Abstract
Background/aim
Involvement of programmed death‐1 (PD‐1) and its ligands has been demonstrated in experimental allergic airway disease. Here, the authors aimed to examine whether PD‐1 and its ligands are involved in the development of experimental allergic conjunctivitis (EC) in mice.
Methods
EC was induced in Balb/c mice by active immunisation with short ragweed pollen (RW) in alum. 10 days later (day 10), the mice were challenged with eye drops containing RW. 24 hours after the challenge, conjunctivas, spleens, and sera were harvested for histological analysis, cytokine assays, and measurement of RW specific Ig levels. The actively immunised mice were treated with anti‐PD‐1, anti‐PD‐L1, anti‐PD‐L2 antibodies (Abs), or normal rat immunoglobulin G (nrIgG) during either the induction (day 0, 2, 4, 6, and 8) or the effector (2 hours before RW challenge on day 10) phase.
Results
Ab treatment during the induction phase did not affect eosinophil infiltration although immune responses were modulated. In contrast, treatment with anti‐PD‐L2 Ab, but not anti‐PD‐1 or anti‐PD‐L1 Ab, during the effector phase significantly increased eosinophil infiltration into the conjunctiva without affecting systemic immune responses.
Conclusions
Similar to allergic airway inflammation, PD‐L2 is involved in the development of EC during the effector phase but not the induction phase.
Keywords: allergic conjunctivitis, mouse, programmed death, ligands
The severity of eosinophil infiltration into the conjunctiva parallels the clinical severity of allergic conjunctivitis (AC), because more eosinophils infiltrate into the conjunctiva in patients with severe types of AC such as vernal keratoconjunctivitis (VKC)1 and atopic keratoconjunctivitis (AKC).2 In addition to eosinophils, T cells may be involved in the development of VKC, since many CD4+ T cells infiltrate the conjunctiva of active VKC patients.3,4 Furthermore, the observations indicating that infiltrating T cells produce cytokines in the conjunctiva5,6 suggest that T cells exert their effects at the inflammatory site, conjunctiva. Mechanistic analyses using rats and mice have confirmed that Ag specific CD4+ T cells play a crucial part in the development of experimental AC (experimental immune mediated blepharoconjunctivitis, EC) in terms of eosinophil infiltration into the conjunctiva.7,8,9 Immunohistochemical analyses of conjunctivas from rats8 and mice9 developing EC demonstrated that eosinophils and T cells predominantly infiltrated into the conjunctiva, thus resembling the conjunctivas in VKC patients.2,3,4 Additional analyses using transgenic and knockout mice have confirmed that Th2 type T cells are putatively involved in the development of EC.10
Activation of naive T cells requires the signals from both the T cell receptor and co‐stimulatory molecules.11 Because EC is mediated by CD4+ T cells and since activation of T cells is required for EC development, we have investigated the involvement of several co‐stimulatory molecules in the development of EC. With regard to proteins within the tumour necrosis factor (TNF) receptor superfamily, a forced stimulation of 4‐1BB by intraperitoneal injection of agonistic anti‐4‐1BB Ab inhibited EC,12 while injection of agonistic anti‐OX40 Ab exacerbated EC.13 In contrast, blockade of B7 related protein 1 (B7RP‐1), which is a member of the B7 family and the ligand of inducible co‐stimulator (ICOS), did not affect the severity of EC.14 Thus, each co‐stimulatory molecule is involved differently in the development of EC.
Programmed death‐1 (PD‐1) is a member of an immunoglobulin (Ig) superfamily member and is related to CD28 and cytotoxic T lymphocyte associated antigen‐4 (CTLA‐4).15 PD‐1 is expressed on activated, but not resting, CD4+ and CD8+ T cells, on B cells and on myeloid cells.16,17 Initially, PD‐1 was identified as being involved in programmed cell death of a T cell hybridoma.16 However, subsequent studies have not supported a direct role in cell death. For example, a recent study indicated that PD‐1 ligand induced PD‐1 expressing cells to arrest the cell cycle in G0/G1.18 Two members of the B7 family, PD‐L1 (B7‐H1) and PD‐L2 (B7‐DC), have been identified to be the ligands for PD‐1.19 PD‐L1‐Ig fusion protein in vitro inhibits proliferation and cytokine production of both resting and previously activated T cells.20 Furthermore, proliferation of PD‐1 deficient T cells was not inhibited by PD‐L1‐Ig fusion proteins, indicating that stimulation through PD‐1 provides a negative signal to T cell activation.20 Thus, it may be that PD‐1 is a negative regulator of the development of EC, which is mediated by CD4+ T cells. In this study, we investigated the involvement of PD‐1 and its ligands in the development of EC.
Materials and methods
Mice
Inbred Balb/c mice (Japan SLC Inc, Hamamatsu, Shizuoka, Japan) were kept in specific pathogen free conditions at the animal facility of Kochi Medical School; 6–12 weeks old female mice were used in these studies. All research adhered to the Association for Research in Vision and Ophthalmology statement for the use of animals in ophthalmic and vision research.
Reagents
Short ragweed pollen (RW) was purchased from Polysciences, Inc (Warrington, PA, USA). RW extract was obtained from LSL Co Ltd (Tokyo, Japan). Aluminium hydroxide (alum) was purchased from Sigma (St Louis, MO, USA). Antagonistic anti‐PD‐1 mAb (RMP1‐14, rat IgG2a),21 anti‐PD‐L1 mAb (MIH6, rat IgG2a),21,22,23 and anti‐PD‐L2 mAb (TY25, rat IgG2a)21,22 Abs were prepared as described previously and were purified from ascitic fluid using a Protein G column. The preparations contained less than 100 pg endotoxin/ml. Normal rat IgG (nrIgG) was purchased from MP Biomedicals Inc (Aurora, OH, USA).
EC induction by active immunisation and treatment with Abs
RW adsorbed on alum was injected into the left hind footpad and at the base of the tail. A volume of 50 μl of the emulsion (50 μg of RW and 2 mg of alum) was injected into each site. The mice were injected intraperitoneally with 200 μg of purified anti‐PD‐1, anti‐PD‐L1, anti‐PD‐L2 Ab, or control rat IgG (n = 10 per group) on days 0, 2, 4, 6, and 8 after RW immunisation. On day 10, the eyes of the immunised mice were challenged with RW in PBS (2 mg in 10 μl per eye). In a separate experiment, the actively immunised mice (n = 10 per group) were injected intraperitoneally with 200 μg of each Ab on day 10 only, 2 hours before RW challenge. Twenty four hours after the RW challenge, the eyes, sera, and spleens were harvested for histological analysis, measurement of RW specific Ig levels, and cytokine assays, respectively.
Histological analysis
The eyes, including the conjunctivas, were harvested along with the lid margin by cutting with razor blades and scissors, and then fixed in 10% buffered formalin. Vertical 2 μm thick sections were cut and stained with Giemsa. Infiltrating eosinophils in the lamina propria mucosae of the tarsal and bulbar conjunctivas in the entire section were counted by two observers given blind samples. The sections counted were those from the central portion of the eye, which included the pupil and optic nerve head. Data are expressed as infiltrating eosinophil numbers per section. Since the counts vary depending on the severity of inflammation (when inflammation is severe, the thickness of lamina propria mucosae increases), the cell count data are expressed as infiltrating eosinophil numbers divided by area (mm2) as measured by Scion Image (Scion Corporation, Frederick, MD, USA). The data were presented as an average (SEM) of all the mice examined.
Measurement of cytokines in the culture supernatants
RBC depleted splenocytes (107 cells/ml) were cultured for 48 hours with RW extract (25 μg/ml) in 96 well flat bottom plates in a final volume of 0.2 ml RPMI 1640 medium supplemented with 10% fetal calf serum (FCS, ICN Biomedical Japan Co, Tokyo, Japan), 2‐mercaptoethanol (2‐ME, 5×10−5 M), l‐glutamine (2 mM), penicillin (100 U/ml), and streptomycin (100 μg/ml). The levels of IL‐4, IL‐5, IL‐10, IL‐13, and IFN‐γ in the culture supernatants were measured with commercially available ELISA kits (Duoset; R&D Systems, Minneapolis, MN, USA) according to the manufacturer's recommendations.
Measurement of RW specific Igs in serum
Twenty four hours after RW challenge, blood was collected and serum was prepared. RW specific Ig levels in serum were assessed by ELISA. Briefly, EIA plates (Costar, Corning, NY, USA) were coated with RW extract (1 μg/ml) at 4°C overnight. After blocking with 1% bovine serum albumin in phosphate buffered saline (PBS), serum samples were added and incubated for 2 hours at room temperature or overnight at 4°C. As the control, the quantified RW specific IgE and pooled hyperimmune serum were used. The plates were washed with PBS plus 0.05% Tween 20 (PBS/T) (Wako, Osaka, Japan) and incubated for 2 hours at room temperature with alkaline phosphatase (ALP) conjugated goat Ab specific for IgG1 or IgG2a (Zymed, San Francisco, CA, USA). In the case of IgE, biotin conjugated rat anti‐mouse IgE (BD Biosciences, Franklin Lakes, NJ, USA) was added to each well for 2 hours at room temperature. After washing, avidin‐ALP (Sigma) was added to each well for 1 hour. After washing with PBS/T, p‐nitrophenyl phosphate (p‐nitrophenyl phosphate liquid substrate system, Sigma) was added to each well and allowed to develop for 15 minutes Absorbance was measured at 405 nm. IgG1 and IgG2a concentrations were expressed as units (U)/ml relative to the pooled hyperimmune serum, while IgE concentrations were shown as ng/ml.
Immunohistochemistry
Sections were prepared for immunohistochemistry by following the previously described method.8 In brief, the eyes from naive or actively immunised mice that were developing EC without Ab treatment were immediately frozen in 3% carboxymethyl cellulose (CMC) gel. Sections 4 μm thick were prepared and fixed in methanol. Endogenous peroxidase activity was inhibited by incubation with 0.1% NaN3 and 0.3% H2O2 in distilled water for 10 minutes at room temperature. The samples were then exposed to anti‐PD‐L2 (TY25) for 30 minutes and subsequently to biotinylated anti‐rat Abs for another 30 minutes. As a negative control, samples were incubated with secondary Abs (biotinylated anti‐rat Abs) without exposing to anti‐PD‐L2 Ab. All slides were subjected to an avidin‐biotin‐complex kit (Vector laboratories Inc, USA) and then developed with 3,3′‐diaminobenzidine tetrahydrochloride (Sigma, St Louis, MO, USA).
Statistical analysis
Significant differences in serum Ig levels, cytokine production, and infiltrating eosinophil numbers between the Ab treated and nrIgG treated groups were determined by Student's t test. p Values less than 0.05 were considered significant.
Results
Treatment with anti‐PD‐1, anti‐PD‐L1, or anti‐PD‐L2 Ab during the induction phase did not affect the infiltration of eosinophils into the conjunctiva
To investigate whether PD‐1, PD‐L1, or PD‐L2 is involved in the development of EC during the induction phase, actively immunised mice were intraperitoneally injected with each Ab every other day from days 0 to 8. There were no significant differences in the numbers of infiltrating eosinophils into the conjunctiva among the four groups (fig 1).
Figure 1 Treatment with anti‐PD‐1, anti‐PD‐L1, or anti‐PD‐L2 Ab during the induction phase did not affect eosinophil infiltration into the conjunctiva. Balb/c mice were immunised with RW in alum. Ten days later (day 10), the mice were challenged with RW in eye drops and their conjunctivas were harvested 24 hours later for histological analysis by Giemsa staining. The mice were intraperitoneally injected with nrIgG, anti‐PD‐1, anti‐PD‐L1, or anti‐PD‐L2 Ab during the induction phase (200 μg per injection on days 0, 2, 4, 6, and 8). The infiltrating eosinophils were counted and the data are expressed as eosinophils per mm2 (n = 10 per group).
Treatment with anti‐PD‐1, anti‐PD‐L1, or anti‐PD‐L2 Ab during the induction phase affected both cellular and humoral immune responses
Since Ab treatment did not affect eosinophil infiltration into the conjunctiva, compared to the nrIgG treatment, we conducted control studies to exclude the possibility that the treatment with Abs exerted no effects. We evaluated both cellular and humoral immune responses. Treatment with anti‐PD‐1, anti‐PD‐L1 or anti‐PD‐L2 Abs significantly increased IL‐4, IL‐5, IL‐10, and IL‐13 production by splenocytes in response to the RW stimulation in vitro, especially when anti‐PD‐L2 Ab was injected (fig 2A). RW specific IgE levels in serum were not significantly different among the four groups, while RW specific IgG1 and IgG2a levels were significantly upregulated by treatment with anti‐PD‐L2 Ab (fig 2B). Thus, the treatment with anti‐PD‐1, anti‐PD‐L1, or anti‐PD‐L2 Ab significantly affected systemic immune responses.
Figure 2 Treatment with anti‐PD‐1, anti‐PD‐L1, or anti‐PD‐L2 Ab during induction phase affected systemic immune responses. Spleens and sera were collected at the time of harvesting the conjunctivas from actively immunised mice treated with nrIgG, anti‐PD‐1, anti‐PD‐L1, or anti‐PD‐L2 Ab during the induction phase as described in the legend to figure 1. The IL‐4, IL‐5, IL‐10, IL‐13, and IFN‐γ levels in the culture supernatants of splenocytes stimulated with RW were measured by ELISA (A). RW specific IgE, IgG1, and IgG2a levels were evaluated by ELISA (B). *p<0.05, **p<0.01, compared with nrIgG treated group.
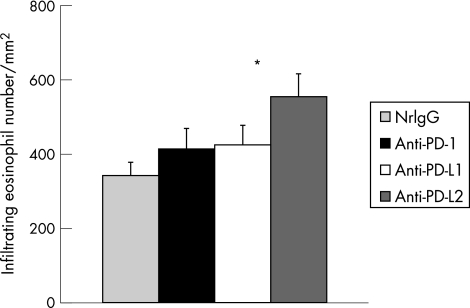
Treatment with anti‐PD‐L2 Ab during the effector phase increased eosinophil infiltration into the conjunctiva without significantly affecting systemic immune responses
To investigate whether PD‐1, PD‐L1, or PD‐L2 is involved in the development of EC during the effector phase, actively immunised mice were intraperitoneally injected with each Ab on day 10, 2 hours before RW challenge. Anti‐PD‐L2 Ab treatment significantly increased eosinophil infiltration (fig 3). Analyses of RW specific cytokine production (fig 4A) and RW specific Ig levels (fig 4B) demonstrated that no significant differences were observed among the four groups.
Figure 3 Treatment with anti‐PD‐L2 Ab during the effector phase increased eosinophil infiltration into the conjunctiva. Balb/c mice were immunised with RW in alum. Ten days later (day 10), the mice were challenged with RW in eye drops and their conjunctivas were harvested 24 hours later for histological analysis by Giemsa staining. The mice were intraperitoneally injected with 200 μg of nrIgG, anti‐PD‐1, anti‐PD‐L1, or anti‐PD‐L2 Ab during the effector phase on day 10, 2 hours before RW challenge. The infiltrating eosinophils were counted and the data are expressed as eosinophils per mm2 (n = 10 per group). *p<0.05, compared with nrIgG treated group.
Figure 4 Treatment with anti‐PD‐1, anti‐PD‐L1, or anti‐PD‐L2 Ab during the effector phase did not significantly affect systemic immune responses. Spleens and sera were collected at the time of harvesting the conjunctivas from actively immunised mice treated with nrIgG, anti‐PD‐1, anti‐PD‐L1, or anti‐PD‐L2 Ab during the effector phase as described in the legend to figure 3. The IL‐4, IL‐5, IL‐10, IL‐13, and IFN‐γ levels in the culture supernatants of splenocytes stimulated with RW were measured by ELISA (A). RW specific IgE, IgG1, and IgG2a levels were evaluated by ELISA (B).
PD‐L2 expression in the conjunctiva was upregulated by the induction of EC
Finally, to confirm the expression of PD‐L2 in the conjunctiva, conjunctivas from naive and EC developing mice were harvested for immunohistochemical analysis. A small number of PD‐L2 expressing cells were detected in the conjunctiva of naive mice (fig 5A). In contrast, PD‐L2 expressing cells were increased by the induction of EC (fig 5B). Negative controls did not show any positive signals (data not shown).
Figure 5 PD‐L2 expression in the conjunctiva was upregulated by the induction of EC. Conjunctivas from naive (A) and EC developing mice (B) were harvested for immunohistochemical analysis using anti‐PD‐L2 Ab (TY25). PD‐L2 expressing cells were detected in the conjunctiva of naive mice (A). By induction of EC, PD‐L2 expressing cells increased in the conjunctiva (B). Bar = 20 μm. One representative of four mice is shown. Arrows indicate Ab stained cells.
Discussion
In murine experimental asthma, the involvement of PD‐1 and its ligands has been demonstrated.21 Consistent with this previous report demonstrating that treatment with anti‐PD‐1 Ab did not affect asthmatic responses,21 we show here that treatment with anti‐PD‐1 Ab had no effect on EC during either the induction or the effector phase. In contrast, treatment with anti‐PD‐L2 Ab during the effector phase increased inflammation in the lung,21 a result in good agreement with our observation that anti‐PD‐L2 Ab treatment during the effector phase increased eosinophil infiltration into the conjunctiva. It has also been reported that injection of murine PD‐L2‐Fc fusion protein increased cell infiltration to bronchoalveolar lavage fluid.24 Recent studies have shown that PD‐L2‐Fc binds and co‐stimulates naive CD4+ T cells from PD‐1 deficient mice.25,26 Thus, our data further support the possibility that PD‐L2 interacts not only with PD‐1 but also with some receptor other than PD‐1.
Treatment with anti‐PD‐1, anti‐PD‐L1, or anti‐PD‐L2 Ab during the induction phase significantly affected RW specific immune responses. Particularly, treatment with anti‐PD‐L2 Ab markedly increased RW specific Th2 cytokine production and RW specific IgGs in serum. However, these changes in immune responses did not affect eosinophil infiltration into the conjunctiva. It remains unclear why the upregulated Th2 immune responses (IL‐4, IL‐5, and IL‐13) did not affect the eosinophil infiltration. It is of note that IL‐10, which regulates allergic reactions,27 was also upregulated in the anti‐PD‐L2 Ab treated group and the upregulation of IL‐10 might have suppressed the effector function of Th2 immune responses. Further study will be necessary to elucidate the role of IL‐10 in the development of EC.
Importantly, the upregulation of eosinophil infiltration by the treatment with anti‐PD‐L2 Ab was noted during the effector phase but not the induction phase indicating that the interaction between PD‐L2 and its putative receptor other than PD‐1 is important for the recruitment of eosinophils into the conjunctiva. In fact, using immunohistochemical methods, we found that expression of PD‐L2 in the conjunctiva was upregulated by the induction of EC. Although we have not examined which types of cells express PD‐L2 in the conjunctiva, it was reported that expression of PD‐L2 is restricted in macrophages and dendritic cells.28 Thus, the interaction between PD‐L2 presumably expressed in macrophages and dendritic cells and its receptor occurring in the conjunctiva is likely to be involved in eosinophil infiltration. Thus, PD‐L2 may be a therapeutic candidate for the inhibition of eosinophil infiltration into the conjunctiva in severe AC.
Acknowledgements
We thank Ms Waka Ishida and Kazuyo Fukata for their excellent technical help. This work was supported in part by grant in aid for scientific research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (AF).
Abbreviations
Abs - antibodies
AC - allergic conjunctivitis
AKC - atopic keratoconjunctivitis
ALP - alkaline phosphatase
B7RP‐1 - B7 related protein 1
CTLA - cytotoxic T lymphocyte associated antigen
EC - experimental allergic conjunctivitis
ICOS - inducible co‐stimulator
nrIgG - normal rat immunoglobulin G
PBS - phosphate buffered saline
PD - programmed death
PD‐L2 - programmed death‐ligand 2
RW - ragweed pollen
TNF - tumour necrosis factor
VKC - vernal keratoconjunctivitis
References
- 1.Bonini S, Bonini S, Lambiase A.et al Vernal keratoconjunctivitis revisited: a case series of 195 patients with long‐term followup. Ophthalmology 20001071157–1163. [DOI] [PubMed] [Google Scholar]
- 2.Fukagawa K, Nakajima T, Tsubota K.et al Presence of eotaxin in tears of patients with atopic keratoconjunctivitis with severe corneal damage. J Allergy Clin Immunol 19991031220–1221. [DOI] [PubMed] [Google Scholar]
- 3.Metz D P, Hingorani M, Calder V L.et al T‐cell cytokines in chronic allergic eye disease. J Allergy Clin Immunol 1997100817–824. [DOI] [PubMed] [Google Scholar]
- 4.Avunduk A M, Avunduk M C, Dayanir V.et al A flow cytometric study about the immunopathology of vernal keratoconjunctivitis. J Allergy Clin Immunol 1998101821–824. [DOI] [PubMed] [Google Scholar]
- 5.Leonardi A, DeFranchis G, Zancanaro F.et al Identification of local Th2 and Th0 lymphocytes in vernal conjunctivitis by cytokine flow cytometry. Invest Ophthalmol Vis Sci 1999403036–3040. [PubMed] [Google Scholar]
- 6.Matsuura N, Uchio E, Nakazawa M.et al Predominance of infiltrating IL‐4‐producing T cells in conjunctiva of patients with allergic conjunctival disease. Curr Eye Res 200429235–243. [DOI] [PubMed] [Google Scholar]
- 7.Fukushima A, Fukata K, Ozaki A.et al Exertion of the suppressive effects of IFN‐γ on experimental immune mediated blepharoconjunctivitis in brown Norway rats during the induction phase but not the effector phase. Br J Ophthalmol 2002861166–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukushima A, Ozaki A, Fukata K.et al Ag‐specific recognition, activation, and effector function of T cells in the conjunctiva with experimental immune‐mediated blepharoconjunctivitis. Invest Ophthalmol Vis Sci 2003444366–4374. [DOI] [PubMed] [Google Scholar]
- 9.Fukushima A, Yamaguchi T, Ishida W.et al Genetic background determines susceptibility to experimental immune‐mediated blepharoconjunctivitis: comparison of Balb/c and C57BL/6 mice. Exp Eye Res (in press) [DOI] [PubMed]
- 10.Ozaki A, Seki Y, Fukushima A.et al The control of allergic conjunctivitis by suppressor of cytokine signaling (SOCS)3 and SOCS5 in a murine model. J Immunol 20051755489–5497. [DOI] [PubMed] [Google Scholar]
- 11.June C H, Ledbetter J A, Gillespie M M.et al T‐cell proliferation involving the CD28 pathway is associated with cyclosporine‐resistant interleukin 2 gene expression. Mol Cell Biol 198774472–4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukushima A, Yamaguchi T, Ishida W.et al Engagement of 4‐1BB inhibits the development of experimental allergic conjunctivitis in mice. J Immunol 20051754897–4903. [DOI] [PubMed] [Google Scholar]
- 13.Fukushima A, Yamaguchi T, Ishida W.et al Roles of OX40 in the development of murine experimental allergic conjunctivitis: exacerbation and attenuation of eosniophil infiltration by stimulation and blocking of OX40. Invest Ophthalmol Vis Sci. (in press) [DOI] [PubMed]
- 14.Fukushima A, Yamaguchi T, Ishida W.et al The interaction between ICOS and B7RP‐1 is not required for the development of experimental murine allergic conjunctivitis. Biochem Biophys Res Commun 20053381726–1731. [DOI] [PubMed] [Google Scholar]
- 15.Ishida Y, Agata Y, Shibahara K.et al Induced expression of PD‐1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 1992113887–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agata Y, Kawasaki A, Nishimura H.et al Expression of the PD‐1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol 19968765–772. [DOI] [PubMed] [Google Scholar]
- 17.Vibhakar R, Juan G, Traganos F.et al Activation‐induced expression of human programmed death‐1 gene in T‐lymphocytes. Exp Cell Res 199723225–28. [DOI] [PubMed] [Google Scholar]
- 18.Latchman Y, Wood C R, Chernova T.et al PD‐L2 is a second ligand for PD‐1 and inhibits T cell activation. Nat Immunol 20012261–268. [DOI] [PubMed] [Google Scholar]
- 19.Greenwald R J, Freeman G J, Sharpe A H. The B7 family revisited. Annu Rev Immunol 200523515–548. [DOI] [PubMed] [Google Scholar]
- 20.Carter L, Fouser L A, Jussif J.et al PD‐1:PD‐L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL‐2. Eur J Immunol 200232634–643. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto K, Inoue H, Nakano T.et al B7‐DC regulates asthmatic response by an IFN‐gamma‐dependent mechanism. J Immunol 20041722530–2541. [DOI] [PubMed] [Google Scholar]
- 22.Yamazaki T, Akiba H, Iwai H.et al Expression of programmed death 1 ligands by murine T cells and APC. J Immunol 20021695538–5545. [DOI] [PubMed] [Google Scholar]
- 23.Tsushima F, Iwai H, Otsuki N.et al Preferential contribution of B7‐H1 to programmed death‐1‐mediated regulation of hapten‐specific allergic inflammatory responses. Eur J Immunol 2003332773–2782. [DOI] [PubMed] [Google Scholar]
- 24.Oflazoglu E, Swart D A, Anders‐Bartholo P.et al Paradoxical role of programmed death‐1 ligand 2 in Th2 immune responses in vitro and in a mouse asthma model in vivo. Eur J Immunol 2004343326–3336. [DOI] [PubMed] [Google Scholar]
- 25.Wang S, Bajorath J, Flies D B.et al Molecular modeling and functional mapping of B7‐H1 and B7‐DC uncouple costimulatory function from PD‐1 interaction. J Exp Med 20031971083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin T, Kennedy G, Gorski K.et al Cooperative B7‐1/2 (CD80/CD86) and B7‐DC costimulation of CD4+ T cells independent of the PD‐1 receptor. J Exp Med 200319831–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellinghausen I, Knop J, Saloga J. The role of interleukin 10 in the regulation of allergic immune responses. Int Arch Allergy Immunol 200112697–101. [DOI] [PubMed] [Google Scholar]
- 28.Greenwald R J, Freeman G J, Sharpe A H. The B7 family revisited. Annu Rev Immunol 200523515–548. [DOI] [PubMed] [Google Scholar]