Abstract
Background
Differentiation patterns of the neural retina and its retinal vasculature are not well matched. The foveal region differentiates first, however the central retina is not vascularised until late in gestation. The authors explored the hypothesis that higher rates of endothelial cell proliferation in the choriocapillaris of the central retina might compensate for the slow growth of central retinal vessels, providing supplementary nutrients to the region during the early stages of neuronal maturation.
Methods
Frozen sections of five human fetal eyes (14–18.5 weeks' gestation), were examined for Ki‐67 and CD34 immunoreactivity using confocal microscopy. Measurements of choriocapillaris area and the number of proliferating choroidal endothelial cells were used to calculate the rate of choroidal endothelial proliferation at five different chorioretinal locations.
Results
The choriocapillaris area is consistently greater in the foveal region than at other locations and increases progressively with age. A higher rate of endothelial cell proliferation was found in parts of the choriocapillaris associated with the undifferentiated (proliferating) neural retina, compared with the differentiated, central region.
Conclusion
The findings suggest that mechanisms regulating proliferation and growth of the choroidal vasculature are independent of differentiation in the neural retina, and are thus profoundly different from mechanisms that regulate formation of the retinal vasculature.
Keywords: choroid, retinal development, angiogenesis, primates
The choroid develops early, with primitive endothelium lined elements present in the mesenchyme surrounding the anterior optic cup as early as 29 days' gestation, and its development into a loosely stratified aggregation of vessels is largely complete by 24 weeks' gestation.1,2 The choriocapillaris is the innermost capillary layer of the choroid. It comprises a close, thin walled, highly permeable network of endothelial cells (EC) with little or no basement membrane material, and supplies the neural retina by diffusion across the retinal pigmented epithelium (RPE) and Bruch's membrane. Little is known about the mechanisms that regulate development of the choroid (see, however, Steinle et al3).
In contrast, development of the retinal vasculature is better understood and the mechanisms regulating its growth and development are well documented. The retinal vessels form later than those in the choroid, the first vessels forming at the optic disc at approximately 14 weeks' gestation, the final stage of development being formation of the perifoveal capillary plexus just after birth.4,5,6,7,8,9 Initially the retinal vessels grow in a lobular arrangement, each lobule defining the territories of one of the quadrantic arteries of the mature retina.8,10,11,12 Nasal to the optic disc the superior and inferior lobes merge along the equator at approximately 20 weeks' gestation. Temporally, the superior and inferior lobes of vasculature skirt around the foveal region to meet along the equator, peripheral to the foveal region, at approximately 25 weeks' gestation.9,13,14 The long trajectory of the temporal vessels, combined with a slow growth rate,15 results in the central retina remaining avascular for a much longer period than other parts of the retina. The central fovea itself is not normally vascularised at any stage of development.13,14
It has been argued that development of the retinal circulation is determined by the ability/inability of the choroid to deliver nutrients to the retina.16,17 Indeed, it is widely accepted that formation of the retinal vasculature is induced by a transient hypoxia associated with increased metabolic activity in maturing retinal neurons and photoreceptors, resulting in the proliferation and migration of retinal endothelial cells, mediated by growth factors including hypoxia inducible factor‐1alpha (HIF1‐α) and vascular endothelial growth factor (VEGF).18,19,20,21,22 Because the retina matures from centre (fovea) to periphery, the differentiation patterns of the neural retina and its retinal vasculature are not well matched. In humans, all of the retinal layers are evident and there is a full complement of cells at the incipient fovea by 11 weeks' gestation,23,24 but the perifoveal capillary bed does not begin to form until 25 weeks' gestation and is not complete until after birth. This delay in central retinal vascularisation means that the metabolic demands of rapidly maturing central neurons are supplied by diffusion from the choroid, along with some diffusion from the hyaloid vessels via the vitreal body, until relatively late in development.10,25
We hypothesised that during the period when the central retina is largely avascular, metabolic activity in central retinal neurons might be supported by increased capacity in the choriocapillaris, reflected by increased proliferation of choriocapillaris EC. Consistent with the accepted mechanism of retinal vascularisation,4,19 we predicted that maturation of neurons at the incipient fovea and adjacent retina might drive EC proliferation in the adjacent choriocapillaris during the early phases of retinal vascular development. The results, however, indicate that proliferation of choroidal EC is not regulated by maturation of retinal neurons.
Materials and methods
Specimens
Five human fetal eyes, aged 14, 15, 17, 17.5, and 18.5 weeks' gestation, were obtained from terminations of pregnancy with informed maternal consent, following approval from the human ethics committee, University of Sydney. The gestational age was determined by preoperative obstetric ultrasound and postmortem ocular morphometry. Following removal of the anterior segment and vitreous, eye cups were fixed in 2% paraformaldehyde/0.1 M phosphate buffered saline (PBS; pH 7.4), washed in PBS, and placed in 30% sucrose/PBS (4°C). Eye cups were embedded in TissueTek OCT (Sakura Finetek, CA, USA), snap frozen in liquid nitrogen cooled isopentane (BDH, Sydney, Australia), and stored at −80°C. Transverse frozen sections (20 µm) were cut on a Leitz 1720 cryostat and collected onto poly‐l‐lysine (Sigma‐Aldrich, Sydney, Australia) and gelatin coated slides (BDH). Only sections passing through the incipient fovea and the optic disc were used for analysis.
Immunohistochemistry
Sections were placed in 0.01 M citrate buffer (pH 6.0)/0.4% saponin at 80°C for 5 minutes to enhance antigen detection, cooled to room temperature, rinsed, and blocked in 10% normal goat serum/PBS for 60 minutes. Sections were incubated in polyclonal rabbit anti‐human Ki‐67 antibody (1:50) to label proliferating cells, and monoclonal mouse anti‐human CD34 antibody (1:100) to label vascular endothelium,12 at 4°C overnight. After rinsing in PBS, sections were incubated for 60 minutes in goat anti‐rabbit Alexa‐488 (1:1000; Molecular Probes) to detect bound anti‐Ki‐67 and goat anti‐mouse Alexa‐594 (1:1000; Molecular Probes) to detect bound anti‐CD34. Sections were then rinsed in PBS, mounted in glycerol/DABCO (triethylenediamine; Sigma), coverslipped, and sealed with nail varnish. A negative control, omitting the primary antibody, was included in each experiment.
Confocal microscopy
For each specimen, three sections from three different slides were analysed. Fifteen areas/section were sampled (135 sample areas/specimen) and subsequently grouped into five chorioretinal regions—incipient fovea (foveal, F), peripheral (nasal, N; temporal, T), and transitional regions (nasofoveal, NF; temporofoveal, TF) using morphological criteria (fig 1A). The incipient fovea was identified by (1) the absence of proliferating cells, (2) the presence of cone photoreceptors only (no rods), and (3) the presence of all characteristic layers of the neural retina.23 The peripheral regions (T and N) were identified as having only a differentiated ganglion cell layer (GCL), the deeper retina having no layers and comprising a maximum density of Ki‐67 immunoreactive (IR) cells.26 The transitional regions (TF and NF), like the peripheral ones, had a fully differentiated GCL and partially differentiated outer retina containing relatively few (<15) Ki‐67‐IR cells.
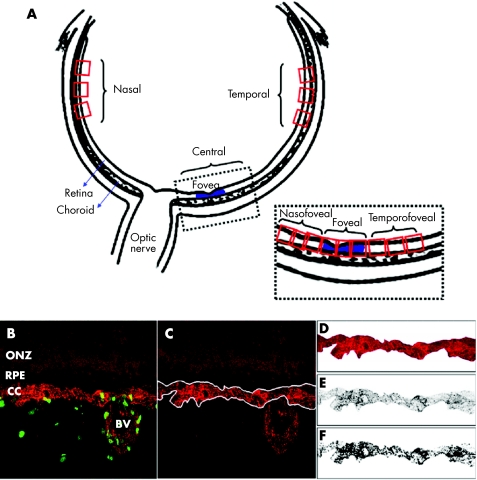
Figure 1 (A) Diagrammatic representation of sample areas analysed; inset showing central region (fovea shaded in blue). (B) Adobe Photoshop image (250 µm × 250 µm; 512 pixels × 512 pixels) of a 17 weeks' gestation chorioretinal sample showing Ki‐67‐IR proliferating cells (green) and the CD34‐IR choriocapillaris endothelium (red). (C) Single red channel, after blue and green channels are filled with black. The choriocapillaris is selected as shown by the white outline. (D) Cropped image of the selected choriocapillaris. (E) The inverted cropped image is imported into NIH Image in grey scale. (F) Thresholding of the image into binary form, where red pixels above background are changed into black pixels. Measurement macros were then used to calculate the vascular area of the choriocapillaris. ONZ, outer neuroblastic zone; RPE, retinal pigmented epithelium; CC, choriocapillaris; BV, blood vessel.
Sections were imaged using a Leica upright scanning laser confocal microscope (Leica TCSNT software, version 1.6.587). An argon‐krypton laser with dual filters for maximum excitation (488 nm and 594 nm) was used to visualise anti‐Ki‐67‐IR proliferating cells and anti‐CD34‐IR vascular endothelium respectively. Each sample area (250 µm×250 µm) was viewed using a 40× oil immersion objective. Ten optical z‐sections at 1 µm intervals were collected from each sample location (1350 images/specimen). Photomultiplier gain, offset, aperture, and laser power settings were standardised and maintained for all measurements for comparisons between specimens.
Image analysis
Counting proliferating cells
Immunolabelled confocal images were opened in Adobe Photoshop 5.0.2 (fig 1B) with z‐sections of each field comprising different layers of each image. Proliferating cells were counted in each layer and identified as cells with a green labelled (Ki‐67‐IR) nucleus within the red stained (CD34‐IR) blood vessel wall, present in at least three adjacent layers. Each double labelled cell was counted only once.
Estimating choriocapillaris area
Layers were flattened and green and blue channels filled with black to allow visualisation of the single red channel (fig 1C). The length of choriocapillaris was a constant of 250 µm (fig 1D). The identified region of choriocapillaris adjacent to Bruch's membrane and containing no large vessels was copied, pasted into a new Photoshop document, colour inverted, saved in TIFF format and imported into NIH Image software (version 1.62, http://rsb.info.nih.gov/nih‐image/) with the units set to pixels (fig 1E). Three estimates of area, which varied by no more than 4%, were taken from each flattened image and the average area recorded in pixels, and converted to micrometres (µm2).
The amount of red in the background of each image was determined by sampling at least three vessel free areas. After thresholding, each image was converted into binary form (fig 1F) and measurement macros used to calculate the vascular area. Counts of proliferating cells v CD34‐IR choriocapillaris area were recorded in Microsoft Excel according to gestational age and chorioretinal region. The rate of cell proliferation in the choriocapillaris was calculated: (number of proliferating cells in a sample region)/(choriocapillaris area).
Statistical analysis
After normalising the data we used ANOVA to compare the fovea with other locations (NF, N, TF, and T) at each age for (a) mean choriocapillaris area, and (b) mean rates of choriocapillaris proliferation. To elucidate trends in choriocapillaris development we grouped the data based on degree of retinal differentiation. Before 16 weeks' gestation only a very small area of central retina is differentiated; after this age the differentiated region grows rapidly in size, beyond the incipient macular region. We use “<16 weeks' gestation” and “>16 weeks' gestation” to indicate these groups, respectively. Grouped data were tested for significance using the Kruskal‐Wallis and the Conover Inman post hoc tests (StatsDirect, p<0.05).
Results
Immunolabelling
Antibody to CD34 (red) consistently labelled cells in the choroid and choriocapillaris (fig 2A and B). Few retinal blood vessels were identified at 14 and 15 weeks' gestation. In the 17–18.5 weeks' gestation specimens, EC labelling was evident at the nerve fibre layer (NFL)/GCL interface, temporal to the optic disc. No labelling was present in control sections when primary antibody was omitted.
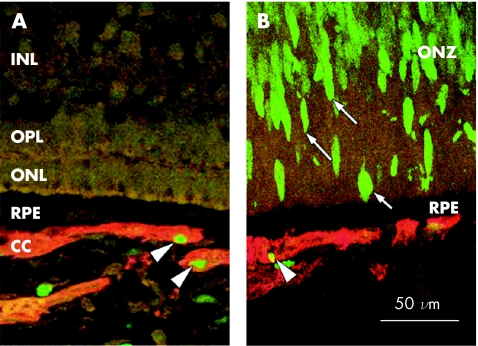
Figure 2 Cell proliferation in 18.5 weeks' gestation human chorioretinal locations. (A) In the incipient fovea (“foveal” location), no Ki‐67 proliferating cells (green) are present in the retina but are apparent in the CD34‐IR choriocapillaris (red; arrowheads). (B) Numerous Ki‐67‐IR proliferating cells (green, arrows) are seen in the outer neuroblastic zone in a peripheral sample. In the choriocapillaris, proliferating cell nuclei are generally seen in vessel walls away from the retina (arrowhead). CC, choriocapillaris; INL, inner nuclear layer; ONL, outer nuclear layer; ONZ, outer neuroblastic zone; OPL, outer plexiform layer; RPE, retinal pigmented epithelium.
Ki‐67‐IR (green), indicative of proliferating cells, was seen consistently throughout the choroid within the vascular endothelium and stroma (fig 2A and B) and in the nuclei of neuroblasts in undifferentiated retina of all specimens (fig 2B). In the choriocapillaris, the proliferating cells were most commonly seen on the sclerad aspect of vessel walls, away from the RPE. Proliferating cells were also seen in the optic nerve, in presumed EC in inner retina of some specimens, but rarely in the RPE. No labelling was present in control sections with the primary antibody omitted.
Choriocapillaris area
The choriocapillaris endothelium appeared as CD34‐IR, small calibre profiles oriented parallel to the RPE (fig 1). Vessels outside the choriocapillaris could be identified travelling obliquely over several sections, were generally larger in diameter, but not readily identifiable as belonging to either Sattler's or Haller's layer.
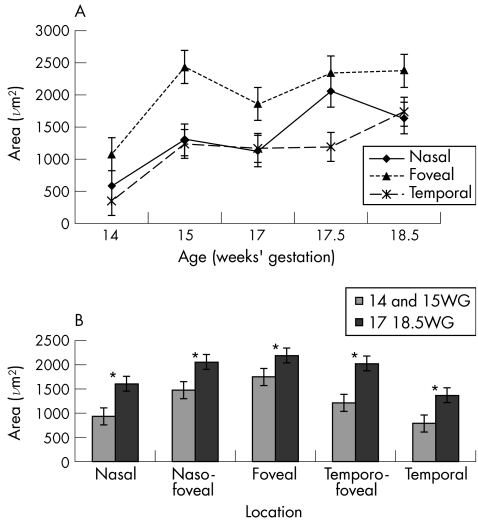
Choriocapillaris area by location and age is illustrated in figure 3. Measurements from the transitional areas (NF and TF) are omitted from figure 3A for clarity. When choriocapillaris area at the foveal location is compared with nasal and temporal locations the differences are statistically significant (p<0.001, ANOVA), except for the foveal versus nasal samples at 17.5 weeks' gestation (fig 3A). The grouped data also indicate a significant increase in choriocapillaris area with age at all locations (p<0.05, Kruskal‐Wallis test and Conover Inman post hoc test) (fig 3B).
Figure 3 Analysis of choriocapillaris endothelial area, by location and age. (A) Graphs showing the area of CD34 immunolabelling in the choriocapillaris SE), shown at three of the five locations analysed—nasal, temporal, and foveal—for clarity. The graphs show that (a) there is a steady increase in choriocapillaris area over the age range studied, and (b) the area of choriocapillaris at the incipient fovea is greater than in the peripheral locations at all the ages analysed. Foveal choriocapillaris area is significantly different from the other locations by ANOVA (p<0.001) at each age except one sample at 17.5 weeks' gestation (F v N). (B) Data from all five locations in which measurements from the two youngest retinas (14 and 15 weeks' gestation) (WG) are grouped and compared with data from the three older retinas (17–18.5 weeks' gestation). The data show that in the transitional locations (NF and TF) choriocapillaris area is greater than in the periphery, but not as high as in the fovea, in both age groups. Thus, the data indicate a progressive decrease in choriocapillaris area from central to peripheral locations. *Denotes significant differences, p<0.05 (Kruskal‐Wallis test and Conover Inman post hoc test).
EC proliferation
There were fewer proliferating cells in the choriocapillaris underlying the foveal region compared with other locations at all ages (data not shown). In the <16 weeks' gestation group peak numbers of proliferating cells were observed at NF and TF locations (2.33 cells/field (SE 0.33); 1.67 (SE 0.21) but in the >16 weeks' gestation group peak numbers were in the peripheral chorioretinal locations (N, 1.67 (SE 0.24); T, 2.22 (SE 0.15)). This suggests a proliferation gradient in the choroid, but did not reach statistical significance, probably because of the relatively small sample size.
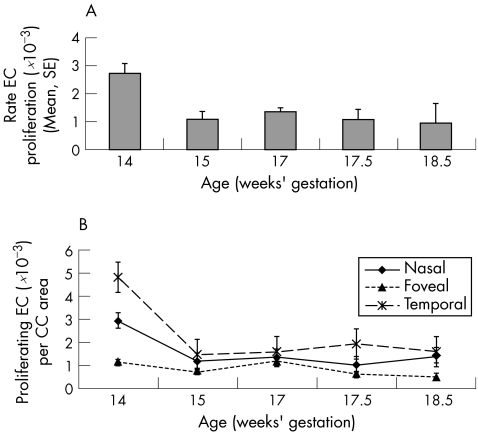
The rate of cell proliferation was calculated as the number of proliferating cells/unit of choriocapillaris EC area. The highest mean rate of choriocapillaris EC proliferation occurred at 14 weeks' gestation, and declined as a function of age (fig 4A). Analysis by age and location indicated that the lowest rates of proliferation were in central locations compared with peripheral (N and T) locations at all ages (fig 4B), reaching statistical significance in samples at 14 weeks' gestation (F v N; F v T), 17.5 weeks' gestation (F v T), and 18.5 weeks' gestation (F v N; F v T) (ANOVA, p<0.001).
Figure 4 Rates of choriocapillaris EC proliferation (SE) per unit area of choriocapillaris. (A) The mean rate of EC proliferation in the choriocapillaris (all locations) is shown for each eye. The data indicate a reduction in the proliferation rate, with increasing age. (B) Graphs showing the rate of EC proliferation at three locations at each age. Nasofoveal and temporofoveal locations are omitted for clarity. The data suggest that (a) there is a decline in the rate of proliferation with age at the foveal and peripheral locations, and (b) rates of proliferation at the fovea are less than in the periphery at all ages studied. Proliferation rates at the foveal locations are significantly different from the other locations at 14 weeks' gestation (F v N; F v T), 17.5 weeks' gestation (F v T), and 18.5 weeks' gestation (F v N; F v T) by ANOVA (p<0.001).
Discussion
Our data support three new findings. Firstly, the area of the choriocapillaris endothelium is greater in the foveal region at all ages studied, and declines toward the periphery. Choriocapillaris area also increases, at all locations analysed, as development progresses (fig 3). Secondly, the rate of choriocapillaris EC proliferation declines dramatically over the period studied (14–18.5 weeks' gestation, fig 4A). Thirdly, when the rate of EC proliferation is calculated (number of proliferating cells/unit area of choriocapillaris), the lowest rates of EC proliferation are at the incipient fovea for all ages (fig 4B).
The larger area of choriocapillaris endothelium in the foveal region indicates that, like the retina, the central choriocapillaris is specialised very early in development. This observation is consistent with the choriocapillaris specialisation seen underlying the foveal region of the adult human eye that appears to facilitate a higher rate of oxygen and nutrient delivery to central photoreceptors per unit time, compared with other parts of the retina.27,28 While our data suggest the choriocapillaris area increases as development progresses neither the full time course of this expansion, nor at what stage choriocapillaris area stabilises, is established. Analysis of proliferating EC by location indicates there may be a wave of proliferation passing towards the periphery over the time course of the present study (NF/TF<16 weeks' gestation compared with N/T>16 weeks' gestation). Although not statistically significant, this is an unexpected finding worthy of further investigation. One possible explanation for the larger areas of choriocapillaris at foveal locations earlier in development (<14 weeks' gestation), is that peak EC proliferation occurs centrally, establishing a substantial choriocapillaris that is added to by subsequent EC proliferation. At this stage suitable specimens are not available to validate this suggestion.
Most significantly, our data indicate that EC proliferation in the choriocapillaris does not appear to be promoted by increased metabolic activity in central retinal neurons. Such a mechanism is widely thought to regulate development of the retinal vasculature.19 If a similar mechanism regulated development of the choriocapillaris we would expect peak EC proliferation at foveal locations, which would increase with increasing age—at least up until a significant retinal vasculature is established in temporal retina. However, we find reduced EC proliferation at foveal locations at all ages studied. Furthermore, the rate of EC proliferation at foveal locations is stable between 14 and 18.5 weeks' gestation (0.6–1.2×10−3 proliferating cells/µm2 choriocapillaris), when the central retina is undergoing rapid maturation,29,30 suggesting no dynamic relation between neuronal maturation in the differentiating retina and choroid during this period.
Earlier studies in this laboratory suggest that a factor(s) expressed in central retina inhibit retinal vessel growth in the foveal region14,31 and may reduce EC proliferation rates.17 Such factors may also act on the adjacent choriocapillaris, resulting in the reduced rates of EC proliferation in the “foveal” chorioretinal location described here. While such an effect cannot be discounted, other evidence regarding EC growth, origins and phenotypes need to be considered.
In very early human ocular development (<10 weeks' gestation) both VEGF and the KDR receptors are expressed at relatively high levels in the choroid, while at stages comparable with this study, very little VEGF or KDR is detected.32 Consistent with this, little VEGF mRNA expression is seen in the choroid of human and monkey eyes at equivalent ages of ⩾14 weeks' gestation,8,31 although VEGF mRNA is detected in the RPE.8 Although conditional inactivation of VEGF expression in the RPE results in absence of the choriocapillaris and microphthalmia in mice,33 in vitro studies show that VEGF has only mild effects on choroidal EC proliferation compared with strong effects for FGF‐2.34 Furthermore, choroidal EC, in contrast with retinal EC, display increased proliferation and migration when stimulated with nerve growth factor.35
Other evidence indicates divergent lineages of EC with different response characteristics. Lineage negative haematopoietic, bone marrow derived stem cells give rise to endothelial precursor cells that can integrate into peripheral vasculature36,37,38,39,40 and, when injected intravitreally, selectively seek out astrocytes and are incorporated into retinal vessels.41,42 Bone marrow stromal cells (BMSC) non‐haematopoietic pluripotent cells—can give rise to a variety of mesenchymal phenotypes, including EC.43,44 BMSC derived EC are more responsive to fibroblast growth factor (FGF‐2) than VEGF, proliferating at double the rate.45 Given the time lag of at least 10 weeks between initial formation of the choroid and vascularisation of the retina, and that formation of a retinal vasculature is not a constant feature of vertebrate eye development, the possibility that choriocapillaris EC and retinal EC derive from different lineages appears strong. This may explain some of the differences in response characteristics of retinal and choroidal EC, including the lack of response of choriocapillaris EC to the metabolic demands of the differentiating retina described here.
Acknowledgements
The authors thank personnel at the Prince of Wales Hospital Department of Endocrinology for coordinating the donor programme, and Richard Stump for advice on statistical analyses. We thank all donors for making tissues available for research. Funded by NHMRC grant no 307848 (AA), NHRMC Project Grant no 153825 (JP and MM), Sydney Foundation for Medical Research (MM), and the ARC Centre of Excellence in Vision Science at the ANU.
Abbreviations
BMSC - bone marrow stromal cells
EC - endothelial cells
F - foveal
FGF - fibroblast growth factor
GCL - ganglion cell layer
HIF1‐α - hypoxia inducible factor‐1alpha
IR - immunoreactive
N - nasal
NFL - nerve fibre layer
phosphate buffered saline -
BS - PBS
RPE - retinal pigmented epithelium
T - temporal
VEGF - vascular endothelial growth factor
References
- 1.Ozanics V, Rayborn M E, Sagun D. Observations on the ultrastructure of the developing primate choroid coat. Exp Eye Res 19782625–45. [DOI] [PubMed] [Google Scholar]
- 2.Heimann K. The development of the choroid in man. Ophthalmic Res 19723257–273. [Google Scholar]
- 3.Steinle J J, Zamora D O, Rosenbaum J T.et al Beta 3‐adrenergic receptors mediate choroidal endothelial cell invasion, proliferation, and cell elongation. Exp Eye Res 20058083–91. [DOI] [PubMed] [Google Scholar]
- 4.Michaelson I C. The mode of development of the vascular system of the retina, with some observations on its significance for certain retinal diseases. Trans Ophthalmol Soc UK 194868137–181. [Google Scholar]
- 5.Nilhausen K. The vasoformative tissue in the foetal retina with particular reference to the histochemical demonstration of its alkaline phosphatase activity. Acta Ophthalmol 19583665–70. [DOI] [PubMed] [Google Scholar]
- 6.Ashton N. Retinal angiogenesis in the human embryo. Br Med Bull 197026103–106. [DOI] [PubMed] [Google Scholar]
- 7.Mann I.The development of the human eye. (First published 1928). 3rd ed. New York: Grune and Stratton, 1964
- 8.Provis J M, Leech J, Diaz C M.et al Development of the human retinal vasculature: cellular relations and VEGF expression. Exp Eye Res 199765555–568. [DOI] [PubMed] [Google Scholar]
- 9.Provis J M. Development of the primate retinal vasculature. Prog Ret Eye Res 200120799–821. [DOI] [PubMed] [Google Scholar]
- 10.Michaelson I C.Retinal circulation in man and animals. Springfield, IL: Charles C Thomas, 1954
- 11.Patz A. The effect of oxygen on immature retinal vessels. In: Vascular disorders of the eye 196616–27.
- 12.Sandercoe T M, Madigan M C, Billson F A.et al Astrocyte proliferation during development of the human retinal vasculature. Exp Eye Res 199969511–523. [DOI] [PubMed] [Google Scholar]
- 13.Gariano R F, Iruela A M, Hendrickson A E. Vascular development in primate retina: 1. Comparison of laminar plexus formation in monkey and human. Invest Ophthalmol Vis Sci 1994353442–3455. [PubMed] [Google Scholar]
- 14.Provis J M, Sandercoe T, Hendrickson A E. Astrocytes and blood vessels define the foveal rim during primate retinal development. Invest Ophthalmol Vis Sci 2000412827–2836. [PubMed] [Google Scholar]
- 15.Engerman R L. Development of the macular circulation. Invest Ophthalmol 197615835–840. [PubMed] [Google Scholar]
- 16.Chase J. The evolution of retinal vascularization in mammals. Ophthalmology 1982891518–1525. [DOI] [PubMed] [Google Scholar]
- 17.Stone J, Sandercoe T, Provis J. Mechanisms of the formation and stability of retinal blood vessels. In: Tombran‐Tink J, Barnstable C, eds. Ocular angiogenesis: diseases, mechanisms and therapeutics. Totowa, NJ: Humana Press (in press),
- 18.Stone J, Itin A, Alon T.et al Development of retinal vasculature is mediated by hypoxia‐induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci 1995154738–4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stone J, Maslim J. Mechanisms of retinal angiogenesis. Prog Ret Eye Res 199716157–181. [Google Scholar]
- 20.Ozaki H, Yu A Y, Della N.et al Hypoxia inducible factor‐1alpha is increased in ischemic retina: temporal and spatial correlation with VEGF expression. Invest Ophthalmol Vis Sci 199940182–189. [PubMed] [Google Scholar]
- 21.Semenza G L. HIF‐1: using two hands to flip the angiogenic switch. Cancer Metastasis Reviews 20001959–65. [DOI] [PubMed] [Google Scholar]
- 22.Morita M, Ohneda O, Yamashita T.et al HLF/HIF‐2alpha is a key factor in retinopathy of prematurity in association with erythropoietin. EMBO J 2003221134–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Provis J M, van Driel D, Billson F A B.et al Development of the human retina: patterns of cell distribution and redistribution in the ganglion cell layer. J Comp Neurol 1985233429–451. [DOI] [PubMed] [Google Scholar]
- 24.Linberg K A, Fisher S K. A burst of differentiation in the outer posterior retina of the eleven‐week human fetus: an ultrastructural study. Vis Neurosci 1990543–60. [DOI] [PubMed] [Google Scholar]
- 25.Bernstein M H, Hollenberg M J. Fine structure of the choriocapillaris and retinal capillaries. Invest Ophthalmol 196541016–1025. [PubMed] [Google Scholar]
- 26.Walcott J C, Provis J M. Muller cells express the neuronal progenitor cell marker nestin in both differentiated and undifferentiated human foetal retina. Clin Exp Ophthalmol 200331246–249. [DOI] [PubMed] [Google Scholar]
- 27.Fryczkowski A W, Sherman M D. Scanning electron microscopy of human ocular vascular casts: the submacular choriocapillaris. Acta Anat (Basel) 1988132265–269. [DOI] [PubMed] [Google Scholar]
- 28.Provis J M, Diaz C M, Dreher B. Ontogeny of the primate fovea: a central issue in retinal development. Prog Neurobiol 199854549–580. [DOI] [PubMed] [Google Scholar]
- 29.Xiao M, Hendrickson A. Spatial and temporal expression of short, long/medium, or both opsins in human fetal cones. J Comp Neurol 2000425545–559. [PubMed] [Google Scholar]
- 30.Georges P, Cornish E, Provis J.et al Müller cell expression of glutamate cycle‐related proteins & anti‐apoptotic proteins in early human retinal development. Br J Ophthalmol 200690223–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandercoe T M, Geller S F, Hendrickson A E.et al VEGF expression by ganglion cells in central retina before formation of the foveal depression in monkey retina: evidence of developmental hypoxia. J Comp Neurol 200346242–54. [DOI] [PubMed] [Google Scholar]
- 32.Gogat K, Le Gat L, Van Den Berghe L.et al VEGF and KDR gene expression during human embryonic and fetal eye development. Invest Ophthalmol Vis Sci 2004457–14. [DOI] [PubMed] [Google Scholar]
- 33.Marneros A G, Fan J, Yokoyama Y.et al Vascular endothelial growth factor expression in the retinal pigment epithelium is essential for choriocapillaris development and visual function. Am J Pathol 20051671451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zubilewicz A, Hecquet C, Jeanny J C.et al Two distinct signalling pathways are involved in FGF2‐stimulated proliferation of choriocapillary endothelial cells: a comparative study with VEGF. Oncogene 2001201403–1413. [DOI] [PubMed] [Google Scholar]
- 35.Steinle J J, Granger H J. Nerve growth factor regulates human choroidal, but not retinal, endothelial cell migration and proliferation. Autonomic Neuroscience—Basic and Clinical 200310857–62. [DOI] [PubMed] [Google Scholar]
- 36.Asahara T, Murohara T, Sullivan A.et al Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997275964–967. [DOI] [PubMed] [Google Scholar]
- 37.Kalka C, Masuda H, Takahashi T.et al Vascular endothelial growth factor(165) gene transfer augments circulating endothelial progenitor cells in human subjects. Circulation Res 2000861198–1202. [DOI] [PubMed] [Google Scholar]
- 38.Kalka C, Tehrani H, Laudenberg B.et al VEGF gene transfer mobilizes endothelial progenitor cells in patients with inoperable coronary disease. Ann Thoracic Surg 200070829–834. [DOI] [PubMed] [Google Scholar]
- 39.Isner J M, Kalka C, Kawamoto A.et al Bone marrow as a source of endothelial cells for natural and iatrogenic vascular repair. Ann NY Acad Sciences 200195375–84. [DOI] [PubMed] [Google Scholar]
- 40.Csaky K, Baffi J, Byrnes G.et al Recruitment of marrow‐derived endothelial cells to experimental choroidal neovascularization by local expression of vascular endothelial growth factor. Exp Eye Res 2004781107–1116. [DOI] [PubMed] [Google Scholar]
- 41.Otani A, Kinder K, Ewalt K.et al Bone marrow‐derived stem cells target retinal astrocytes and can promote or inhibit retinal angiogenesis. [comment]. Nature Med 200281004–1010. [DOI] [PubMed] [Google Scholar]
- 42.Otani A, Dorrell M, Kinder K.et al Rescue of retinal degeneration by intravitreally injected adult bone marrow‐derived lineage‐negative hematopoietic stem cells. J Clin Invest 2004114765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prockop D. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 19972771–74. [DOI] [PubMed] [Google Scholar]
- 44.Reyes M, Dudek A, Jahagirdar B.et al Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest 2002109337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Annabi B, Naud E, Lee Y.et al Vascular progenitors derived from murine bone marrow stromal cells are regulated by fibroblast growth factor and are avidly recruited by vascularizing tumors. J Cell Biochem 2004911146–1158. [DOI] [PubMed] [Google Scholar]