Abstract
Background
Owing to the improvement of modern intraocular lenses (IOLs) in terms of design and material, posterior capsule opacification (PCO) usually takes 2–3 years to develop. Thus, long term clinical evaluation of new implants is important.
Methods
As part of a prospective, non‐randomised FDA trial, the Rayner Centerflex, a foldable hydrophilic acrylic, single piece IOL was implanted in one eye of 83 patients (mean age 73.5 (SD 7.0) years). Over 3 years postoperatively, a standardised FDA protocol concerning IOL safety and efficacy was followed including evaluation of spherical equivalent (SE), best corrected distance visual acuity (BCDVA), endothelial cell count (ECC), flare meter values, PCO development, and anterior capsule shrinkage.
Results
Postoperatively, mean SE was stable ranging between −0.3D and 0.17D. After 1–2 months, all patients achieved a BCDVA of 20/40 or better. At 3–6 months postoperatively, mean ECC decreased from 2612 (SD 346) cells/mm2 to 2380 (316) cells/mm2. Mean PCO score for the entire optic increased from 0.20 (0.20) months (3–6 months postoperatively) to 0.87 (0.57) resulting in a Nd:YAG rate of 29.41% after 3 years. No anterior capsule shrinkage was found.
Conclusion
The Centerflex showed excellent functional results, low values for endothelial cell loss and inflammatory signs, and no anterior capsule shrinkage. PCO formation was higher compared to other IOLs, which could be explained by the incomplete sharp edge at the optic‐haptic junctions representing an “Achilles' heel” for cell ingrowth.
Keywords: posterior capsule opacification, intraocular lens, hydrophilic acrylic, single piece intraocular lens
Development of posterior capsule opacification (PCO) is still a very common complication after cataract surgery.1,2,3,4,5 Neodymium:YAG (Nd:YAG) laser capsulotomy is usually effective, but is associated with significant cost and complications.6 Over the last decades, different IOL models have been developed and improved in terms of design and material. A sharp optic edge in combination with a capsular bend formation prevent secondary cataract formation significantly compared to round edged intraocular lenses (IOLs).7,8,9,10,11,12,13,14,15
Hydrophilic acrylic material offers the advantage of good uveal biocompatibility as well as low silicone oil adherence and is therefore suitable for patients with a history of uveitis, diabetes, or high myopia.16,17,18,19,20
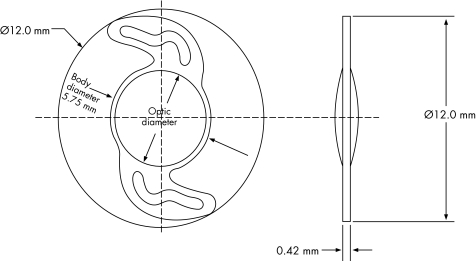
Because of the improvement of modern IOLs, PCO usually takes 2–3 years to develop. Thus, long term clinical evaluation of new implants is important. We investigated the performance of a single piece, hydrophilic acrylic foldable IOL (Rayner Centerflex 570H) over 3 years postoperatively. This IOL has a total diameter of 12 mm and an optic diameter of 5.75 mm, which is equiconvex and designed with sharp edges excluding the optic‐haptic junctions (fig 1). We analysed this IOL model in terms of functional results, endothelial cell count, flare meter values, PCO development, and anterior capsule shrinkage.
Fig 1 Centerflex IOL.
Materials and methods
We conducted a prospective, clinical, non‐randomised FDA trial enrolling 83 patients (54 female, 29 male) with a mean age of 73.5 (SD 7.0) years (range 58–86 years) who presented for cataract surgery. Only one eye of each patient was included in the study. Ethics committee approval was achieved for this study design and written informed consent was obtained from all patients.
After uneventful phacoemulsification with topical (n = 57) or general anaesthesia (n = 26), the IOLs were implanted using injector or forceps. The mean value of implanted IOL power was 20.8D (2.9D) (range 14–30D). Clinical examinations were performed preoperatively, 1 day, 1–2 weeks, 1–2 months, 6 months, 1 year, 2 years, and 3 years after surgery. Patient compliance was extremely good; however, it was not possible to evaluate every parameter at all time points because of illness or quality of pictures.
Functional results
We were able to examine 51 patients for all follow up visits over a period of 3 years postoperatively and we evaluated the development of spherical equivalent (SE) and best corrected distance visual acuity (BCDVA).
Endothelial cell count and flare meter evaluation
Endothelial cell count measurements of 67 patients were performed up to 3–6 months after surgery using the Tomey corneal endothelial microscope V.1200. Inflammatory reactions were evaluated using the Kowa laser flare meter FM‐500 (68 patients; up to 3–6 months postoperatively).
Posterior capsule opacification and anterior capsule shrinkage
Development of PCO was analysed 6 months, 1 year, and 3 years postoperatively. Using the image analysis program EPCO 2000, PCO scores (scale from 0 to 4) were calculated for the entire optic, the central 3 mm zone, and within the capsulorhexis. Nd:YAG laser capsulotomy rate was calculated 3 years postoperatively. Criteria for performing a YAG capsulotomy were either reduction of BCDVA of less than 20/40 or significant patient complaints regarding quality of vision (for example, glare) associated with moderate to severe PCO formation. Anterior capsule shrinkage was analysed by measuring the overlapping between IOL optic and anterior capsule.
Statistical analysis was performed using the Wilcoxon test and a p value <0.05 was considered statistically significant.
Results
Functional results
SE was stable over time with mean values ranging between −0.3 and 0.17 D (table 1). After 2 months postoperatively, all patients achieved a BCDVA of 20/40 or better; 94% of patients achieved 20/40 or better after 2 or 3 years (table 1).
Table 1 Development of different parameters over time (SD).
| Preoperative | Postoperative | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 day | 1–2 days | 1–2 weeks | 1–2 months | 3–6 months | 1 year | 2 years | 3 years | |
| Mean SE (D) | −0.34 (2.42) n = 51 | −0.3 (1.11) n = 51 | −0.13 (1.01) n = 51 | −0.12 (1.19) n = 51 | −0.02 (1.19) n = 51 | 0.17 (1.24) n = 51 | −0.06 (1.23) n = 51 | −0.04 (1.2) n = 51 |
| Mean BCDVA >20/40 | 20/60 n = 51 | 20/32 n = 51 | 20/30 n = 51 | 20/25 n = 51 | 20/25 n = 51 | 20/25 n = 51 | 20/25 n = 51 | 20/25 n = 51 |
| 35.29% | 86.0% | 90.2% | 100% | 100% | 100% | 94.0% | 94.12% | |
| Mean ECC (cells/mm2) | 2612 (346) n = 67 | – | 2484 (332) n = 58 | 2346 (381) n = 67 | 2380 (316) n = 45 | – | – | – |
| Mean flare (photons/ms) | 10.3 (0.7) n = 68 | 17.8 (1.0) n = 68 | 17.9 (1.5) n = 67 | 11.1 (0.8) n = 63 | 9.9 (0.6) n = 37 | – | – | – |
| Mean PCO (total IOL optic) | – | – | – | – | 0.20 (0.20) n = 36 | 0.35 (0.22) n = 31 | – | 0.87 (0.57) n = 40 |
| Mean PCO (3 mm zone) | – | – | – | – | 0.11 (0.20) n = 43 | 0.31 (0.25) n = 36 | – | 0.69 (0.63) n = 40 |
| Mean PCO (CR) | – | – | – | – | 0.14 (0.21) n = 36 | 0.20 (0.29) n = 31 | – | 0.86 (0.59) n = 40 |
| Mean overlapping (%) | – | 25.0 (9.1) n = 62 | – | – | 28.6 (10.5) n = 38 | 22.5 (17.4) n = 30 | – | 25.1 (11.2) n = 40 |
SE, spherical equivalent, D, dioptre, BCDVA, best corrected distance visual acuity, ECC, endothelial cell count, PCO, posterior capsule opacification, IOL, intraocular lens, CR, capsulorhexis, n, number of eyes.
Endothelial cell count and flare meter evaluation
Mean endothelial cell count (ECC) decreased from 2612 (346) cells/mm2 to 2380 (316) cells/mm2 after 3–6 months postoperatively (table 1). However, the intraindividual ECC changes were not statistically significant (p>0.05, Wilcoxon test).
Mean flare meter values (photons/ms) were 10.3 (0.7) preoperatively which increased to 17.9 (1.5) 2 weeks postoperatively and returned to initial values 3–6 months after surgery (table 1). There were statistically significant differences between the intraindividual changes 1–2 days and 1–2 weeks after surgery compared to the preoperative data (p<0.01, Wilcoxon test).
Posterior capsule opacification and anterior capsule shrinkage
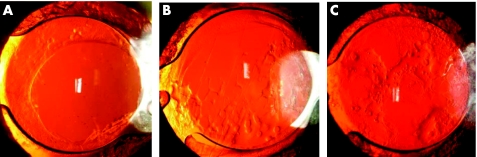
Mean PCO values for the entire optic increased from 0.20 (0.20) to 0.87 (0.57) (n = 40) 3 years after surgery (table 1). For the central 3 mm zone, mean PCO score was 0.11 (0.20) after 3–6 months and 0.69 (0.63) after 3 years (table 1). Within the capsulorhexis, we calculated a mean PCO value of 0.14 (0.21) (3–6 months) increasing to 0.86 (0.59) (3 years) postoperatively (table 1). The Nd:YAG laser capsulotomy rate was 29.41% (15 of 51 eyes) 3 years after surgery. The number of Nd:YAG laser treatments performed by each of the study time points is shown in table 2. Figure 2 illustrates IOLs with mild, moderate, and severe PCO development 3 years after surgery (fig 2).
Table 2 Nd:YAG laser capsulotomy treatments performed by each of the study follow up points.
| Follow up | Number of eyes | Nd:YAG laser capsulotomy rate (%) |
|---|---|---|
| 6 months | 2 of 51 | 3.92 |
| 12 months | 4 of 51 | 7.84 |
| 24 months | 10 of 51 | 19.61 |
| 36 months | 15 of 51 | 29.41 |
Fig 2 Centerflex 570H IOLs 3 years postoperately: mild (A), moderate (B), severe PCO development (C).
The mean overlap between anterior capsule and IOL optic was stable ranging around 25% and thus indicating no anterior capsule shrinkage (table 1).
Discussion
Hydrophilic acrylic IOL material offers some advantages. The material is very smooth and thus induces only mild traumas to the corneal incision during the implantation process. Another advantage of this material is its little adherence to silicone oil and its good uveal biocompatibility.16,17,18,19,20 Our evaluation of inflammatory signs postoperatively showed very good results with mean flare meter values increasing from only 10–18 photons/ms within the first 2 weeks postoperatively.
Measurement of corneal ECC revealed no statistically significant difference. Endothelial cell loss after cataract surgery has been reported from 6.7% to 27% after implantation of different IOLs.21,22
In the literature the frequency of PCO development is described with 5–30% within the first 5 years postoperatively.5 It has been shown that PCO development depends on different factors.4,16,23,24
Systemic diseases—Diabetes and ocular pathologies such as retinitis pigmentosa, pseudoexfoliative glaucoma or uveitis increase the expression of PCO.5
Surgery techniques—Hydrodissection, cortical clean up, lens implantation in the capsular bag as well as complete overlapping of the capsulorhexis reduces PCO formation.4,13,16,23,24
IOL material—High PCO values were seen in three piece PMMA IOLs, moderate PCO values were found in hydrophilic acrylic lenses, and mild PCO values were found in hydrophobic acrylic IOLs and silicone IOLs.25
IOL design—Nishi described the preventive effect of a sharp optic edge in combination with a capsular bend formation on PCO development. Owing to this barrier effect, lens epithelial cell ingrowth is inhibited.7,8,9,10,11,12,13,14,15 Vargas et al reported a good barrier effect for the Centerflex IOL design.26 Furthermore, haptic design influences PCO formation since a tight contact between posterior IOL surface and posterior lens capsule facilitates capsular bending, thus reducing PCO expression.27,28 Even though the Centerflex has no haptic angulation incorporated in the design, Schmidbauer and co‐authors could show that the special sharp edge design and the thick lens optic of the Centerflex compensate for the disadvantages of the missing haptic angulation.29
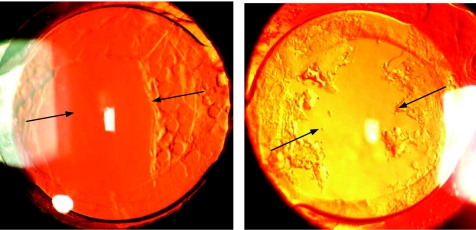
Recently, reports have been published which indicated that a lack of the square edge at the optic‐haptic junction of single piece IOLs contributes to cell ingrowth along the haptics representing an “Achilles' heel,”30,31,32 which we also noticed in this study (fig 3). However, it is not clear yet whether the missing sharp edge at the optic‐haptic junction is the main gateway for migrating lens epithelial cells in single piece IOL models since authors who compared the three piece versus single piece IOLs have reported both more PCO for the single piece IOLs32,33 and no significant differences so far.34,35 Additionally, wide haptic roots as implemented in the Centerflex design might prevent or minimise the capsular bending process if the capsular leaves fail to adhere to each other. Nishi and coauthors could demonstrate that the three piece Acyrsof with narrow haptics is superior to the single piece IOL with wider haptic roots in terms of PCO prevention.28,32,36
Fig 3 Centerflex 570H IOLs: lens epithelial cell migration along the haptics because of a discontinuous square edge design at the optic‐haptic junction.
In conclusion, more studies are necessary to investigate how haptic design especially in terms of width and discontinuous sharp optic rim of single piece IOLs influences PCO formation and lens epithelial cell migration. As far as our study is concerned, it is also possible that other factors besides IOL (for example, surgical factors, patient population) may have contributed to the high PCO rates design since there was no control for this trial.
Nevertheless, the Centerflex showed excellent long term functional results, low values for endothelial cell loss, and inflammatory signs as well as no anterior capsule shrinkage.
Abbreviations
BCDVA - best corrected distance visual acuity
ECC - endothelial cell count
IOLs - intraocular lenses
Nd:YAG - neodymium:YAG
PCO - posterior capsule opacification
SE - spherical equivalent
Footnotes
Competing interest: none declared.
References
- 1.Apple D J, Solomon D K, Tetz M R.et al Posterior capsule opacification. Major review. Surv Ophthalmol 19923773–116. [DOI] [PubMed] [Google Scholar]
- 2.Auffarth G U, Rabsilber T M, Reuland A J. [New methods for the prevention of posterior capsule opacification]. Ophthalmologe 2005102579–586. [DOI] [PubMed] [Google Scholar]
- 3.Spalton D J. Posterior capsular opacification after cataract surgery. Eye 199913489–492. [DOI] [PubMed] [Google Scholar]
- 4.Pandey S K, Apple D J, Werner L.et al Posterior capsule opacification: a review of the aetiopathogenesis, experimental and clinical studies and factors for prevention. Indian J Ophthalmol 20045299–112. [PubMed] [Google Scholar]
- 5.Auffarth G U, Becker K A. Cataracta secundaria. Histopathologische Grundlagen, Evaluierungsmethoden und Präventionsmöglichkeiten. Ophthalmo‐Chirurgie 200214108–119. [Google Scholar]
- 6.Aslam T M, Devlin H, Dhillon B. Use of Nd:YAG capsulotomy. Surv Ophthalmol 200348594–612. [DOI] [PubMed] [Google Scholar]
- 7.Nishi O. Posterior capsule opacification. Part 1: Experimental investigations. J Cataract Refract Surg 199925106–117. [DOI] [PubMed] [Google Scholar]
- 8.Nishi O, Nishi K, Sakanishi K. Inhibition of migrating lens epithelial cells at the capsular bend created by the rectangular optic edge of a posterior chamber intraocular lens. Ophthlamic Surg Lasers 199829587–594. [PubMed] [Google Scholar]
- 9.Nishi O, Nishi K. Preventing posterior capsule opacification by creating a discontinuous sharp bend in the capsule. J Cataract Refract Surg 199925521–526. [DOI] [PubMed] [Google Scholar]
- 10.Nishi O, Nishi K, Wickström K. Preventing lens epithelial cell migration using intraocular lenses with sharp rectangular edges. J Cataract Refract Surg 2000261543–1549. [DOI] [PubMed] [Google Scholar]
- 11.Nishi O, Nishi K, Akura J.et al Effect of round edged acrylic intraocular lenses on preventing posterior capsule opacification. J Cataract Refract Surg 200127608–613. [DOI] [PubMed] [Google Scholar]
- 12.Peng Q, Visessook N, Apple D J.et al Surgical prevention of posterior capsule opacification. Part 3: Intraocular lens optic barrier effect as a second line of defense. J Cataract Refract Surg 200026198–213. [DOI] [PubMed] [Google Scholar]
- 13.Auffarth G U, Golescu A, Becker K A.et al Quantification of posterior capsule opacification with round and sharp edge intraocular lenses. Ophthalmology 2003110772–780. [DOI] [PubMed] [Google Scholar]
- 14.Buehl W, Findl O, Menapace R.et al Effect of an acrylic intraocular lens with a sharp posterior optic edge on posterior capsule opacification. J Cataract Refract Surg 2002281105–1111. [DOI] [PubMed] [Google Scholar]
- 15.Kruger A J, Schauersberger J, Abela C.et al Two year results: sharp versus rounded optic edges on silicone lenses. J Cataract Refract Surg 200026566–570. [DOI] [PubMed] [Google Scholar]
- 16.Trivedi R H, Werner L, Apple D J.et al Post cataract‐intraocular lens (IOL) surgery opacification. Eye 200216217–241. [DOI] [PubMed] [Google Scholar]
- 17.Arthur S N, Peng Q, Apple D J.et al Effect of heparin surface modification in reducing silicone oil adherence to various intraocular lenses. J Cataract Refract Surg 2001271662–1669. [DOI] [PubMed] [Google Scholar]
- 18.Apple D J, Isaacs R T, Kent D G.et al Silicone oil adhesion to intraocular lenses: an experimental study comparing various biomaterials. J Cataract Refract Surg 199723536–544. [DOI] [PubMed] [Google Scholar]
- 19.Abela‐Formanek C, Amon M, Schauersberger J.et al Results of hydrophilic acrylic, hydrophobic acrylic, and silicone intraocular lenses in uveitic eyes with cataract: comparison to a control group. J Cataract Refract Surg 2002281141–1152. [DOI] [PubMed] [Google Scholar]
- 20.Abela‐Formanek C, Amon M, Schild G.et al Uveal and capsular biocompatibility of hydrophilic acrylic, hydrophobic acrylic, and silicone intraocular lenses. J Cataract Refract Surg 20022850–61. [DOI] [PubMed] [Google Scholar]
- 21.Dick H B, Kohnen T, Jacobi F K.et al Long‐term endothelial cell loss following phacoemulsification through a temporal clear corneal incision. J Cataract Refract Surg 19962263–71. [DOI] [PubMed] [Google Scholar]
- 22.Kohlhaas M, Stahlhut O, Tholuck J.et al [Changes in corneal thickness and endothelial cell density after cataract extraction using phacoemulsification]. Ophthalmologe 199794515–518. [DOI] [PubMed] [Google Scholar]
- 23.Apple D J, Peng Q, Visessook N.et al A Surgical prevention of posterior capsule opacification. Part 1: Progress in eliminating this complication of cataract surgery. J Cataract Refract Surg 200026180–187. [DOI] [PubMed] [Google Scholar]
- 24.Peng Q, Apple D J, Visessook N.et al A Surgical prevention of posterior capsule opacification. Part 2: Enhancement of cortical cleanup by focusing on hydrodissection. J Cataract Refract Surg 200026188–197. [DOI] [PubMed] [Google Scholar]
- 25.Auffarth G U, Becker K A, Martin M. Morphologische Nachstar‐Auswertung: Eine Datenbankanalyse von 1000 Augen. In: Auffarth GU, Kohnen Th, Völcker HE, Demeler U, eds. 16th Meeting of the German speaking society for IOL Implantation and Refractive Surgery. Bremen: Biermann Verlag 2002;231–5
- 26.Vargas L G, Peng Q, Apple D J.et al Evaluation of 3 modern single‐piece foldable intraocular lenses. Clinicopathological study of posterior capsule opacification in a rabbit model. J Cataract Refract Surg 2002281241–1250. [DOI] [PubMed] [Google Scholar]
- 27.Wesendahl T A, Hunold W, Auffarth G U.et al [Area of contact of the artificial lens and posterior capsule. Systematic study of various haptic parameters]. Ophthalmologe 199491680–684. [PubMed] [Google Scholar]
- 28.Nishi O. [Influence of intraocular lens material and design on the development of posterior capsule opacification]. Ophthalmologe 2005102572–578. [DOI] [PubMed] [Google Scholar]
- 29.Schmidbauer J M, Escobar‐Gomez M, Apple D J.et al Effect of haptic angulation on posterior capsule opacification in modern foldable lenses with a square, truncated optic edge. J Cataract Refract Surg 2002281251–1255. [DOI] [PubMed] [Google Scholar]
- 30.Werner L, Mamalis N, Pandey S K.et al Posterior capsule opacification in rabbit eyes implanted with hydrophilic acrylic intraocular lenses with enhanced square edge. J Cataract Refract Surg 2004302403–2409. [DOI] [PubMed] [Google Scholar]
- 31.Sugita M, Kato S, Sugita G.et al Migration of lens epithelial cells through haptic root of single‐piece acrylic‐foldable intraocular lens. Am J Ophthalmol 2004137377–379. [DOI] [PubMed] [Google Scholar]
- 32.Nishi O, Nishi K, Osakabe Y. Evaluation of posterior capsule opacification using a new posterior view method in rabbits: single‐piece acrylic versus 3‐piece acrylic intraocular lens. J Cataract Refract Surg 2005312369–2374. [DOI] [PubMed] [Google Scholar]
- 33.Wallin T R, Hinckley M, Nilson C.et al A clinical comparison of single‐piece and three‐piece truncated hydrophobic acrylic intraocular lenses. Am J Ophthalmol 2003136614–619. [DOI] [PubMed] [Google Scholar]
- 34.Nejima R, Miyata K, Honbou M.et al A prospective, randomised comparison of single and three piece acrylic foldable intraocular lenses. Br J Ophthalmol 200488746–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bender L E, Nimsgern C, Jose R.et al Effect of 1‐piece and 3‐piece Acrysof intraocular lenses on the development of posterior capsule opacification after cataract surgery. J Cataract and Refract Surg 200430786–789. [DOI] [PubMed] [Google Scholar]
- 36.Nishi O, Nishi K. Effect of the optic size of a single‐piece acrylic intraocular lens on posterior capsule opacification. J Cataract Refract Surg 200329348–353. [DOI] [PubMed] [Google Scholar]