Abstract
Aim
To review the medical records of seven children with a delayed diagnosis of cerebral visual impairment.
Methods
The charts of a consecutive series of children examined in a university based ophthalmology clinic with a delayed diagnosis of cerebral visual impairment were reviewed. Their neuroimaging studies were evaluated by a paediatric neuroradiologist.
Results
The seven patients ranged in age from 2 years to 10 years at the time of presentation and had acuities ranging from 20/40 to 20/400. Their visual fields ranged from full visual fields to homonymous hemianopias. Neuroimaging findings ranged from subtle occipital lobe abnormalities to obvious cerebral volume loss.
Conclusions
Cerebral visual impairment can be cryptic in children with mild visual impairment. Neuroimaging studies and visual field testing can help to differentiate this condition from other causes of visual impairment.
Keywords: cerebral visual impairment, neuroimaging, periventricular leucomalacia, striate cortex, children
Cerebral visual impairment (CVI), also sometimes referred to as cortical visual impairment, is one of the most common causes of blindness in children.1,2,3 It usually occurs secondary to a perinatal insult, and in most cases these children have other neurological problems.4 CVI is a term used to describe a visual disorder due to abnormalities in the retrochiasmatic visual pathways. The findings and presentation of patients with CVI can be highly variable, and because of the difficulty in determining visual function in young children, the diagnosis may be missed initially. Complete cortical blindness can be caused by destruction of the optic radiations and/or the striate cortex, but this is rare.5 CVI most commonly occurs in children as a result of hypoxic‐ischaemic brain injuries, especially during the perinatal period. The gestational age of the child at the time of the insult largely determines the type of injury that will develop.6,7 Full term infants usually develop watershed infarcts of the cerebral cortex, whereas premature infants more commonly sustain injuries to the periventricular white matter resulting in periventricular leucomalacia (PVL).8 Visual impairment in children with PVL is due to injuries to the optic radiations, whereas visual impairment in full term children is usually secondary to infarctions of the striate cortex.9,10 In most cases, children with CVI have other neurological problems that raise the suspicion of CVI; however, in some cases their neurological problems may be fairly subtle and the diagnosis of CVI may not be suspected.11 In addition, some children with CVI have abnormalities of the anterior visual pathways such as optic atrophy, which may further obfuscate the diagnosis of CVI. We report the clinical and neuroimaging findings of seven children in whom the diagnosis of CVI was initially missed by another ophthalmologist.
Patients and methods
After obtaining institutional review board approval, the charts of seven consecutive children in whom the diagnosis of CVI was initially missed by one or more ophthalmologists were reviewed (table 1).
Table 1 Clinical findings.
| Patient no | Age (years, at time of diagnosis)/sex | BCVA (right eye/left eye) at time of diagnosis | Age (years) at last follow up | Last measured acuity (BCVA) | Visual field findings | Neuroimaging | Suspected cause of CVI |
|---|---|---|---|---|---|---|---|
| 1 | 10 M | 20/20020/200 | 10 | 20/20020/200 | Full | Bilateral frontal and occipital infarcts | Perinatal sepsis |
| 2 | 7 M | 20/6020/100 | 9 | 20/20020/200 | Generalised constriction right eye>left eye | Porencephalic dilatation of the left occipital horn | Haemophilus influenzae meningitis |
| 3 | 10 M | 20/40020/400 | 20 | 20/400CF 4 ft | Left homonymous hemianopsia | Right occipital lobe and posterior temporal lobe encephalomalacia | Anoxic injury during infancy |
| 4 | 7 F | 20/40‐120/60‐2 | 15 | 20/4020/50‐2 | Full | Left occipital encephalomalacia with ex vacuo dilatation of the left occipital horn | Unknown |
| 5 | 2 ½ M | CSMCSM | 8 | 20/4020/60 | Right homonymous hemianopsia | Left hemispheric encephalomalacia | Closed head injury at 8 months of age |
| 6 | 5 M | CSM 20/40‐2 20/40‐3 | 7 | 20/50‐220/50‐1 | Full | Absent septum pellucidumand abnormalities of occipital lobes | Hydrocephalus |
| 7 | 4 M | CSMCSM | 15 | 20/60‐220/70‐2 | Full | Subtle, bilateral occipital lobe atrophy | Unknown |
BCVA, best corrected visual acuity.
The children ranged in age from 2 years to 10 years at the time the diagnosis of CVI was established. Six of the seven patients were male. All neuroimaging studies were reviewed by a paediatric neuroradiologist. The patient's vision at presentation was compared with their vision at subsequent follow up examinations.
Case presentations
Patient 1
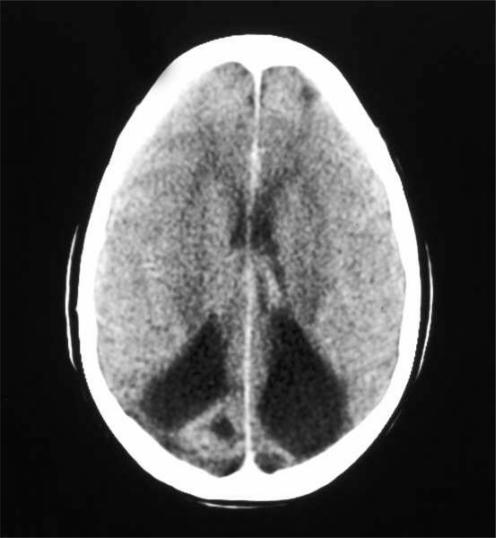
A 10 year old boy with unexplained decreased vision was referred for evaluation. He was born after a full term pregnancy complicated by sepsis secondary to premature rupture of the amniotic membranes at the time of birth. His best corrected visual acuity (BCVA) was 20/200 in both eyes. His refraction was plano in both eyes. An electroretinogram (ERG) was normal. Pattern reversal visually evoked potentials (VEPs) were highly attenuated in both eyes. A computed tomography (CT) scan (fig 1) revealed old bilateral frontal and occipital lobe infarcts that were probably related to his perinatal sepsis.
Figure 1 Patient 1. Computed tomography, brain, axial, demonstrates chronic, bilateral occipital infarcts with encephalomalacia and ex vacuo dilatation of the occipital horns of both ventricles.
Patient 2
A 6 year old boy was referred for electrophysiological testing to rule out a retinal dystrophy. He had been noted to hold objects close to his face since infancy. He had Haemophilus influenzae meningitis when 2 months of age, but his subsequent development had been normal. His BCVA was 20/60 in the right eye and 20/100 in the left eye. His cycloplegic refraction was +0.75D, in both eyes. He had slightly constricted visual fields, the right greater than the left. An ERG was normal. A magnetic resonance imaging (MRI) scan revealed porencephalic dilatation of the left occipitial horn and pitting of the left occipital cortex.
Patient 3
A 10 year old boy was referred for evaluation of decreased vision. He had an apnoeic episode when 6 weeks of age and subsequently developed a seizure disorder and strabismus. He was treated with seizure medications until 2 years of age, but had been seizure free since then. He underwent strabismus surgery to correct an esotropia when 15 months of age. He had great difficulty keeping up with the other students in his class at school. His BCVA was 20/400 in both eyes. He had a refractive error of +0.50D in each eye. An MRI revealed significant encephalomalacia of the right occipital lobe with mild volume loss and gliosis of the left occipital lobe (fig 2). He was subsequently taught to read using braille which resulted in a dramatic improvement in his school performance. At his last follow up examination when 20 years of age, his vision was 20/400 in the right eye and counting fingers (CF) at 4 feet in the left eye. He had a complete left homonymous hemianopsia using Goldmann perimetry and exotropia. His ocular examination was otherwise normal.
Figure 2 Patient 3. Magnetic resonance imaging, T2, fast spin echo, brain, axial, shows an area of encephalomacia of the right occipital lobes with evidence of contralateral injury, mild volume loss, and gliosis.
Patient 4
A 7 year old girl was referred for evaluation of decreased vision. She had undergone three previous strabismus surgeries. She was the product of a full term pregnancy and her medical history was unremarkable. Her BCVA was 20/40‐1 in the right eye and 20/60‐2 in the left eye. Her refraction was plano +1.00 × 075, in the right eye and plano +1.00 × 100 in the left eye. She had an A‐pattern exotropia with bilateral superior oblique overaction and dissociated vertical deviation. Her optic discs and retinas were normal in appearance. Goldmann visual fields were normal in both eyes. An MRI revealed a focal area of encephalomalacia in the left occipital lobe with ex vacuo dilatation of the occipital horn of the left lateral ventricle (fig 3).
Figure 3 Patient 4. Magnetic resonance imaging, T1, three dimensional, FSPGR, brain, axial, showing focal area of encephalomalacia of the left occipital lobe, with ex vacuo dilatation of the occipital horn of the left lateral ventricle.
Patient 5
A 2½ year old boy was evaluated for decreased vision. He had a history of a closed head injury when 8 months of age after falling out of bed. He developed a right hemiparesis and a seizure disorder after the accident. His visual acuity was central, steady, and maintained in both eyes. He had bilateral overaction of the inferior oblique muscles, possibly secondary to bilateral fourth nerve palsies. His fundi were normal. When 8 years of age, his BCVA was 20/40 in the right eye and 20/60 in the left eye. An MRI revealed extensive left hemispheric encephalomalacia and milder abnormalities in the left parietal and occipital lobes (fig 4). He had a right homonymous hemianoptic defect on Goldmann visual field testing.
Figure 4 Patient 5. Magnetic resonance imaging, T2, fast spin echo, brain, axial, shows extensive encephalomalacia of the left occipital and temporal lobes
Patient 6
A 5 year old boy with a history of spina bifida and hydrocephalus was referred for evaluation of an exotropia. He had previously undergone a ventriculo‐peritoneal shunting procedure. His BCVA was 20/40‐2, with a refraction of +2.50 sphere in the right eye, and 20/40‐3, with a refraction of +2.50 +1.00 × 030 in the left eye. He had an intermittent left exotropia with mild, bilateral inferior oblique overaction. One year later, his BCVA was 20/60 in each eye. An MRI revealed an absent septum pellucidum but normal optic nerves. There were also multiple dysplastic areas, including both occipital lobes.
Patient 7
A 4 year old boy was referred for evaluation of a large angle exotropia. He also had a complex partial seizure disorder and delayed development. A MRI revealed subtle bilateral occipital lobe abnormalities (fig 5). When 6 years of age, his BCVA was 20/60 in both eyes. When 15 years of age, his BCVA was 20/60‐2 in the right eye and 20/70‐2 in the left eye. Goldmann visual fields were full in both eyes.
Figure 5 Patient 7. Magnetic resonance imaging, T2, fast spin echo, brain, axial, shows bilateral atrophy and gliosis of the occipital lobes.
Discussion
Cerebral visual impairment should be considered in the differential diagnosis of any child with unexplained decreased vision. In particular, it should be suspected in a child who is unable to achieve the expected visual acuity based on their ocular examination, while taking into account their mental or verbal abilities. Electrophysiological testing can be helpful in determining which patients may have an additional reason for their decreased vision. Ultimately, a neuroimaging study of the visual pathways is usually required to establish the diagnosis of CVI. Computed tomography may be adequate in patients with severe abnormalities, but MRI with T2, fast spin echo with gadolinium is necessary to diagnosis more subtle abnormalities.12
The diagnosis of CVI was probably missed in our patients because four had not undergone any previous neuroimaging studies, and all had relatively minor neurological problems. While two had a history of seizures, the seizures had resolved, and the children were no longer on seizure medication. One child also had hydrocephalus, but his visual disability was so minor that it had not been appreciated. All of the children were ambulatory, and none had cerebral palsy. All of the school aged children were attending schools in their local communities, although some were in special education classes. In contrast, Huo and co‐workers4 reported that 75% of the children in their series with CVI had other neurological deficits and 50% had seizures. Rogers and co‐workers13 reported cerebral palsy in 53% of the children in their study with CVI.
A probable aetiology for CVI was established in five of the seven patients. These aetiologies included perinatal sepsis, meningitis, a prolonged episode of apnoea, a closed head injury, and hydrocephalus.13,14,15,16,17,18,19,20 None of the children in our series had known hypoxic‐ischaemic insults during the perinatal period. Hypoxic‐ischaemic insults generally result in more catastrophic brain injuries with profound visual impairment and severe neurological handicaps.19 Given the relatively subtle visual and neurological problems of our patients, this was not unexpected. In most children, an aetiology for CVI can be elicited with a careful history. In a review of 170 children with CVI, Huo et al4 found 9.4% to be idiopathic. We could not establish an aetiology for the striate cortex abnormalities in two of the patients in our series.
The visual acuity was preserved much better than expected for the children in our series compared to other series of children with CVI. Six of our seven patients (86%) had a visual acuity of 20/200 or better. In contrast, Hoyt21 reported that only 10% of the children in his series with CVI had a visual acuity of 20/200 or better, although this was a much larger series including all patients with CVI, not just those presenting with a difficult diagnosis. CVI generally affects the visual acuity equally in both eyes. The only exception to this rule in our series was patient 3. The more severely reduced visual acuity in this patient's left eye was probably caused by strabismic amblyopia superimposed on his CVI. One patient had a complete homonymous hemianopsia suggesting that the occipital lobe injuries were asymmetrical. Patient 3 had a dramatic improvement in his school performance, once the extent of his visual difficulties was fully appreciated, and he was taught to read using braille. Children with CVI often have profound visual perceptive problems that make it difficult for them to read efficiently, even when their visual acuity would seem to be adequate for reading by sight.13
The small number of patients in our series makes description of trends and generalisations impossible, but these cases emphasise that the presentation of patients with CVI may be cryptic, later than expected, and visual function may be quite good. The diagnosis of CVI should be considered in any child in whom there are no ocular findings to account for their decreased vision. A high quality MR scan as well as a careful medical history is critical in establishing the diagnosis of CVI.
Acknowledgements
Supported in part by NIH Departmental Core Grant EY06360 and Research to Prevent Blindness, Inc (SRL).
Abbreviations
CVI - cerebral visual impairment
ERG - electroretinogram
MRI - magnetic resonance imaging
PVL - periventricular leucomalacia
VEPs - visually evoked potentials
References
- 1.Good W V, Jan J E, Luis D.et al Cortical visual impairment in children. Surv Ophthalmol 199438351–364. [DOI] [PubMed] [Google Scholar]
- 2.Foster A. Childhood blindness. Eye 19882(Suppl)S27–S36. [DOI] [PubMed] [Google Scholar]
- 3.Goggin M, O'Keefe M. Childhood blindness in the Republic of Ireland: a national survey. Br J Ophthalmol 199175425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huo R, Burden S K, Hoyt C S.et al Chronic cortical visual impairment in children: aetiology, prognosis, and associated neurological deficits. Br J Ophthalmol 199983670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoerig P, Cowey A. Blindsight in man and monkey. Brain 1997120535–559. [DOI] [PubMed] [Google Scholar]
- 6.Keeney S, Adock E W, McArdle C B. Prospective observations of 100 high‐risk neonates by high‐field (1.5 Tesla) magnetic resonance imaging of the central nervous system. II. Lesions associated with hypoxic‐ischaemic encephalopathy. Pediatrics 199187431–438. [PubMed] [Google Scholar]
- 7.Barkovich A J, Westmark D, Rerriero D M.et al Perinatal asphyxia: MR findings in the first 10 days. Am J Neuroradiol 199516427–438. [PMC free article] [PubMed] [Google Scholar]
- 8.Eken P, de Vries L S, von der Graaf Y.et al Haemorrghagic‐ischaemic lesions of the neonatal brain: correlation between cerebral visual impairment, neurodevelopmental outcome, and MRI in infancy. Dev Med Child Neurol 19953741–55. [DOI] [PubMed] [Google Scholar]
- 9.Casteels I, Demaerel P, Spileers W.et al Cortical visual impairment following perinatal hypoxia: clinicoradiologic correlation using magnetic resonance imaging. J Pediatr Ophthalmol Strabismus 199734297–305. [DOI] [PubMed] [Google Scholar]
- 10.Uggetti C, Egitto M G, Fazzi E.et al Cerebral visual impairment in periventricular leukomalacia: MR correlation. Am J Neuroradiol 199617979–985. [PMC free article] [PubMed] [Google Scholar]
- 11.Cioni G, Fazzi B, Ipata A E.et al Correlation between cerebral visual impairment and magnetic resonance imaging in children with neonatal encephalopathy. Dev Med Child Neurol 199638120–132. [DOI] [PubMed] [Google Scholar]
- 12.Van Nieuwenhuizen O, Abbing P R, Zeidses des Plantes B G.et al Role of MRI scanning in the diagnosis of cerebral visual disturbance. Pediatr Neurol 19862363–366. [DOI] [PubMed] [Google Scholar]
- 13.James J E, Wong P K H. The child with cortical visual impairment. Sem Ophthalmol 19916194–200. [Google Scholar]
- 14.Rogers M. Vision impairment in Liverpool: prevalence and morbidity. Arch Dis Chld 199674299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lambert S R, Hoyt C S, Jan J E.et al Visual recovery from hypoxic cortical blindness during childhood: CT and MR predictors. Arch Ophthalmol 19871051371–1377. [DOI] [PubMed] [Google Scholar]
- 16.Van Hof‐van D J, Cioni G, Bertuccelli B.et al Visual outcome at 5 years of newborn infants at risk of cerebral visual impairment. Dev Med Child Neurol 199840302–309. [DOI] [PubMed] [Google Scholar]
- 17.Roland E H, James E J, Hill A.et al Cortical visual impairment following birth asphyxia. Pediatr Neurol 19862133–137. [DOI] [PubMed] [Google Scholar]
- 18.DeSousa A L, Kleiman M B, Mealey J., Jr Quadriplegia and cortical blindness in hemophilus influenzae meningitis. J Pediatr 197893253–254. [DOI] [PubMed] [Google Scholar]
- 19.Ackroyd R S. Cortical blindness following bacterial meningitis: a case report with reassessment of prognosis and aetiology. Dev Med Child Neurol 198426227–230. [DOI] [PubMed] [Google Scholar]
- 20.Dutton G, Ballantyne J, Boyd G.et al Cortical visual dysfunction in children: a clinical study. Eye 199610302–309. [DOI] [PubMed] [Google Scholar]
- 21.Hoyt C S. Visual function in the brain‐damaged child. Eye 200317369–384. [DOI] [PubMed] [Google Scholar]