour | None |
BM, bone marrow; DLBCL, diffuse large B cell lymphoma; F, female; LN, lymph nodes; LS, lacrimal sac; M, male; MALT, extranodal marginal zone B cell lymphoma of mucosa associated lymphoid tissue; NA, not available.
*This patient had symptoms from the lacrimal sac more than 6 months before diagnosis was made.
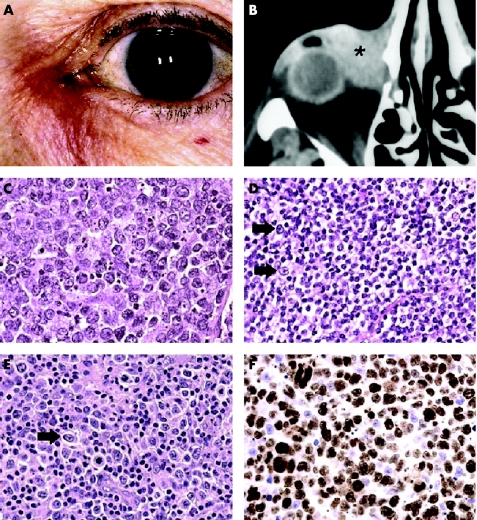
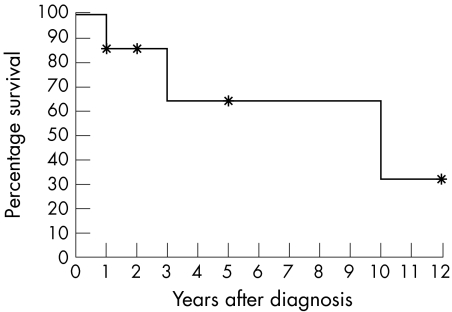
The most common presenting symptoms were epiphora in 12 patients (85%; 95% CI 62% to 100%) and swelling in the lacrimal sac region in 11 patients (79%; 95% CI 53% to 100%; fig 1A). The tumour was associated with dacryocystitis in three patients (21%; 95% CI 0% to 45%). Signs at presentation were a visible tumour in all cases (95% CI 91% to 100%; figs 1A and B) and obstruction of the lacrimal sac in six patients (43%; 95% CI 14% to 72%). The 5 year overall survival was 65% (fig 2).
Figure 1 (A) Redness and swelling of the lacrimal sac region in a 62 year old female (patient K) with transitional MALT lymphoma. Note that the tumour extends above the medial canthal ligament. (B) Computed tomography showing a tumour (asterisk) in the lacrimal sac of a 75 year old patient (patient E). The tumour was a diffuse large B cell lymphoma that regressed completely following chemotherapy and radiation therapy. (C) DLBCL characterised by large centroblasts with vesicular nuclei and prominent nucleoli in a 59 year old female (patient B) (haematoxylin and eosin, × 400). (D) A MALT lymphoma in a 71 year old male (patient G) characterised by a diffuse pattern of small centrocyte‐like cells and occasional larger centroblastic cells (arrows) (haematoxylin and eosin, × 400). (E) Transitional MALT lymphoma showing a mixture of small centrocyte‐like cells and large centroblasts (arrow). Note the increased number of centroblasts compared with the MALT lymphoma (haematoxylin and eosin, × 400). (F) A case of DLBCL showing the high proportion of cells in mitosis (90%) detected by labelling with MIB‐1 antibodies (brown nuclear staining) (×400).
Figure 2 Kaplan‐Meier overall survival curve for seven patients with primary lacrimal sac lymphoma. The 5 year overall survival was 65%. *Censored patient.
Review of histopathological specimens showed three types of lesion; DLBCL (fig 1C) consisting of sheets of large B lymphocytes, usually centroblastic in appearance, was diagnosed in five patients (33%) and MALT lymphoma (fig 1D) predominated by small, centrocyte‐like cells in five patients (33%). Three of the tumours (20%) had features between MALT lymphoma and DLBCL and was designated “transitional MALT lymphoma” (fig 1E). They consisted of centrocyte‐like cells admixed with a larger number of large B cells than found in a classic MALT lymphoma. The large cells were scattered among the centrocytes as single cells or, less frequently, as small clusters of up to 10 cells, but sheets of large B cells were not present. Hence, these lymphomas could not be classified as DLBCL, and they were tentatively designated MALT lymphoma in transition, assuming that the presence of increased numbers of large cells reflected incipient transformation to DLBCL.
Finally, two tumours remained unclassified B cell lymphomas because too many morphological details had been lost during preparation.
Diffuse large B cell lymphoma
Clinical data
The median age was 69 years (range, 45–95; table 1). Two patients whose stage was known had only involvement of the lacrimal sac at time of diagnosis (stage I).
Immunohistochemical reactions
The tumour cells were positive for CD20 and CD79α, and negative for CD3, CD5, CD23, and cyclin D‐1. Variable reaction was seen with CD10 (1/5), CD43 (1/5), bcl‐2 (2/5), bcl‐6 (2/5), and MUM‐1 (3/5). MIB‐1 positive cells ranged from 25% to 90%.
Treatment
One patient who was 95 years old at presentation, received palliative radiation therapy and the stage of disease was not investigated (table 2). He died from his disease 1 year later. The other two patients received radiation and chemotherapy. One obtained complete remission and is alive after 5 years. Follow up data from the other patient are lacking.
Table 2 Treatment and outcome of 10 primary lacrimal sac lymphomas in a predominantly white population.
| Case | Diagnosis | Treatment | Ann Arbor stage of disease (diagnosis → follow up → last follow up) | Follow up period (years) | Alive/dead (years after diagnosis) | ||
|---|---|---|---|---|---|---|---|
| A | DLBCL | Palliative RT | Not investigated because of old age | None | DOD (1) | ||
| B | DLBCL | Surgery + CHOP × 3 + RT 30 Gy | I → NA | NA | NA | ||
| E | DLBCL | CHOP × 4 + RT 40 Gy | I → CR | 5 | Alive (5) | ||
| F | MALT | Incomplete excision. Refused further treatment | I → I | 1 | Alive (1) | ||
| G | MALT | Surgery + RT regional and other LN, 40 Gy + CHOP × 6 | I → IV | 7 | Dead, cause unknown (10) | ||
| J | MALT | RT 45 Gy | I → CR | 12 | Alive (12) | ||
| K | Transitional MALT | Rituximab dose NA | I → II → CR | 2 | NA | ||
| L | Transitional MALT | Surgery + CHOP × 3 | II* → IV | 3 | DOD (3) | ||
| N | B cell, unclassified | RT dose NA | I → NA | NA | NA | ||
| O | B cell, unclassified | CHOP × 3 + RT 40 Gy | I → CR | NA | NA |
CHOP, cyclophosphamide + hydroxydoxorubicine + oncovine + prednisone; CR, complete remission; DLBCL, diffuse large B cell lymphoma; DOD, dead of disease; Gy, Gray; LN, lymph nodes; MALT, extranodal marginal zone B cell lymphoma of mucosa associated lymphoid tissue; NA, not available; RT, radiation therapy.
*This patient had symptoms from the lacrimal sac more than 6 months before diagnosis was made.
MALT lymphoma
Clinical data
The median age was 71 years (range 69–93; table 1). All three patients whose stage was known had only involvement of the lacrimal sac at time of diagnosis (stage I).
Immunohistochemical reactions
Positive reaction was seen with CD20, CD79α, and bcl‐2, whereas the tumour cells were negative for CD3, CD5, CD10, CD23, cyclin‐D1, and bcl‐6 in all cases. Two of five tumours furthermore reacted with CD43 and MUM‐1. Proliferation index detected by labelling with MIB‐1 varied from 5% to 10%.
Treatment
One patient underwent incomplete excision of the tumour, but was alive without evidence of progression after 1 year (table 2). One patient achieved complete remission (CR) after radiation therapy, and the third patient showed progression with involvement of regional lymph nodes and the bone marrow 8 months after diagnosis despite initial radiation therapy. Additional chemotherapy did not produce any objective response either.
“Transitional MALT lymphoma”
Clinical data
The median age was 80 years (range 62–85; table 1). Of two patients whose stage was known, one had only involvement of the lacrimal sac (stage I). The other patient had a history of over 6 months of lacrimal sac obstruction, and showed at time of diagnosis involvement of regional lymph nodes (stage II).
Immunohistochemical reactions
The tumour cells were positive for CD20, CD79α, and MUM‐1 negative for CD3, CD5, CD10, CD23, bcl‐6, and cyclin D‐1. Variable reaction was seen with CD43 (1/3) and bcl‐2 (2/3). MIB‐1 positive cells ranged from 10% to 15%.
Treatment
One patient had regional spread of disease 6 months after diagnosis (table 2). She was treated with rituximab and achieved CR. The other patient progressed to stage IV, despite surgery and three courses of systemic combination therapy.
Unclassified B cell lymphoma
Clinical data
This subgroup consisted of one male, 57 years old, and one female, 64 years old. Both patients presented in stage I (table 2).
Immunohistochemical reactions
The tumour cells were CD20 and CD79α positive.
Treatment
One patient was treated with radiation therapy. The other achieved CR with three courses of chemotherapy and adjuvant radiation therapy.
Discussion
Lymphoma arising in the lacrimal sac is infrequent. In the literature, fewer than 50 cases of primary lacrimal sac lymphoma have been described over a period of 30 years. Previously there has been no global consensus concerning lymphoma classification until the Revised European‐American Classification of Lymphoid Neoplasms (REAL)12 followed by the WHO classification of tumours of haematopoietic and lymphoid tissues3 were published. Nineteen of the patients living in Europe, United States, and Japan have been diagnosed according to the REAL or the WHO classification systems,8,13,14,15,16,17,18,19,20,21 and are thus comparable to our study. Clinical data were reported for 12 of these patients (table 3). Of our 15 patients, only six were diagnosed during the past 10 years.
Table 3 Published cases with primary lacrimal sac lymphoma.
| Case | Diagnosis | Sex | Age | Symptoms | Stage* at diagnosis | Treatment | Follow up (months) | Authors | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | DLBCL | F | 70 | Epiphora, swelling | I | RT 50 Gy + CHOP × 1 | CR (26) | Nakamura et al15 | |
| 2 | DLBCL angiotropic | F | 87 | Epiphora, swelling, dacryocystitis | I | Excision | CR (24) | O'Connor et al19 | |
| 3 | DLBCL | M | 21 | Bloody epiphora, swelling | I | CHOP × 8 | CR (7) | Saccogna et al17 | |
| 4 | DLBCL | F | 72 | Epiphora, swelling, dacryocystitis | De Palma et al20 | ||||
| 5–6 | DLBCL | NA | NA | NA | I | NA | NA | McKelvie et al13 | |
| 7 | DLBCL | F | 74 | Epiphora, swelling, proptosis | I | RT + CHOP × 3 | Relapse LN (24) | Parmar and Rose16 | |
| 8 | DLBCL | F | 63 | Epiphora, swelling | I | RT + CT | CR (24) | Parmar and Rose16 | |
| 9 | MALT | F | 50 | Epiphora, swelling | I | RT | CR (16) | Parmar and Rose16 | |
| 10 | MALT | F | 56 | Epiphora, swelling | I | RT | CR (46) | Parmar and Rose16 | |
| 11 | MALT | F | 81 | Epiphora, swelling | I | Chlorambucil | CR (36) | Parmar and Rose16 | |
| 12 | MALT | M | 10 | Epiphora, swelling | I | CHOP × 2 | CR (30) | Schefler et al18 | |
| 13 | MALT | F | 55 | NA | NA | NA | Alive (98) | Takada et al21 | |
| 14–17 | MALT | NA | NA | NA | NA | NA | NA | Anderson et al8 | |
| 18 | Plasmacytoma | NA | NA | NA | NA | NA | NA | Anderson et al8 | |
| 19 | NK/T | F | 51 | Swelling | II† | CHOP × 6 | DOD (9) | Mori et al14 |
CHOP, cyclophosphamide + hydroxydoxorubicine + oncovine + prednisone; CR, complete remission; DLBCL, diffuse large B cell lymphoma; DOD, dead of disease; F, female; Gy, Gray; LN, lymph nodes; M, male; MALT, extranodal marginal zone B cell lymphoma of mucosa associated lymphoid tissue; NK/T, extranodal NK/T cell lymphoma; NA, not available; RT, radiation therapy.
*Ann Arbor stage.
†At presentation the lymphoma was localised to the lacrimal sac, 4 weeks later it extended into the ethmoidal sinus.
Epiphora and swelling that can be associated with dacryocystitis are the predominant symptoms of lacrimal sac lymphoma (tables 1 and 3). Blood in the tears has been reported only once.17 Two patients reported in the literature were much younger than our patients (10 years and 21 years at diagnosis), and the median age for all reported cases was 12 years younger than in our series (59 years and 71 years, respectively). This may reflect either reporting bias in literature that is largely based on single case reports, or the fact that race can influence susceptibility for different types of lymphoma. Our study should provide the most reliable estimate so far for a predominantly white population.
Of the 19 primary lacrimal sac lymphomas in the literature, eight were classified as DLBCL and nine as MALT lymphoma (table 3). Taken together with our study, it seems that DLBCL and MALT lymphoma are equally common in the lacrimal sac and both account for approximately one half of all cases. DLBCL is over‐represented in the lacrimal sac compared to other adnexal structures, where DLBCL is seen in only 8–13%.4,5,6,13,22
The ocular adnexae, including the tear drainage system are derived from the same ectoderm23 and a difference in ontogenesis is not a plausible cause for the over‐representation of DLBCL.
Lymphomagenesis depends on the biology and function of the resident lymphocytes and on lymphocyte homing to that specific site.24 The function of the resident lymphocytes is probably the same in the lacrimal sac and the conjunctiva because their lamina propria contain abundant lymphocytes and share characteristics of MALT equivalent to the lymphoid tissue of the gastrointestinal tract.25 Interestingly, the majority of sinonasal lymphomas in white people are DLBCL.26,27,28 However, the lymphoid tissue of the sinonasal region is not thought to be part of the MALT system.24 Possibly there is a difference in homing of the lymphocytes, meaning that the pathways for activation of naive lymphocytes as a response to antigenic stimulation are different in the sinonasal region and in the lacrimal sac, compared with the remaining ocular adnexa.
DLBCL may represent transformation/progression of a less aggressive lymphoma, such as MALT lymphoma.10,29,30 In our study, three tumours were classified intermediate between MALT lymphoma and DLBCL, and this observation makes it possible that transformation is contributing to the large proportion of DLBCL in the lacrimal sac. However, longitudinal studies of individual patients to support this assumption are not available. Recent studies of gastric MALT lymphoma have shown that at least two entities of MALT lymphoma exist depending on the genetic alterations in the tumour cells; (1) t(11;18) positive lymphoma, which is cytogenetically stable and represent 18% to 60% of cases, and (2) t(11;18) negative lymphoma which shows chromosomal instability and acquire various genetic abnormalities, identical to some of the aberrations found in DLBCL.31,32 Furthermore, t(11;18) has not been found in either de novo or transformed high grade lymphomas. These observations indicate that transformation from low grade to high grade lymphoma is caused by accumulation of additional genetic aberrations and that only t (11;18) negative MALT lymphoma may transform into DLBCL.31,32,33 In the ocular region the great majority of MALT lymphoma is t(11;18) negative.21,31,34 Therefore, the risk of transformation is present.
It has been suggested that persistent antigenic stimulation is required for tumour progression of MALT lymphomas.30 Infections in the lacrimal sac are difficult to treat and often become chronic leading to persistent antigenic stimulation. Dacryocystitis was reported in two of five DLBCL patients in the present study and in two of six DLBCL patients in other publications.19,20 Possibly the somewhat hidden lacrimal sac location may delay the diagnosis and thereby increase the probability of further genetic alterations leading to transformation from MALT lymphoma to DLBCL.
Primary treatment with surgery, irradiation, and chemotherapy alone or in combination led to complete remission in eight patients reported in the literature (table 3). These data are in accordance with our study (table 2). Five year overall survival for seven patients in our study was 65% and two of eight patients died of their disease, one with DLBCL and the other with “transitional MALT lymphoma” despite treatment with systemic chemotherapy. A third patient with MALT lymphoma progressed 8 months after diagnosis to stage IV and did not respond to surgery, chemotherapy, and radiation therapy. Nevertheless, he survived for 10 years before dying of an unknown cause. Such a course is not uncommon in stage IV MALT lymphoma.7,22. Other larger studies of lymphoma in the ocular region have shown that lymphoma related death rate varies from 2% to 12% in MALT lymphoma and from 38% to 48% in DLBCL.13,22
Treatment both in our study and in previous studies consists of a mixture of surgery, chemotherapy, and radiation therapy. No commonly agreed treatment regimen for periocular lymphoma exists. The choice of treatment is primarily based on clinical trials of lymphomas in other sites because of the limited number of patients with periocular lymphoma. Considering the aggressive behaviour of DLBCL, which is common in the lacrimal sac region, referral of the patients to a medical oncologist should not be postponed.
Acknowledgements
The study was supported by grants from the Velux Foundation, the Synoptik Foundation, and the Foundation of 17.12.1981.
Abbreviations
CHOP - cyclophosphamide + hydroxydoxorubicine + oncovine + prednisone
CR - complete remission
DLBCL - diffuse large B cell lymphoma
EOPS - European Ophthalmic Pathology Society
EORTC - European Organization for Research and Treatment of Cancer
MALT - mucosa associated lymphoid tissue
REAL - Revised European‐American Classification of Lymphoid Neoplasms
Footnotes
Competing interests: none.
Presented at European Association for Vision and Eye Research, Vilamoura, Portugal, September 2004.
References
- 1.Knowles D M, Jakobiec F A. Ocular adnexal lymphoid neoplasms: clinical, histopathologic, electron microscopic, and immunologic characteristics. Hum Pathol 198213148–162. [DOI] [PubMed] [Google Scholar]
- 2.Specht C S. Benign and malignant lymphoid tumors, leukemia and histiocytic lesions. In: Albert DM, Jakobiec FA, eds. Principles and practice of ophthalmology. Philadelphia: WB Saunders, 19943328–3350.
- 3.Jaffe E S, Harris N L, Stein H.et alWorld Health Organization classification of tumours. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyons: IARC Press, 2001
- 4.Coupland S E, Krause L, Delecluse H J.et al Lymphoproliferative lesions of the ocular adnexa. Analysis of 112 cases. Ophthalmology 19981051430–1441. [DOI] [PubMed] [Google Scholar]
- 5.Coupland S E, Hellmich M, Auw‐Haedrich C.et al Prognostic value of cell‐cycle markers in ocular adnexal lymphoma: an assessment of 230 cases. Graefes Arch Clin Exp Ophthalmol 2004242130–145. [DOI] [PubMed] [Google Scholar]
- 6.White W L, Ferry J A, Harris N L.et al Ocular adnexal lymphoma. A clinicopathologic study with identification of lymphomas of mucosa‐associated lymphoid tissue type. Ophthalmology 19951021994–2006. [DOI] [PubMed] [Google Scholar]
- 7.Isaacson P G, Berger F, Müller‐Hermeling H K.et al Extranodal marginal zone B‐cell lymphoma of mucosa‐associated lymphoid tissue (MALT lymphoma). In: Jaffe ES, Harris NL, Stein H, et al, eds. World Health Organization classification of tumours. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyons: IARC Press, 2001157–160.
- 8.Anderson N G, Wojno T H, Grossniklaus H E. Clinicopathologic findings from lacrimal sac biopsy specimens obtained during dacryocystorhinostomy. Ophthal Plast Reconstr Surg 200319173–176. [DOI] [PubMed] [Google Scholar]
- 9.Stefanyszyn M A, Hidayat A A, Pe'er J J.et al Lacrimal sac tumors. Ophthal Plast Reconstr Surg 199410169–184. [DOI] [PubMed] [Google Scholar]
- 10.Gatter K C, Warnke R A. Diffuse large B‐cell lymphoma. In: Jaffe ES, Harris NL, Stein H, et al., eds. World Health Organization classification of tumours. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyons: IARC Press, 2001171–174.
- 11.Carbone P P, Kaplan H S, Musshoff K.et al Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res 1971311860–1861. [PubMed] [Google Scholar]
- 12.Harris N L, Jaffe E S, Stein H.et al A revised European‐American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 1994841361–1392. [PubMed] [Google Scholar]
- 13.McKelvie P A, McNab A, Francis I C.et al Ocular adnexal lymphoproliferative disease: a series of 73 cases. Clin Experiment Ophthalmol 200129387–393. [DOI] [PubMed] [Google Scholar]
- 14.Mori T, Tokuhira M, Mori S.et al Primary natural killer cell lymphoma of the lacrimal sac. Ann Hematol 200180607–610. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura K, Uehara S, Omagari J.et al Primary non‐Hodgkin's lymphoma of the lacrimal sac: a case report and a review of the literature. Cancer 1997802151–2155. [DOI] [PubMed] [Google Scholar]
- 16.Parmar D N, Rose G E. Management of lacrimal sac tumours. Eye 200317599–606. [DOI] [PubMed] [Google Scholar]
- 17.Saccogna P W, Strauss M, Bardenstein D S. Lymphoma of the nasolacrimal drainage system. Otolaryngol Head Neck Surg 1994111647–651. [DOI] [PubMed] [Google Scholar]
- 18.Schefler A C, Shields C L, Shields J A.et al Lacrimal sac lymphoma in a child. Arch Ophthalmol 20031211330–1333. [DOI] [PubMed] [Google Scholar]
- 19.O'Connor S R, Tan J H, Walewska R.et al Angiotropic lymphoma occurring in a lacrimal sac oncocytoma. J Clin Pathol 200255787–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Palma P, Ravalli L, Modestino R.et al Primary lacrimal sac B‐cell immunoblastic lymphoma simulating an acute dacryocystitis. Orbit 200322171–175. [DOI] [PubMed] [Google Scholar]
- 21.Takada S, Yoshino T, Taniwaki M.et al Involvement of the chromosomal translocation t(11;18) in some mucosa‐associated lymphoid tissue lymphomas and diffuse large B‐cell lymphomas of the ocular adnexa: evidence from multiplex reverse transcriptase‐polymerase chain reaction and fluorescence in situ hybridization on using formalin‐fixed, paraffin‐embedded specimens. Mod Pathol 200316445–452. [DOI] [PubMed] [Google Scholar]
- 22.Jenkins C, Rose G E, Bunce C.et al Histological features of ocular adnexal lymphoma (REAL classification) and their association with patient morbidity and survival. Br J Ophthalmol 200084907–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sevel D. Development and congenital abnormalities of the nasolacrimal apparatus. J Pediatr Ophthalmol Strabismus 19811813–19. [DOI] [PubMed] [Google Scholar]
- 24.Jaffe E S. Lymphoid lesions of the head and neck: a model of lymphocyte homing and lymphomagenesis. Mod Pathol 200215255–263. [DOI] [PubMed] [Google Scholar]
- 25.Paulsen F P, Paulsen J L, Thale A B.et al Organized mucosa‐associated lymphoid tissue in human nasolacrimal ducts. Adv Exp Med Biol 2002506873–876. [DOI] [PubMed] [Google Scholar]
- 26.Cuadra‐Garcia I, Proulx G M, Wu C L.et al Sinonasal lymphoma: a clinicopathologic analysis of 58 cases from the Massachusetts General Hospital. Am J Surg Pathol 1999231356–1369. [DOI] [PubMed] [Google Scholar]
- 27.Abbondanzo S L, Wenig B M. Non‐Hodgkin's lymphoma of the sinonasal tract. A clinicopathologic and immunophenotypic study of 120 cases. Cancer 1995751281–1291. [DOI] [PubMed] [Google Scholar]
- 28.Proulx G M, Caudra‐Garcia I, Ferry J.et al Lymphoma of the nasal cavity and paranasal sinuses: treatment and outcome of early‐stage disease. Am J Clin Oncol 2003266–11. [DOI] [PubMed] [Google Scholar]
- 29.Horning S J, Rosenberg S A. The natural history of initially untreated low‐grade non‐Hodgkin's lymphomas. N Engl J Med 19843111471–1475. [DOI] [PubMed] [Google Scholar]
- 30.Matolcsy A. High‐grade transformation of low‐grade non‐Hodgkin's lymphomas: mechanisms of tumor progression. Leuk Lymphoma 199934251–259. [DOI] [PubMed] [Google Scholar]
- 31.Ott G, Katzenberger T, Greiner A.et al The t(11;18)(q21;q21) chromosome translocation is a frequent and specific aberration in low‐grade but not high‐grade malignant non‐Hodgkin's lymphomas of the mucosa‐associated lymphoid tissue (MALT‐) type. Cancer Res 1997573944–3948. [PubMed] [Google Scholar]
- 32.Starostik P, Patzner J, Greiner A.et al Gastric marginal zone B‐cell lymphomas of MALT type develop along 2 distinct pathogenetic pathways. Blood 2002993–9. [DOI] [PubMed] [Google Scholar]
- 33.Gascoyne R D. Molecular pathogenesis of mucosal‐associated lymphoid tissue (MALT) lymphoma. Leuk Lymphoma 200344(Suppl 3)13–20. [DOI] [PubMed] [Google Scholar]
- 34.Streubel B, Simonitsch‐Klupp I, Mullauer L.et al Variable frequencies of MALT lymphoma‐associated genetic aberrations in MALT lymphomas of different sites. Leukemia 2004181722–1726. [DOI] [PubMed] [Google Scholar]