Abstract
Aims
To determine the prevalence of intraocular pressure (IOP) alterations following intravitreal injection of triamcinolone acetonide (IVTA) and to assess possible risk factors of IOP elevation in eyes receiving single and/or repeat injections.
Methods
Retrospective, consecutive case series. 570 consecutive eyes of 536 patients who received a single IVTA injection (4 mg/0.1 ml) and a second set of 43 eyes of 40 patients who received a second injection. Retrospective review of all IVTA cases performed by three vitreoretinal surgeons over a 42 month period beginning in 2000. The main outcome measure was change in IOP defined as absolute value of IOP elevation (5 mm Hg or higher, 10 mm Hg or higher), and percentage of baseline (30% or higher increase from baseline IOP).
Results
Of the 528 eyes receiving single injections, 281 (53.2%) had an IOP elevation; 267 eyes (50.6%) experienced an elevation of IOP of at least 30%, and 245 (45.8%) and 75 (14.2%) eyes had an increase of 5 mm Hg or 10 mm Hg or more, respectively. Baseline IOP greater than 16 mm Hg is a risk factor for post‐injection IOP elevation. Of the 43 eyes which received a second injection, 28 (65.1%) experienced an increase in IOP of at least 30% of baseline. Filtering surgery was required in five (0.094%) of the single and one (2.3%) of repeat injection eyes.
Conclusions
Elevated IOP after IVTA is common and patients should be monitored beyond 6 months post‐injection. Patients with a baseline IOP more than 16 mm Hg or receiving a second injection should be carefully monitored for an elevated IOP.
Keywords: intravitreal triamcinolone, steroid induced glaucoma
Intravitreal triamcinolone acetonide (IVTA) has been used for the treatment of pseudophakic macular oedema,1,2 exudative macular degeneration,3,4,5 macular oedema following central retinal vein occlusion,6 diffuse diabetic macular oedema,7,8 iris neovascularisation,9 and idiopathic juxtafoveal telangiectasis.10 Reported complications include elevated intraocular pressure (IOP), cataract, pseudohypopyon, and endophthalmitis.1,2,3,4,5,6,7,8,9,10,11 Studies investigating the efficacy of IVTA have noted the prevalence of IOP elevations to be between 28–77%.12,13,14,15,16,17,18,19,20 The wide range in prevalence may be because of varying definitions of IOP increase (>5 mm Hg from baseline versus >21 mm Hg versus no clear definition), sample size (n = 9–113), length of follow up (6 weeks–36 months), and triamcinolone dose (4 mg versus 25 mg).
The purpose of this study was to determine the prevalence of IOP alterations following an IVTA injection(s), and assess possible risk factors for development of IOP alteration in a large case series.
Methods
Following institutional review board approval (No 03‐566), we retrospectively reviewed all cases of IVTA injections performed by three vitreoretinal surgeons over a 42 month period beginning in 2000. Eyes were included if a pre‐injection and at least one follow up IOP measurement were recorded. Eyes with a history of previous IVTA injections were analysed separately. Any eye not having a baseline (that is, pre‐injection) IOP value and at least one postoperative IOP value were excluded.
The injection technique was similar for all patients. Informed consent was obtained. Betadine 5% was applied to the conjunctival fornix and eyelashes. Anaesthesia consisted of topical tetracaine and a sterile cotton tipped applicator soaked in lidocaine 2% applied at the inferior pars plana injection site (4 mm posterior to the limbus in phakic eyes/3.5 mm in pseudophakic eyes). Triamcinolone acetonide (4 mg in 0.1 ml) (Kenalog 40, Bristol‐Myers Squibb) was injected through the inferior pars plana into the vitreous cavity. Indirect ophthalmoscopy confirmed proper intravitreal localisation of the suspension and perfusion of the optic nerve head. IOP was measured 15 minutes later and an anterior chamber paracentesis was performed if there was an elevation of IOP greater than 35 mm Hg for more than 1 hour.
The main outcome measure was an IOP increase, defined both by absolute value of IOP elevation (5 mm Hg or higher, 10 mm Hg or higher), and percentage of baseline IOP (that is, an increase equal to 30% of the pre‐injection IOP or higher). These definitions are consistent with other studies of steroid induced glaucoma and generally represent a clinically significant IOP elevation.21,22 IOP measurements were obtained at 4–7 days, 1, 3, 6, 9, and 12 months postoperatively. At the baseline and each postoperative IOP recording, the number of glaucoma medications the eye was receiving was recorded. The addition of a medication in response to an elevated IOP is reflected in the number of medications at the next follow up interval. For the group of repeat IVTA injection eyes, the last follow up for the initial injection was defined as the baseline IOP reading for the second injection.
Additional data were collected including: age, eye injected, sex, indication for injection, use and number of glaucoma medications, pre‐injection use of topical steroids, the need for cataract surgery, steroid induced glaucoma requiring surgery (refractory to medical therapy), need for repeated IVTA, and pre‐existing glaucoma, diabetes, and hypertension. In some cases, the retina specialist prescribed topical prednisolone acetate four times daily for 4–6 weeks before the injection as a provocative IOP test.
Statistical methods
All calculations were performed using SPSS version 10.0. Descriptive statistics, Kaplan‐Meier survival curves, and Cox proportional analysis, were used for estimating time to event models in the presence of censored data (that is, eyes that never achieved the event of interest an IOP increase). Kaplan‐Meier estimates with their associated confidence intervals were computed to estimate the probability of survival, defined as not experiencing an IOP increase, over the course of 1 year of follow up. We used Cox proportional hazard regression to study the effect of baseline and demographic variables on survival. Cox proportional hazard analysis for duration of survival to each end point of interest, fail by 5 mm Hg, fail by 10 mm Hg, and fail by an increase in IOP of 30% of baseline, was performed. Each equation included the following variables: pre‐existing glaucoma, age, sex, hypertension, diabetes, steroid use at baseline, number of antiglaucoma medications at baseline, and IOP at baseline. The χ2 test was used to compare the prevalence of an IOP elevation in single versus repeat injections.
Results
We reviewed 570 consecutive eyes of 536 patients who received a single IVTA injection and a second set of 43 eyes of 40 patients who received more than one injection. Forty two eyes (7.4%) of 42 patients were excluded owing to the absence of either a baseline IOP or subsequent follow up IOP measurements; all excluded were from the single injection group. Overall, the eyes which received more than one IVTA injection were at a greater risk of developing a postoperative IOP elevation (p = 0.05).
Single injections
Our sample was mostly older women (table 1). The average length of follow up was 5.67 (SD 3.63) months for those eyes that received one IVTA injection.
Table 1 Baseline characteristics.
| Single IVTA injection (%) | Repeated IVTA Injections (%) | Comparison of single v repeated p values | |
|---|---|---|---|
| Sample size | 528 | 43 | |
| Sex | 0.70 | ||
| Male | 242 (45.8%) | 21 (48.8%) | |
| Female | 286 (54.2%) | 22 (51.2%) | |
| Age | |||
| Mean | 70.26 | 69.53 | 0.70 |
| Range | 14–95 | 44–89 | |
| SD | 11.77 | 10.55 | |
| Pre‐existing glaucoma | 90 (17.0%) | 7 (16.3%) | 0.89 |
| Use of antiglaucoma drugs | 43 (47.8%) | 6 (85.7%) | 0.007 |
| Average number of drugs (SD) | 0.29 (0.72) | 0.56 (0.98) | |
| Hypertension | 297 (56.3%) | 18 (41.9) | 0.07 |
| Diabetes | 298 (56.4%) | 29 (67.4) | 0.16 |
| Use of topical steroids | 29 (5.5%) | 4 (9.3) | 0.52 |
| Baseline IOP (mm Hg) (SD) | 15.51 (3.63) | 15.53 (3.24) (range 4–22) | 0.97 |
| Baseline VA (logMAR) (SD) | 0.809 (0.477) | 0.879 (0.367) | 0.44 |
| Diagnoses | |||
| NPDR | 270 (51.1%) | 18 (41.9%) | |
| PDR | 89 (16.9%) | 8 (18.6%) | |
| BRVO/CRVO | 69 (13.1%) | 5 (11.6%) | |
| CMO | 39 (7.4%) | 11 (25.6%) | |
| Uveitis | 3 (0.6%) | None | |
| Other | 58 (11%) | 1 (2.3%) |
NPDR, non‐proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; BRVO, branch retinal vein occlusion; CRVO, central retinal vein occlusion; CMO, cystoid macular oedema.
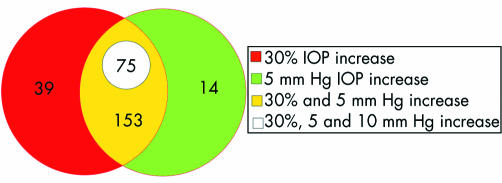
Of the 528 single IVTA cases, 281 eyes (53.2%) had an IOP elevation; 267 eyes (50.6%) experienced an elevation of IOP of 30% or more than their preinjection IOP; 245 eyes (45.8%) experienced an elevation of 5 mm Hg or more, and 75 eyes (14.2%) experienced an IOP rise of 10 mm Hg or more during the course of the study. The mean survival times were >6 months for all failure criteria (fig 1). In all 75 eyes which experienced a greater than 10 mm Hg increase in IOP, the elevation represented more than a 30% increase of their baseline IOP (that is, pre‐injection) (fig 2). However, 14 eyes experienced an elevation greater than 5 mm Hg (but less than 10 mm Hg), where this change represented less than 30% of their baseline IOP. Conversely, there were 39 eyes that experienced an elevation of greater than 30% of their baseline, but the absolute change was less than 5 mm Hg.
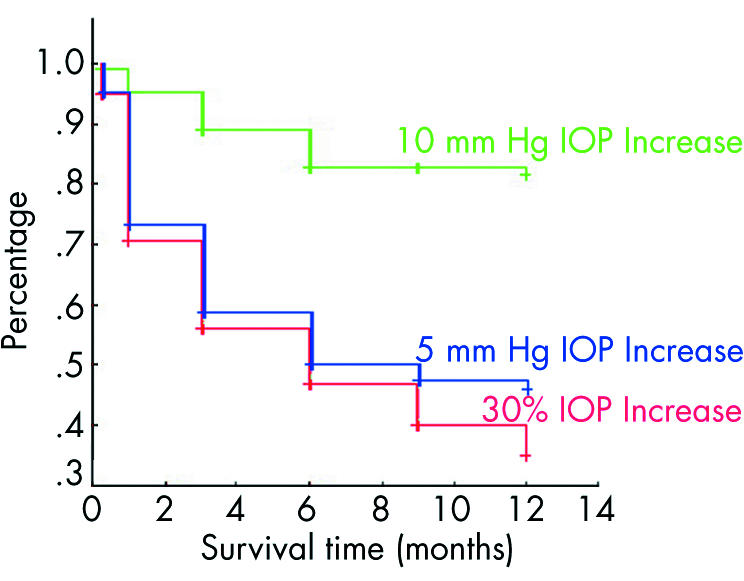
Figure 1 Kaplan‐Meier survival graph for single IVTA injections. All data points are censored. The mean and median survival times, where applicable, were mean 7.12 (SE 0.23) months, 95% CI 6.67 to 7.57, and median 9 months for an increase of 5 mm Hg or more; for 10 mm Hg or more, the mean was 10.55 (SE 0.16) months, 95% CI 10.24 to 10.86, but the median could not be calculated (since less than half of cases failed); for an increase of 30% or more the mean was 6.66 (SE 0.23) months, 95% CI 6.21 to 7.11, and median survival time was 6 months.
Figure 2 Venn diagram showing overlapping definitions of IOP elevation. The 39 eyes in the red coloured area had >30% rise which was less than 5 mm Hg. In all eyes that had a 10 mm Hg, the elevation represented more than a 30% increase of baseline IOP.
Cox proportional hazard analysis did not show any variables associated with the survival time to a 10 mm Hg IOP increase (table 2).
Table 2 p Values from Cox proportional hazard regression equations for single and repeat injections.
| IOP increase after single injection | IOP increase after repeat injection | |||||
|---|---|---|---|---|---|---|
| >5 mm Hg | >10 mm Hg | >30% mm Hg | >5 mm Hg | >10 mm Hg | >30% mm Hg | |
| Baseline IOP | 0.0001 | 0.251 | 0.0001 | 0.135 | 0.013 | 0.049 |
| Age | 0.152 | 0.815 | 0.227 | 0.914 | 0.838 | 0.715 |
| Sex | 0.764 | 0.289 | 0.957 | 0.106 | 0.787 | 0.012 |
| Eye | 0.743 | 0.690 | 0.686 | 0.770 | 0.457 | 0.799 |
| Glaucoma treatment | 0.056 | 0.536 | 0.362 | 0.596 | 0.355 | 0.935 |
| Use of glaucoma drugs | 0.57 | 0.955 | 0.937 | |||
| HTN | 0.632 | 0.903 | 0.126 | 0.910 | 0.492 | 0.957 |
| DM | 0.118 | 0.488 | 0.135 | 0.245 | 0.968 | 0.137 |
| Gl drugs | 0.502 | 0.409 | 0.745 | 0.877 | 0.366 | 0.568 |
| Topical steroids | 0.051 | 0.699 | 0.354 | 0.619 | 0.977 | 0.301 |
| First injection: | ||||||
| Fail by 5% | 0.967 | 0.548 | 0.700 | |||
| Fail by 10% | 0.519 | 0.886 | 0.495 | |||
| Fail by 30% | 0.885 | 0.802 | 0.699 | |||
HTN, hypertension; DM, diabetes mellitus.
For both the 5 mm Hg increase and 30% IOP increase failure criteria, only baseline IOP (p = 0.0001 and p = 0.0001, respectively) significantly predicted for survival time after adjusting for all other variables. A lower pre‐injection IOP was associated with a longer survival time. A baseline IOP 1 mm Hg above the mean was associated with a decreased chance of survival at any given time point that was 0.904 (for a greater than 5 mm Hg elevation) and 0.865 (for an elevation greater than 30% of baseline IOP) the rate for a baseline IOP 1 mm Hg lower. In other words, an eye with a baseline IOP 1 mm Hg higher than the group mean of 15.51 mm Hg had an increased chance of an elevated IOP—that is, 1.096 (for a 5 mm Hg elevation) and 1.135 (for an elevation greater than 30% of baseline IOP), greater than an eye with a baseline IOP 1 mm Hg lower.
Eyes with pre‐existing glaucoma (single IVTA)
The baseline IOP for patients with pre‐existing glaucoma (n = 90) was 17.36 (4.22) mm Hg which was different than those without glaucoma (n = 437; 15.12 (3.37) mm Hg; p<0.001). However, the glaucoma status did not predict an IOP elevation in either univariate or multivariate analyses.
Eyes needing anti‐glaucoma treatment
In all, 124 of the 528 eyes (23.5%) of the single IVTA group had IOP lowering medications added after the injection. Of these 124 eyes, 21 (16.9%) had a medication added at 1 week, 49 (39.5%) at month 1, 32 (25.8%) at month 3, 18 (14.5%) at month 6, and four (3.2%) at month 9. The average duration of increased medication use was 5.64 months (SD 3.05, range 0.75–11.75 months). Five eyes (0.9%) required filtration surgery for elevated IOPs refractory to medications for greater than 2 months.
Repeat injections
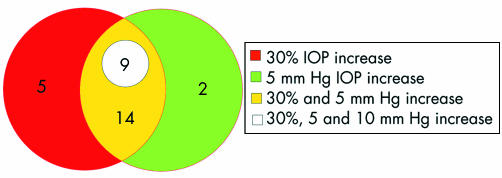
Of the 43 eyes, analysed separately, which received a second injection, 30 (69.7%) experienced an IOP elevation; 28 (65.1%) had an IOP elevation that was 30% or greater than baseline, 25 (58%) experienced an elevation of 5 mm Hg or more, and nine (21%) experienced an IOP rise of 10 mm Hg or more after the second injection. The average length of follow up was 6.41 (3.83) months. Mean survival times were 5.57, 9.66, and 3 months, respectively, for an increase greater than 5 mm Hg, greater than 10 mm Hg, and an elevation of 30% or greater than baseline (fig 3). The IOP elevation of greater than 10 mm Hg also represented more than 30% of the baseline IOP in the nine affected eyes (fig 4). In five eyes which experienced an elevation greater than 30% of baseline, the increase was less than 5 mm Hg. Two eyes with an elevation more than 5 mm Hg, but less than 10 mm Hg, the elevation represented less than 30% of their baseline IOP.
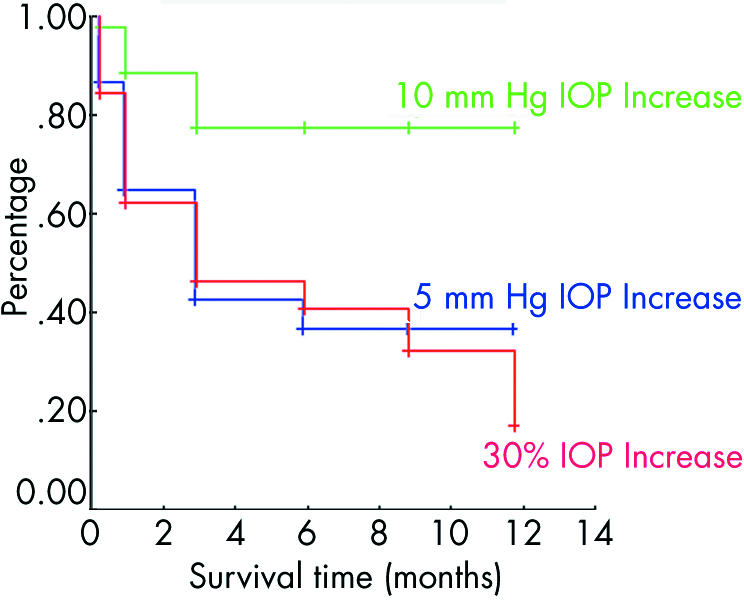
Figure 3 Kaplan‐Meier survival graph for repeated IVTA injections. All data points are censored. Mean and median survival times were a mean of 5.57 (SE 0.80) months, 95% CI 3.99 to 7.14, and median survival was 3 months for an increase greater than 5 mm Hg; the mean was 9.66, (SE 0.69) months, 95% CI 8.32 to 11.01, but a median could not be calculated for failure of more than 10 mm Hg; the mean was 5.60 (SE 0.80) months, 95% CI 4.03 to 7.17 months, and median survival was 3 months for an elevation of 30% or greater than baseline.
Figure 4 Venn diagram showing overlap for eyes that received more than one IVTA injection. The five eyes in the red area had >30% rise which was less than 5 mm Hg. In all eyes that had a 10 mm Hg rise, the elevation represented more than a 30% increase of baseline IOP.
Variables in the equation for the Cox proportional hazards regression included: pre‐existing glaucoma, sex, hypertension, diabetes, topical steroid before the second IVTA injection, IOP and number of medications at baseline of the second injection, and if the patient failed (that is, experienced an elevation of IOP) after the first injection by any of our three definitions of failure. No variables predicted for duration to failure by 5 mm Hg. Only baseline IOP before the second injection of IVTA was associated with duration of survival to a greater than 10 mm Hg increase in IOP (table 2). Again, higher baseline IOPs had a reduced chance of survival that was just over half (0.58) the survival rate of patients with baseline IOPs 1 mm Hg lower. Finally, sex (p = 0.012) and IOP at baseline of second injection (p = 0.049) significantly predicted duration to experiencing an increase of IOP greater than 30% of baseline. Women were less likely to experience an IOP elevation at a rate nearly three and a half times that of their male counterparts at every follow up time point. Patients with a pre‐injection IOP higher than the mean IOP of 15.5 mm Hg (table 1) had a decreased rate of survival, 0.823 that of patients with a baseline IOP 1 mm Hg lower.
Eyes with pre‐existing glaucoma (repeated IVTA)
In the group of eyes that received second injections, seven eyes (16.3%) had pre‐existing glaucoma. Their average baseline IOP was 16.28 (3.09) mm Hg which was not statistically different from those who did not have glaucoma (16.73 (2.76) mm Hg). Pre‐existing glaucoma was not a risk factor in either univariate or multivariate analyses
Eyes needing antiglaucoma treatment
Of the 43 eyes that received a second injection, nine required the addition of IOP lowering medications (one added at 1 week, four at 1 month, and four at the 3 month visit) following their first injection; five were still using them at their final follow up while four were back to baseline at their last follow up visit. The average duration of medication use was 7.53 months. Six of the seven glaucomatous eyes were using glaucoma medications at baseline, four required an addition of glaucoma medications which were added at 1 week (n = 2, 50%) and 1 month (n = 2, 50%).
At the second injection, 14 eyes had at least one glaucoma medication at baseline. Four required an addition of more drugs (one added at 1 week, one at 3 months, two at 6 months—three were still on the increased regimen at final follow up). Of the 29 that were not using glaucoma medications at the time of the second injection, nine required an addition of glaucoma medications. Of these nine, one had a medication added at 1 week, two at 1 month, three at 3 months, one at 6 months, and one at 12 months. Five were still on these new medications at final follow up while four returned to baseline. The average duration of medication use was 6.08 months. One eye (2.3%) required filtration surgery.
Discussion
In our group of 528 single injection eyes, a baseline IOP higher than 15 mm Hg was a significant risk factor for an IOP elevation similar to Smithen et al's series of 89 eyes.20 Age, sex, diagnosis, topical steroids before triamcinolone injection, and pre‐existing glaucoma, diabetes, or hypertension did not pose a significant risk for the development of steroid induced IOP elevation. In other studies, younger age has also been identified as a risk factor for an IOP rise following a single IVTA treatment.19,23,24
We found subsequent injections were at greater risk for the development of an IOP elevation. However, other groups did not find this to be the case,20,25 except when there had been an IOP elevation following the first injection.23 The discrepancy with our findings may be because of differing definitions of IOP elevations (for example, >24 mm Hg).20
We did not identify glaucoma as a risk factor. However, because of the nature of a retrospective analysis, it is probable that glaucomatous individuals with a high risk were prevented from receiving IVTA. Pre‐existing glaucoma was not a risk factor in other studies.20,23 However, Smithen et al's study was also retrospective, likely had the same selection bias, and had a small number of glaucomatous eyes (n = 12).20 Although prospective and non‐exclusive of pre‐existing glaucoma, Jonas et al's comparison of individuals with pre‐existing glaucoma (n = 6) to those without glaucoma (n = 65) did not show a statistical significance in IOP elevations (67% versus 49%, respectively)—also probably owing to the small sample of glaucomatous eyes.23 In studies of topical corticosteroids, pre‐existing glaucoma is a risk factor for the development of an IOP elevation.26,27 We suspect that eyes with pre‐existing glaucoma would be at greater risk with IVTA. A randomised study that did not exclude glaucomatous eyes is needed.
Pretreatment with prednisolone acetate did not correlate with an IOP rise. This too is likely an artefact of selection bias, since those eyes which had an elevation of IOP were not given IVTA. In our series, the eyes, in which a provocative test of prednisolone acetate did not change the IOP, were not at greater risk of having an IOP elevation from IVTA.
Following 4 mg of IVTA, aqueous humour concentrations peak from 2151–7202 ng/ml with half lives from 76–635 hours.28 The mean elimination half life was 18.6 days in non‐vitrectomised eyes and 3.2 days in eyes that had undergone a vitrectomy.28 Thus, in non‐vitrectomised eyes, it would be expected to find detectable levels for up to 3 months. Following 20–25 mg of IVTA, measurable amounts of the drug have been detected up to 1.5 years later.29 Triamcinolone crystals have been detected using ophthalmoscopy up to 9 months post‐injection.30 Clinically, the duration of triamcinolone action has been estimated to be approximately 8–9 months, reaching peak efficacy at 2–5 months, defined as the time at which the visual acuity and IOP return to baseline.5,8
Our data were obtained from patients injected with 4 mg of IVTA, a dose consistent with many studies12,13,15,18; however, some reports used 20–25 mg.11,14,19,25 Despite the sixfold difference in dosage, the prevalence of IOP elevation is similar: approximately 28–48% with the 4 mg dose12,13,15,18,20 and 50% with the 25 mg triamcinolone dose.11,14,19,25 The range of prevalences in these studies is further influenced by differing definitions of IOP elevation, follow up time, sample size, and baseline characteristics.
This study illustrates the relatively high prevalence of IOP elevations and importance of monitoring beyond 6 months after IVTA. Patients with a baseline IOP more than 16 mm Hg or receiving a second injection should be carefully monitored for an elevated IOP.
Acknowledgements
National Eye Institute; Bethesda, MD (EY 13997‐01) (DJR); Eye Research Institute; Philadelphia, PA (DJR).
Abbreviations
BRVO - branch retinal vein occlusion
CMO - cystoid macular oedema
CRVO - central retinal vein occlusion
IOP - intraocular pressure
IVTA - intravitreal triamcinolone acetonide
NPDR - non‐proliferative diabetic retinopathy
PDR - proliferative diabetic retinopathy
Footnotes
Conflict of interest: none.
References
- 1.Benhamou N, Massin P, Haochine B.et al Intravitreal triamcinolone for refractory pseudophakic macular edema. Am J Ophthalmol 2003135246–249. [DOI] [PubMed] [Google Scholar]
- 2.Conway M D, Canakis C, Livir‐Rallatos C.et al Intravitreal triamcinolone acetonide for refractory chronic pseudophakic cystoid macular edema. J Cataract Refract Surg 20032927–33. [DOI] [PubMed] [Google Scholar]
- 3.Challa J K, Gillies M C, Penfold P L.et al Exudative macular degeneration and intravitreal triamcinolone: 18 month follow up. Aust N Z J Ophthalmol 199826277–281. [DOI] [PubMed] [Google Scholar]
- 4.Gillies M C, Simpson J M, Luo W.et al A randomized clinical trial of a single dose of intravitreal triamcinolone acetonide for neovascular age‐related macular degeneration: one‐year results. Arch Ophthalmol 2003121667–673. [DOI] [PubMed] [Google Scholar]
- 5.Jonas J B, Akkoyun I, Budde W M.et al Intravitreal reinjection of triamcinolone for exudative age‐related macular degeneration. Arch Ophthalmol 2004122218–222. [DOI] [PubMed] [Google Scholar]
- 6.Jonas J B, Kreissig I, Degenrig R F. Intravitreal triamcinolone acetonide as treatment of macular edema in central retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol 2002240782–783. [DOI] [PubMed] [Google Scholar]
- 7.Jonas J B, Kreissig I, Sofker A.et al Intravitreal injection of triamcinolone for diffuse diabetic macular edema. Arch Ophthalmol 200312157–61. [PubMed] [Google Scholar]
- 8.Jonas J B, Degenring R F, Kamppeter B A.et al Duration of the effect of intravitreal triamcinolone acetonide as treatment for diffuse diabetic macular edema. Am J Ophthalmol 2004138158–160. [DOI] [PubMed] [Google Scholar]
- 9.Jonas J B, Hayler J K, Sofker A.et al Regression of neovascular iris vessels by intravitreal injection of crystalline cortisone. J Glaucoma 200110284–287. [DOI] [PubMed] [Google Scholar]
- 10.Alldredge C D, Garretson B R. Intravitreal triamcinolone for the treatment of idiopathic juxtafoveal telangiectasis. Retina 200323113–116. [DOI] [PubMed] [Google Scholar]
- 11.Jonas J B, Kreissig I, Degenring R. Intraocular pressure after intravitreal injection of triamcinolone acetonide. Br J Ophthalmol 20038724–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakri S J, Beer P M. The effect of intravitreal triamcinolone acetonide on intraocular pressure. Ophthalmic Surg Lasers Imaging 200343386–390. [PubMed] [Google Scholar]
- 13.Wingate R J, Beaumont P E. Intravitreal triamcinolone and elevated intraocular pressure. Aust N Z J Ophthal 199927431–432. [DOI] [PubMed] [Google Scholar]
- 14.Jonas J B, Kreissig I, Degenring R. Intraocular pressure after intravitreal injection of triamcinolone acetonide. Br J Ophthalmol 20038724–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillies M C, Simpson J M, Billson F A.et al Safety of an intravitreal injection of triamcinolone: results from a randomized clinical trial. Arch Ophthalmol 2004122336–340. [DOI] [PubMed] [Google Scholar]
- 16.Park C H, Jaffe F J, Fekrat S. Intravitreal triamcinolone acetonide in eyes with cystoid macular edema associated with central retinal vein occlusion. Am J Ophthalmol 2003136419–425. [DOI] [PubMed] [Google Scholar]
- 17.Massin P, Audren F, Haouchine B.et al Intravitreal triamcinolone acetonide fro diabetic diffuse macular edema: preliminary results of a prospective clinical trial. Ophthalmology 2004111224–225. [DOI] [PubMed] [Google Scholar]
- 18.Kaushik S, Gupta V, Gupta A.et al Intractable glaucoma following intravitreal triamcinolone in central retinal vein occlusion. Am J Ophthalmol 2004137758–760. [DOI] [PubMed] [Google Scholar]
- 19.Jonas J B, Degenrigh R F, Kreissig I.et al Intraocular pressure elevation after intravitreal triamcinolone acetonide injection. Ophthalmology 2005112593–598. [DOI] [PubMed] [Google Scholar]
- 20.Smithen L M, Ober M D, Maranan L.et al Intravitreal triamcinolone and intraocular pressure. Am J Ophthalmol 2004138740–743. [DOI] [PubMed] [Google Scholar]
- 21.Becker B, Mills D W. Corticosteroids and intraocular pressure. Arch Ophthalmol 196370500–507. [DOI] [PubMed] [Google Scholar]
- 22.Armaly M F. Dexamethasone ocular hypertension in the clinically normal eye. II. The untreated eye, outflow facility, and concentration. Arch Ophthalmol 196675776–782. [DOI] [PubMed] [Google Scholar]
- 23.Jonas J B, Kreissig I, Degenring R. Intraocular pressure after intravitreal injection of triamcinolone acetonide. Br J Ophthalmol 20038724–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park H Y, Yi K, Kim H K. Intraocular pressure elevation after intravitreal triamcinolone acetonide injection. Korean J Ophthalmol 200519122–127. [DOI] [PubMed] [Google Scholar]
- 25.Jonas J B, Degenring R F, Kreissig I.et al Safety of intravitreal high‐dose reinjections of triamcinolone acetonide. Am J Ophthalmol 20041381054–1055. [DOI] [PubMed] [Google Scholar]
- 26.Becker B. Intraocular pressure response to topical corticosteroids. Invest Ophthalmol 196526198–205. [PubMed] [Google Scholar]
- 27.Armaly M F. Statistical attributes of the steroid hypertensive response in the clinically normal eye. I. The demonstration of three levels of response. Invest Ophthalmol 19654187–197. [PubMed] [Google Scholar]
- 28.Beer P M, Bakri S J, Singh R J.et al Intraocular concentration and pharmacokinetics of triamcinolone acetonide after a single intravitreal injection. Ophthalmology 2003110681–686. [DOI] [PubMed] [Google Scholar]
- 29.Jonas J B. Intraocular availability of triamcinolone acetonide after intravitreal injection. Am J Ophthalmol 2004137560–562. [DOI] [PubMed] [Google Scholar]
- 30.Jonas J B, Kreissig I, Degenring R F. Secondary chronic open angle glaucoma after triamcinolone acetonide. Arch Ophthalmol 2003121729–730. [DOI] [PubMed] [Google Scholar]