Abstract
Background
Limited data are available to guide optimal positioning of glaucoma drainage devices (GDD) in relation to the limbus and optic nerve. The authors aim to provide guidelines for appropriate and safe GDD implantation.
Method
The optimal positioning of five different GDD were evaluated using necropsy eyes of varying axial lengths. The dependent variable that was measured was the maximum distance that a GDD could be placed posterior to the limbus while remaining 2 mm away from the optic nerve.
Results
The average maximum distance posterior to the limbus of the anterior plate edge ranged between 9.0–15.0 mm in the superotemporal quadrant for the GDD tested. The distances for superonasal, inferonasal, and inferotemporal quadrants ranged between 8.0–14.0 mm, 9.0–14.0 mm, and 11.0–17.0 mm, respectively. The Molteno device could be placed most posteriorly while remaining 2 mm away from the nerve. The Ahmed FP7 and S2 were the least amenable to posterior placement before encroaching on the 2 mm limit.
Conclusion
The maximum distance that a GDD can be placed posterior to the limbus, before encroachment around the optic nerve, varies between different devices and quadrants of placement. Taking a measurement of the exact distance of the plate from the limbus during GDD surgery is recommended.
Keywords: glaucoma, drainage device, optic nerve
Glaucoma drainage devices (GDD) are important tools for care of glaucoma refractory to conventional medical and surgical therapies. There are several commercially available GDD and they are available in a variety of sizes (table 1). Despite the long term use of these devices, limited data are available to guide optimal placement of GDD in relation to the limbus and optic nerve. Studies have been published on the placement of the Ahmed valve (New World Medical, Rancho Cucamonga, CA, USA) and its potential for contact with the optic nerve.1,2,3 These reports are single patient case reports or animal studies from which it is difficult to derive information guiding clinical placement during surgery. Little is known about the proper implantation of other commonly used drainage devices including the Molteno D1 (Molteno Ophthalmic Ltd, Dunedin, New Zealand) and the Baerveldt (Advanced Medical Optics, Santa Ana, CA, USA).4,5 We aim to define appropriate distances and sites for safe GDD implantation and compare the various plates in relation to size and shape and the possibility of impingement on the optic nerve.
Materials and methods
Five human cadaver eyes of differing axial lengths were obtained for this study after appropriate institutional review board approval was obtained (Committee for Oversight of Research Involving the Dead (CORID), University of Pittsburgh). Axial lengths ranged from 22.5 mm to 26.0 mm. Tenon's capsule and fat were removed from the surface of each eye. The Molteno single plate GDD (D1), the Baerveldt GDD (250 and 350), and the Ahmed valves (FP7 and S2) were used in this experiment. Each GDD was positioned sequentially on an eye, equidistant between the rectus muscles in each quadrant. The GDD was placed 2 mm anterior to the optic nerve, as measured by surgical callipers, and secured to the sclera through the positioning holes with a single Vicryl suture in a mattress fashion. The positioning of the implant was selected based on a study reporting optic nerve changes in rabbits with GDD implantation closer than 2 mm to the nerve.3 The distance from anterior plate edge to the limbus was measured with callipers three times sequentially and recorded. The measurements were made in the superonasal (SN), superotemporal (ST), inferonasal (IN), and inferotemporal (IT) quadrants for each GDD and for each eye.
Results
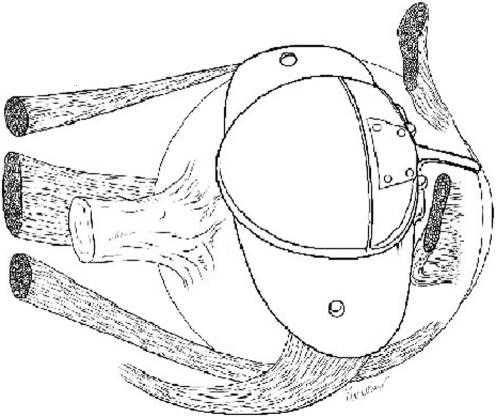
The average maximum distance from limbus to anterior plate edge ranged between 9.0–15.0 mm in the ST quadrant for all of the GDD tested. The anterior plate‐limbus distances for SN, IN, and IT quadrants ranged between 8.0–14.0 mm, 9.0–14.0 mm, and 11.0–17.0 mm, respectively (table 2). The Molteno device could be placed furthest posteriorly to the limbus in all quadrants while remaining 2 mm away from the nerve. The Ahmed FP7 and S2 models required the most anterior placement to avoid encroaching on the 2 mm optic nerve proximity limit (fig 1). The maximum limbus to plate distance for each device on the eyes included in this study was dependent on the eye's axial length, as well as the implant configuration.
Table 2 Measured distance from limbus to anterior plate border.
| Quadrant | Eye axial length | ||||
|---|---|---|---|---|---|
| 22.5 mm | 23.0 mm | 23.0 mm | 24.5 mm | 26.0 mm | |
| Superotemporal | |||||
| Molteno D1 | 13.0 | 13.5 | 13.0 | 14.0 | 15.0 |
| Ahmed (FP7 and S2) | 9.0 | 9.0 | 9.5 | 10.5 | 11.5 |
| Baerveldt | |||||
| 250 | 10.5 | 10.5 | 10.5 | 11.5 | 12.5 |
| 350 | 9.5 | 9.5 | 10.0 | 10.5 | 11.5 |
| Superonasal | |||||
| Molteno D1 | 11.5 | 11.5 | 12.0 | 12.5 | 14.0 |
| Ahmed (FP7 and S2) | 8.0 | 8.0 | 8.5 | 9.5 | 10.5 |
| Baerveldt | |||||
| 250 | 8.5 | 9.0 | 9.5 | 10.0 | 11.0 |
| 350 | 8.0 | 8.0 | 9.0 | 9.5 | 10.5 |
| Inferotemporal | |||||
| Molteno D1 | 15.0 | 15.5 | 16.0 | 16.5 | 17.0 |
| Ahmed (FP7 and S2) | 12.0 | 12.5 | 13.5 | 13.5 | 14.5 |
| Baerveldt | |||||
| 250 | 11.0 | 11.5 | 12.0 | 12.5 | 13.5 |
| 350 | 11.5 | 12.5 | 13.0 | 13.5 | 15.0 |
| Inferonasal | |||||
| Molteno D1 | 12.0 | 13.0 | 13.0 | 13.5 | 14.0 |
| Ahmed (FP7 and S2) | 9.5 | 9.5 | 9.5 | 10.0 | 11.0 |
| Baerveldt | |||||
| 250 | 10.0 | 10.5 | 10.5 | 11.0 | 12.0 |
| 350 | 9.0 | 9.5 | 10.0 | 10.5 | 11.5 |
Figure 1 Location of the Ahmed drainage device in relation to the optic nerve when implanted in the superotemporal quadrant.
The distance between the GDD and the limbus at 2 mm anterior to the optic nerve in the ST quadrant was, on average, greater than the limbus‐GDD distance in the SN quadrant. The mean maximum IN GDD‐limbus and IT GDD‐limbus distances were found to be more closely related to axial length, making GDD implantation in these quadrants harder to generalise across eyes of different axial lengths. The Baerveldt 350 mm2 implant, which has an anterior‐posterior length of 15 mm, could be placed more posteriorly than the overall smaller Ahmed devices, which have an anterior‐posterior length of 16 mm (fig 2), despite the larger surface area of the Baerveldt GDD.
Figure 2 A comparison of the Ahmed and Baerveldt 350 mm2 devices showing more posterior extension of the Ahmed valve towards the optic nerve.
The Ahmed valve was easily sutured in the ST quadrant without extending into the regions of adjacent muscles. The Baerveldt implants, traditionally placed under the recti muscles of the corresponding quadrant, usually encroached into the area of all adjacent muscles. The 350 mm2 Baerveldt extended into the areas of the superior rectus, lateral rectus, and both the superior and inferior oblique muscles when placed in the ST quadrant. The 250 mm2, when placed in the ST quadrant, reached the areas around the superior and lateral recti as well as the superior oblique muscle without abutting the inferior oblique insertion site.
When placed into the IT quadrant, all of the tested devices except the Molteno consistently came into contact with the insertion site of the inferior oblique muscle. Insertion of all GDD in the IN quadrant was less likely to demonstrate impingement on the adjacent inferior oblique muscle.
Discussion
Molteno introduced the concept of draining aqueous fluid away from the limbus to a posterior plate in 1969.6,7 The introduction of this model allowed for increasing the efficacy of drainage devices while at the same time decreasing the complication rates associated with early exposure of plates placed too close to the limbus. The devices that followed, including the Ahmed and Baerveldt implants, were based on the same concept of placing the plate relatively posterior to the limbus. While this led to a decrease in device exposure rates, the possibility of causing dysfunction of extraocular muscle movements and optic neuropathy from compression of the optic nerve increased.
Leen and colleagues reported a case of SN quadrant Ahmed valve (S2 model) implantation in a patient who suffered from a chemical burn with secondary glaucoma and subsequent enucleation of the involved eye 2 months later.1 On gross examination of the enucleated eye, the anterior aspect of the Ahmed plate was noted to be 10 mm posterior to the limbus and the posterior margin of the plate extended to within 1 mm of the optic nerve.
Ayyala and colleagues reported findings in a patient with an eye in which there were Ahmed valves implanted in both the ST and SN quadrants, 9 mm away from the limbus, for treatment of refractory neovascular glaucoma.2 Subsequent complications led to enucleation of the eye, permitting gross and histopathological examination. The authors reported finding the posterior aspect of the plate located 4 mm from the nerve in the ST quadrant and 2 mm away in the SN quadrant. In both cases, the fibrous capsule surrounding the plates did not involve the optic nerve. The authors recommended more anterior implantation of the Ahmed valve in the SN quadrant to avoid potential contact with the optic nerve.
Optic nerve changes following ST quadrant implantation of the Ahmed valve (S3 model) 10–12 mm from the limbus in rabbits has been reported.3 Histological changes of the optic nerve, including microglial loss and astrocyte clustering, were noted in animals with the posterior aspect of the plate either abutting the optic nerve or coming within 1 mm of the nerve sheath. While the anatomy of the rabbit eye is significantly different from that of the human eye, this study illustrates the possible changes that can take place when GDD are placed too close to the optic nerve.
GDD are manufactured in a variety of sizes and shapes and are made of various materials. Posterior placement of the plate is desirable to decrease the potential for anterior exposure and allow for flow of aqueous far from the anterior aspect of the globe. On the other hand, significant optic nerve complications may occur if the GDD are implanted too posteriorly as evidenced by previous animal studies.3 Furthermore, there may be variability in the safe range of placement from eye to eye and quadrant to quadrant. Surgeons must be aware of the differences among the various devices in terms of shape and size. Also, the intermuscular space is variable in each quadrant for implantation of the devices. These factors may be even more important in eyes that are smaller in size, such as in nanophthalmos, paediatric, or hyperopic patients. The current study demonstrates the importance of measuring the location of each device type during surgery to minimise chance of contact with the optic nerve.
The GDD with larger lateral dimensions also appear to be more likely to affect surrounding muscles including both the recti and oblique muscles. Impinging on these muscles can possibly cause double vision and/or discomfort. Those that have larger lateral dimensions may impinge on the oblique muscles even when well centred between rectus muscles. Which muscles may be affected is dependent on the quadrant of placement. Once again, smaller eyes have less distance between these structures and therefore may be at greater risk of impingement.
This current study found, that when placed into the IT quadrant, all of the tested devices except the Molteno consistently came into contact with the insertion site of the inferior oblique muscle. While it is difficult to assess the clinical implication of this phenomenon, it is probably less desirable to have plate‐muscle insertion site contact with the accompanying increased risk of postoperative diplopia. Insertion of GDD in the IN quadrant, while potentially more technically difficult in a clinical setting because of exposure issues, was less likely to demonstrate impingement on the adjacent inferior oblique muscle. These findings are consistent with a previous report comparing IN versus IT quadrant placement of the Baerveldt 350 mm2 drainage device.8
The GDD with the smaller anterior‐posterior dimensions were also less likely to impinge on the 2 mm “safe zone” around the optic nerve. Once again, the Molteno with the smallest dimensions could be placed most posteriorly to the limbus, whereas the Ahmed valves could not be placed as posteriorly, on average, because of the longer anterior‐posterior dimension. Based on the findings in this study, surgeons may not want to consider putting devices other than the Molteno in the SN quadrant, owing to the crowded anatomy of this location. It is probably preferable to place secondary GDD in the inferior quadrants after the traditional ST quadrant has been utilised.
The findings in this study are most important for eyes with smaller axial lengths. Eyes with an axial length under 20 mm require added care owing to the dual risk of posterior impingement on the nerve along with increased risk of anterior placement leading to future exposure. In these cases, the Ahmed devices and Baerveldt 350 mm2 device should be avoided because of their large anterior‐posterior dimension. A Molteno device or Baerveldt 250 mm2 would be more appropriate and allow for safer posterior implantation with less chance of anterior conjunctival erosion and exposure.
It appears that with larger eyes, there is much more room for error and most devices are easily accommodated, especially in the ST and inferior quadrants. However, it may behove surgeons to not always place the GDD a standardised distance back from the limbus or “as posteriorly as possible” in patients with smaller eyes or nanophthalmos. Furthermore, consideration should be given to performing axial length measurements preoperatively, to assess the relative risk of impingement on extraocular structures. Lastly, the findings in this study were based on optimal placement of GDD. If there is non‐centred or asymmetric placement of the plate, the chance of impingement may be magnified.
Acknowledgements
We thank Kira L Lathrop, MAMS, for her artistic contributions to table 1 and figures 1 and 2.
Abbreviations
GDD - glaucoma drainage devices
IN - inferonasal
IT - inferotemporal
SN - superonasal
ST - superotemporal
Footnotes
The authors have no interests or disclosures to report in relation to the content of this manuscript.
References
- 1.Leen M M, Witkop G S, George D P. Anatomic considerations in the implantation of the Ahmed glaucoma valve. Arch Ophthalmol 1996114223–224. [DOI] [PubMed] [Google Scholar]
- 2.Ayyala R S, Layden W E, Slonim C B.et al Anatomic and histopathologic findings following a failed Ahmed glaucoma valve device. Ophthalmic Surg Lasers 200132248–249. [PubMed] [Google Scholar]
- 3.Ayyala R S, Parma S E, Karcioglu Z A. Optic nerve changes following posterior insertion of glaucoma drainage device in rabbit model. J Glaucoma 200413145–148. [DOI] [PubMed] [Google Scholar]
- 4.Rubin B, Chen C C, Burnier M.et al Histopathologic study of the Molteno glaucoma implant in three patients. Am J Ophthalmol 1990110137–379. [DOI] [PubMed] [Google Scholar]
- 5.Minckler D S, Shammas A, Wilcox M.et al Experimental studies of aqueous filtration using the Molteno implant. Trans Am Ophthalmol Soc 198785368–389. [PMC free article] [PubMed] [Google Scholar]
- 6.Molteno A C. New implant for drainage in glaucoma. Animal trial. Br J Ophthalmol 196953161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molteno A C. New implant for drainage in glaucoma. Clinical trial. Br J Ophthalmol 196953606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sidoti P A. Inferonasal placement of aqueous shunts. J Glaucoma 200413520–523. [DOI] [PubMed] [Google Scholar]