Abstract
Tumors arise from normal cells of the body through genetic mutation. Although such genetic mutation often leads to the expression of abnormal antigens, the immune system fails to respond effectively to these antigens; that is, it is tolerant of these antigens. This acquired state of tolerance must be overcome for cancer immunotherapy to succeed. Indoleamine 2,3-dioxygenase (IDO) is one molecular mechanism that contributes to tumor-induced tolerance. IDO helps create a tolerogenic milieu in the tumor and the tumor-draining lymph nodes, both by direct suppression of T cells and enhancement of local Treg-mediated immunosuppression. It can also function as an antagonist to other activators of antitumor immunity. Therefore, strategies to block IDO might enhance the effectiveness of tumor immunotherapy.
Introduction
It is now clear that tumors express many antigens to which the immune system can respond (1–3). Tumor-bearing hosts also possess high-avidity T cells that are specific to these antigens (4). Despite this, however, the host fails to reject the tumor. This occurs because each tumor evolves ways to escape immune surveillance (1). The net result of these escape mechanisms is that the host immune system fails to respond to tumor-associated antigens in a way that causes rejection. In immunologic terms, the host is tolerant of the tumor.
Immunologic tolerance to tumors is not simply a passive process. Although in some cases the immune system seems unaware of the presence of tumor antigens (5, 6), in many cases the immune system is aware of these antigens but is somehow actively rendered tolerant of them. This has been most clearly shown in experiments in which tumors were engineered to express a strongly immunogenic foreign antigen; typically, in such cases, the tumors are not rejected but simply create tolerance of the new antigen (7, 8). Perhaps even more daunting, once this acquired tolerance has been established, immunization with tumor-associated antigens can merely serve to intensify antigen-specific immunosuppression (9, 10). Therefore, tumor-induced tolerance is an acquired state, reflecting active mechanisms of tolerance induction, and it can represent an enormous impediment to the effectiveness of immunotherapeutic approaches to treating cancer.
The molecular mechanisms underlying tumor-induced tolerance are currently the subject of active research, and a number of contributing mechanisms have been identified (11). In this Review, we consider the role of the enzyme indoleamine 2,3-dioxygenase (IDO) as an immunosuppressive and tolerogenic mechanism in cancer. IDO catalyzes the rate-limiting step of tryptophan degradation along the kynurenine pathway (12), and both the reduction in local tryptophan concentration and the production of immunomodulatory tryptophan metabolites contribute to the immunosuppressive effects of IDO (Figure 1). Studies of serum biomarkers (tryptophan and kynurenine concentration) indicate that IDO is chronically activated in many patients with cancer (13) and that IDO activation correlates with more extensive disease (14). However, the mechanistic role of IDO in tumor immunology and its links with other tolerogenic mechanisms have only recently begun to be elucidated.
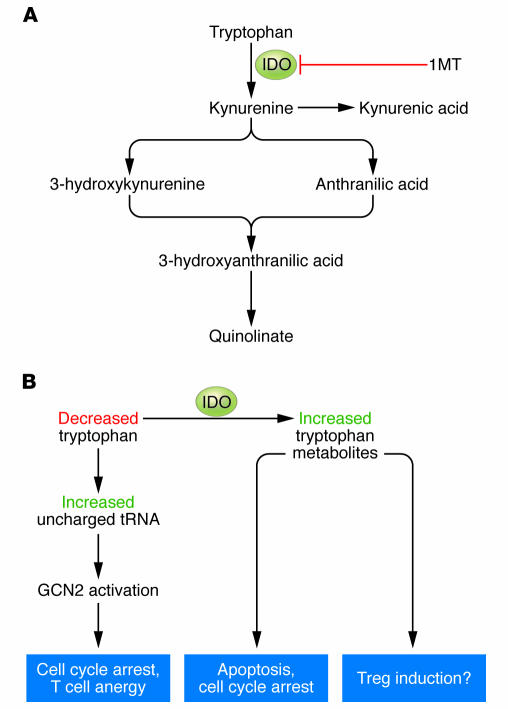
Figure 1. Molecular mechanisms of IDO-induced immunosuppression.
(A) IDO catalyzes the initial and rate-limiting step in the degradation of tryptophan along the kynurenine pathway. Tryptophan metabolites have been shown to have immunomodulatory activity, alone or in combination with the GCN2 signaling pathway. (B) IDO enzymatic activity results in the local depletion of tryptophan and a local increase in the concentration of downstream metabolites. The decrease in tryptophan can cause a rise in the level of uncharged transfer RNA (tRNA) in neighboring T cells, resulting in activation of the amino acid–sensitive GCN2 stress-kinase pathway. In turn, GCN2 signaling can cause cell cycle arrest and anergy induction in responding T cells. The local increase in tryptophan metabolites can cause cell cycle arrest, apoptosis, and (in conjunction with GCN2 signaling) differentiation of new Tregs from uncommitted CD4+ T cells.
IDO as a mechanism of acquired tolerance
Acquired tolerance of tumors is undesirable and harmful to the host, but acquired tolerance of other classes of antigens (such as fetal antigens and harmless foreign antigens at mucosal surfaces) is beneficial and necessary (15). IDO has been implicated as a normal, endogenous mechanism of peripheral tolerance and immunosuppression in a number of settings. It was originally described as contributing to maternal tolerance toward the fetus, as shown by the fact that mice treated early in pregnancy with 1-methyl-tryptophan (1MT), which is an inhibitor of IDO, underwent immune-mediated rejection of allogeneic concepti (16–18). The fetus represents an example of a set of foreign antigens to which the immune system is forced to remain tolerant and therefore is conceptually analogous to tumors in this regard.
More generally, it has now been observed that mice treated with IDO inhibitors become refractory to acquired tolerance induction in a number of settings. For example, blocking IDO with 1MT prevents the induction of tolerance to islet cell allografts by the fusion protein cytotoxic T lymphocyte–associated antigen 4–Ig (CTLA4-Ig) (19, 20), and 1MT blocks the tolerance that normally occurs when foreign antigens are introduced into the anterior chamber of the eye (an immunologically privileged site) (21). In settings where self tolerance has already been disrupted (for example, autoimmune disorders), pharmacologic inhibition of IDO causes marked exacerbation of inflammation and worsened symptoms of disease, as shown in models as diverse as inflammatory bowel disease (22), EAE (23), and experimental allergic asthma (24). Conversely, ectopic overexpression of IDO by gene transfer results in suppression of immune responses. For example, MHC-mismatched lung allografts transfected with IDO are protected from rejection without further immunosuppression (25, 26), and similar results have been reported in corneal transplants (27). Therefore, in vivo, IDO functions as a molecular mechanism contributing to acquired peripheral tolerance.
However, IDO does not seem to be required for the constitutive maintenance of tolerance to self. This is shown by the fact that mice genetically modified to lack IDO (Ido–/– mice) do not develop lethal autoimmune or lymphoproliferative disorders (20) and mice treated systemically for up to 28 days with pharmacologic IDO inhibitors have not been observed to develop spontaneous autoimmunity (22, 28). Therefore, in certain settings, IDO can be very important for tolerance, but the effects of IDO are selective and are narrowly focused on specific forms of acquired peripheral tolerance. This specificity is potentially an advantage when contemplating the clinical use of pharmacologic IDO inhibitors since these would not be predicted to have severe spontaneous autoimmunity as a limiting side effect.
Regulation of IDO expression
IDO can be expressed by multiple cell types in response to inflammation. The regulation of IDO expression (gene, protein, and functional activity) is complex and remains a subject of active investigation. However, two important points have emerged from the literature. First, within the immune system, certain types or subsets of APCs seem to be preferentially disposed to express functional IDO when challenged with proinflammatory stimuli or exposed to signals from activated T cells. In mice, these “IDO-competent” APCs include a subset of plasmacytoid DCs (29), CD8α+ splenic DCs (or a subset thereof) (30, 31), and doubtless other subsets of DCs and macrophages as well. Second, even in APCs that are IDO competent, the actual presence or absence of functional IDO enzymatic activity is tightly regulated by specific maturation and activation signals (31–36).
Conceptually, this ability to upregulate or downregulate IDO in response to external stimuli seems logical, given the need for APCs to sometimes present antigens in an activating fashion and sometimes in a tolerizing fashion, depending on the context. However, this plasticity somewhat complicates the study of IDO-competent APCs in tumor-bearing hosts since both the relevant APC subsets and the specific signals that turn IDO expression on or off in these cells must be identified. It is even less well understood how IDO expression might be regulated in the tumor cells themselves (for example, in response to local inflammatory mediators such as IFN-γ; ref. 37) and whether IDO expressed by tumor cells has the same properties as that expressed by DCs and macrophages.
Molecular mechanisms of IDO-mediated immune suppression
IDO initiates the degradation of tryptophan along the kynurenine pathway (12). IDO and the downstream enzymes in this pathway produce a series of immunosuppressive tryptophan metabolites (Figure 1A). Some of these metabolites suppress T cell proliferation in vitro or cause T cell apoptosis (38–41), and some can affect NK cell function (42). Even if IDO itself is not present, enzymes downstream of IDO in the kynurenine tryptophan degradation pathway can create immunosuppressive metabolites if supplied with kynurenine (43). In addition, in vivo, rats treated with a mixture of tryptophan metabolites showed prolonged graft survival (44); and the drug N-[3′,4′-dimethoxycinnamoyl] anthranilic acid (Tranilast), a synthetic derivative of the tryptophan metabolite anthranilic acid, has been shown to reduce inflammation and reverse paralysis in mice with EAE (45). Therefore, it seems that a number of tryptophan metabolites are immunosuppressive. The molecular mechanism by which these compounds exert their immunologic effects is not known, but at least one recent report has described a receptor able to bind a specific metabolite of tryptophan (kynurenic acid) (46). The biologic function of this orphan G protein–coupled receptor, GPR35, is unknown, but its expression was highest in cells of the immune system and the gut, sites where IDO is known to be expressed (46). Whether there are other such receptors for other tryptophan metabolites and what the biologic effects of such receptors might be in vivo are important questions that deserve further investigation.
In addition to the immunosuppressive effects of tryptophan metabolites, the cellular stress imposed by local depletion of tryptophan also seems to mediate some of the immunosuppressive effects of IDO (Figure 1B). This was first suggested by the observation that some effects of IDO on T cells are reversed by the addition of excess tryptophan in vitro (25, 29, 33, 47). Recently, the stress-responsive kinase general control nonderepressible 2 (GCN2) has been identified as a signaling molecule that enables T cells to sense and respond to stress conditions created by IDO (48, 49). The kinase activity of GCN2 is triggered by a rise in the amount of uncharged transfer RNA (tRNA) in the cell (50); therefore, insufficiency of any amino acid (such as occurs when IDO depletes tryptophan) will activate GCN2 kinase activity and initiate a downstream signaling pathway (51). This results in repression of most protein translation but causes selective upregulation of a small subset of genes that are responsive to signaling by GCN2 (52). These GCN2-responsive genes are different for each cell type, and exactly how this signal transduction pathway modulates immune responses is still under investigation; however, T cells from mice lacking GCN2 are resistant to IDO-induced inhibition of proliferation, and they do not acquire the state of antigen-specific unresponsiveness (anergy) normally induced by exposure to IDO (48). Furthermore, CD4+ T cells from GCN2-deficient mice fail to undergo IDO-induced differentiation into Tregs (48, 49).
Local tryptophan depletion would obviously be a short-range immunosuppressive phenomenon since the total body pool of tryptophan could not be depleted. However, since IDO-expressing APCs and responding T cells are obliged to be in close physical contact, it might be that tryptophan concentrations can be lowered sufficiently in T cells interacting with the IDO-expressing APCs to activate the GCN2 signaling pathway. However, as with the effects of tryptophan metabolites, much of this model of the mechanisms by which local tryptophan depletion elicits immunosuppression remains speculative. It seems probable that both the GCN2 pathway and the metabolite pathways function synergistically to create the full biologic effects of IDO, as has been recently suggested (49).
Potential sites of action for IDO in mediating tolerance to tumors
Multiple mechanisms contribute to tumor-induced tolerance. These can operate in the tumor itself or in key sites where the immune system encounters tumor antigens, such as the tumor-draining lymph nodes. In combination, these tolerogenic mechanisms act to block the initial response to tumor antigens (53, 54), inhibit the ability of activated T cells to kill tumor cells (55), and enhance the suppressive activity of Tregs (56). Emerging evidence suggests that IDO has the potential to contribute to each of these processes, both in the tumor and in the tumor-draining lymph nodes (Figure 2).
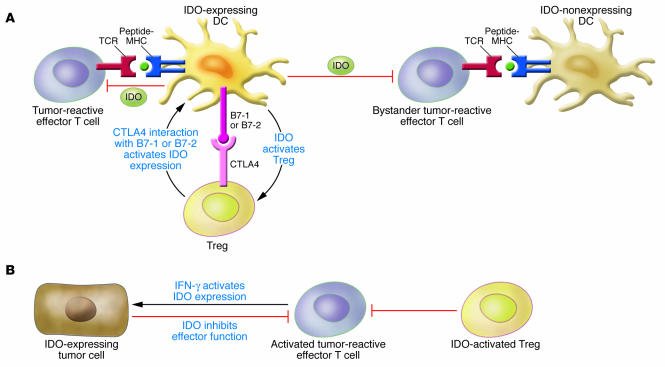
Figure 2. Model of the effects of IDO in tumors and tumor-draining lymph nodes.
(A) In tumor-draining lymph nodes, IDO-expressing DCs directly suppress and anergize tumor-reactive effector T cells responding to antigens presented by IDO+ DCs. Also, through bystander suppression, IDO can inhibit T cell responses to antigens presented by neighboring APCs. One mechanism that can induce IDO expression in DCs is reverse signaling mediated by B7-1 or B7-2 molecules expressed on DCs binding to CTLA4 expressed on Tregs. Reciprocally, IDO expression by DCs can also activate Tregs and drive the differentiation of new Tregs from uncommitted CD4+ T cells. (B) In the tumor microenvironment, tumor cells can either express IDO constitutively or upregulate IDO in response to inflammatory signals generated by activated effector T cells. IDO expression by tumor cells inhibits effector T cells and also activates Tregs to further contribute to the suppressive microenvironment within the tumor.
IDO expression in tumor-draining lymph nodes
Most studies suggest that naive, resting T cells become aware of tumor antigens primarily through presentation of tumor-derived peptides by host APCs rather than by direct presentation of the peptides by the tumor cells themselves (57–61). Classically, antigen presentation to resting T cells occurs in draining lymph nodes (62). Usually, this is thought to lead to T cell activation, but studies now show that this can also lead to potent, antigen-specific tolerance induction, particularly when antigens are presented in lymph nodes draining normal uninflamed tissues or mucosal surfaces (63–65). These studies emphasize that it is often the nature of the lymph node where the antigen is initially presented rather than the nature of the antigen itself, that dictates the choice between tolerance and immunity (66). Since most tumor antigens are first presented in the tumor-draining lymph nodes, these nodes are pivotally positioned to influence the immune response to tumor antigens (67, 68).
IDO is expressed in tumor-draining lymph nodes of humans (32, 36, 69, 70) and mice (29). In humans, the specific cell type expressing IDO has not yet been definitively characterized, but it is clear that these are frequently host cells (not metastatic tumor cells) and that they often display a “plasmacytoid” morphology (69). In mice, the immunosuppressive IDO-expressing cells in tumor-draining lymph nodes are phenotypically similar to mouse plasmacytoid DCs (expressing the cell surface markers CD11c, B220, and Ly6C) but with additional coexpression of the B cell–lineage marker CD19 (29). Similar CD19+ plasmacytoid DCs that express IDO have also been observed in nontumor models of tolerance induction (71, 72), so the CD19+ phenotype seems to represent an IDO-competent subset of DCs. IDO+ DCs from tumor-draining lymph nodes have been shown to suppress T cell responses in vitro and induce antigen-specific T cell anergy when adoptively transferred into new hosts (29, 48). This ability to induce anergy might be conceptually similar to the ability of mouse splenic IDO-expressing CD8α+ DCs to create systemic unresponsiveness to antigens in vivo in other experimental models of tolerance induction (30).
Taken together, these studies suggest that IDO-expressing host DCs found in tumor-draining lymph nodes might help suppress the initiation of immune responses to tumor-derived antigens and perhaps help create systemic tolerance to these antigens. Further work is needed to determine whether tumor-derived antigens are indeed presented by these DCs (or possibly by other DCs within an immunosuppressive milieu created by these IDO-expressing DCs) and whether this leads to tolerance induction.
Prognostic significance of IDO expression in sentinel lymph nodes.
In humans, IDO-expressing cells have been observed by immunohistochemistry in lymph nodes from patients with melanoma and breast, lung, and colon cancer as well as other tumors (ref. 29 and our unpublished observations). To date, however, the only studies of IDO expression in radiographically mapped sentinel lymph nodes (where it is known that the lymph node in question actually drains the tumor) have been performed in patients with malignant melanoma (29, 70). In two studies, IDO expression was associated with substantially worse clinical prognosis (29, 70). However, it will require larger studies to determine whether IDO expression in tumor-draining lymph nodes constitutes an independent prognostic factor in individuals with melanoma and other tumors. For the purposes of therapy, however, it would not be necessary that IDO be statistically independent of other known risk factors: the relevant point is that IDO represents a functional molecular target for which inhibitors exist. Therefore, the key question is whether blocking IDO will improve the immunologic response to tumors, not whether IDO is independent of other risk factors.
Suppression in the tumor microenvironment
A second potential site of IDO activity is the tumor microenvironment itself. Uyttenhove and colleagues have shown by immunohistochemistry that various human tumors express IDO (28). IDO expression by tumor cells has been shown to correlate with a poor clinical prognosis in ovarian carcinoma (73), endometrial carcinoma (74), and colon carcinoma (75). It is not yet known whether this worsened prognosis compared with that of individuals whose tumor cells lack IDO reflects the immunosuppressive effects of IDO or a more general alteration in the biology of the tumor that is associated with IDO expression. However, on the basis of mouse models, it is reasonable to speculate that IDO expression by tumor cells could create an immunosuppressive microenvironment in the tumor. Consistent with this hypothesis, Uyttenhove et al. showed that mouse tumor cells transfected with IDO became resistant to immunologic rejection, even in mice that had been preimmunized against the tumor and that would have fully rejected the same tumor cells not expressing IDO (28). Conceptually, this is analogous to the lung transplant studies cited above, in which ectopic expression of IDO protected antigenically foreign tissues from rejection by the host immune system (25, 26). IDO might be expressed constitutively by tumor cells as part of the genetic changes involved in malignant transformation (18). Alternatively, IDO is known to be inducible in many tumor cell lines by IFN-γ and other inflammatory mediators (37); therefore, IDO might be secondarily induced in tumor cells or in host stromal cells in response to inflammatory cytokines from the initial host response against the tumor (76).
IDO, Tregs, and CTLA4
Tregs are emerging as a key component of acquired tolerance to tumors. Increased Treg activity facilitates tumor growth (77) whereas depletion of Tregs allows effective antitumor immune responses that would otherwise be undetectable or ineffectual to occur (4, 8, 78, 79). IDO has been implicated as a possible immunosuppressive effector mechanism of Tregs. Grohmann and colleagues showed that Tregs can trigger high levels of functional IDO expression in mouse DCs in vitro (80). This occurred through binding of CTLA4 on Tregs to B7-1 and B7-2 on DCs, which transduced a signal in the DCs that upregulated IDO protein expression and functional enzymatic activity (19, 80). A similar ability of CTLA4–B7-1 and/or B7-2 interactions to induce IDO has been shown in human monocyte-derived DCs (31, 33). Therefore, IDO might function as one downstream mechanism by which CTLA4+ Tregs mediate immunosuppression (81).
Some support for this hypothesis comes from recent studies of primates infected with SIV. Infected monkeys have CTLA4+ Tregs that inhibit anti-SIV immune responses, and these same animals also show high levels of systemic IDO expression and functional activity (82). When SIV-infected animals were treated with a blocking antibody specific for CTLA4, they showed a substantial reduction in IDO mRNA in tissues and decreased amounts of IDO metabolites in plasma (82). These results would be consistent with the hypothesis that IDO expression by APCs can be upregulated by CTLA4 expressed on Tregs (although other interpretations are also possible). Further studies in genetically defined knockout and transgenic mice are needed to definitively address this question. However, since tumor-draining lymph nodes can contain many activated Tregs (83–85), Treg-mediated induction of IDO through CTLA4 offers one potential explanation for the increased level of IDO expression in tumor-draining lymph nodes.
Recently, in vitro studies have indicated that IDO expression by DCs can also promote the differentiation of new Tregs from naive CD4+ T cells (49, 86). At present, however, this remains an in vitro observation, and additional studies are needed to demonstrate whether this pathway has an in vivo role in tumor immunology. If this is shown to occur in vivo, however, then IDO and Tregs would be revealed as a closely coupled positive-feedback system, with Tregs inducing IDO and IDO driving differentiation of new Tregs.
IDO inhibitors and chemotherapy
Cytotoxic chemotherapy is now known to place a substantial stress on the state of established tolerance created by the tumor. This is, in part, because chemotherapy causes dying tumor cells to release a wave of tumor-associated antigens, which can then enter the antigen-presentation pathway (87, 88). In addition, many chemotherapy regimens induce a period of transient lymphopenia and homeostatic recovery, during which T cells seem to be more receptive to breaking tolerance (89–92). Finally, certain chemotherapy regimens can at least partially deplete or transiently inactivate tumor-protective Tregs (4, 93–96). Chemotherapeutics have been shown to enhance the effectiveness of immunotherapy in a number of different mouse models of cancer (4, 88, 97–100), and chemotherapy has begun to be incorporated as an element of immunotherapy regimens in some recent clinical trials (101, 102).
Despite these potential benefits, however, most chemotherapeutic agents do not seem to trigger a detectable protective immune response against established human tumors. One possible reason for this failure could be that the tumor is able to rapidly reestablish tolerance to itself following each cycle of chemotherapy. Tumor antigens released by chemotherapy are presented in tumor-draining lymph nodes, and mouse studies show that IDO expression in draining lymph nodes can convert them into an immunosuppressive and tolerance-inducing milieu (29). Therefore, inhibiting IDO in the post-chemotherapy period could potentially delay or disrupt the reacquisition of tolerance to tumor antigens.
Preclinical studies in mouse tumor models demonstrate that 1MT has substantial synergy when combined with a number of chemotherapy drugs (18, 103). This was first demonstrated by Muller and colleagues using a stringent model of autochthonous breast tumors arising in mice engineered to overexpress the oncogenic protein HER2/neu in breast tissue (18). An important feature of this model is that, as in humans, each tumor is genetically different, arising through its own unique series of genetic mutations (104), and therefore must develop its own strategy to escape immune surveillance. Despite this genetic diversity, tumors consistently regressed in size when treated with 1MT in combination with any one of several chemotherapeutics (18). Effective chemotherapeutics in this model included cyclophosphamide, doxorubicin, paclitaxel, and cisplatin. These findings have recently been extended in a second report (103). The synergy between 1MT and chemotherapy was immune mediated, as shown by the fact that the effect of 1MT was lost when tumors were grown in immunodeficient RAG1-deficient hosts (103). Therefore, by a functional definition of tolerance, the combination of 1MT and chemotherapy was able to break tolerance to established tumors, allowing therapeutic antitumor immune responses that formerly would not have been possible to occur. The molecular mechanism of the synergy between IDO inhibitors and chemotherapeutics is still a subject of active investigation. However, from a clinical standpoint, the ability of IDO inhibitors to function in combination with chemotherapy offers a substantial practical advantage because it means that immunotherapy regimens can be tested in clinical trials without denying patients the benefits of standard chemotherapy.
Choosing among available IDO inhibitors
In preclinical studies, the most widely used IDO inhibitor has been 1MT, which exists as both d and l isomers. Most studies to date have employed the racemic mixture (that is, a mixture of the d and l isomers of 1MT), thereby leaving open the question of which isomer would be better suited as an immune-modulating agent in combination with chemotherapy. The l isomer is the more potent inhibitor of IDO activity when tested in assays using the purified IDO enzyme (105) or IDO expressed in cell lines (103). However, using in vitro assays based on T cell responses to IDO-expressing APCs, we and others have found that the d isomer of 1MT is at least equally efficacious (32, 33, 86, 106, 107), a finding that would not have been predicted based solely on assays using the purified enzyme. Direct comparison of the d and l isomers in vitro, and also in vivo in mouse models of cancer, suggested that the d isomer is more effective at reversing the suppression of T cells created by IDO-expressing DCs in the models tested (103). The racemic mixture of 1MT at high concentrations has been reported to display off-target effects (108), and the advantage of the d isomer is perhaps due to reduction in the off-target inhibition of T cell responses that was seen with the l isomer and racemic mixture (103). Furthermore, in vivo mouse tumor studies suggested that the target of the d isomer of 1MT is authentic IDO since the antitumor efficacy of d-1MT was lost when tumors were grown in IDO-deficient (Ido–/–) mice (103).
IDO as a counterregulatory mechanism in cancer immunotherapy
One of the biologic functions of IDO seems to be as a counterregulatory mechanism to suppress excessive immune activation. Such counterregulatory pathways are important in the immune system because uncontrolled immune responses can cause unacceptable damage to the host. At sites of inflammation, IDO expression is not limited to DCs and macrophages but can be found in epithelial cells (27, 109, 110), eosinophils (111), endothelial cells (112), and possibly other cell types as well. Blocking this inflammation-induced IDO can substantially worsen local tissue damage, sometimes to the point of lethality (22). Therefore, IDO is an important endogenous counterregulatory mechanism that helps protect the host. However, virtually all tumor-immunotherapy strategies aim to induce inflammation and immune activation. If endogenous IDO is induced by this therapeutic immune activation (as it is during the physiologic response to pathogens and inflammation), then IDO could potentially antagonize the desired effects of immunotherapy. Emerging evidence suggests that this might be more than a theoretical concern.
Induction of IDO by CpG oligonucleotides
One well-known inducer of IDO is bacterial LPS, a TLR4 ligand (113). Recently, it has been shown that IDO is also induced by ligands for TLR9 (oligodeoxynucleotide containing one or more unmethylated CpG dinucleotides [CpG ODN]) (72, 114). CpG ODN are potent immunologic activators (115) and are currently in phase III clinical trials as an immunostimulatory agent in patients with lung cancer (116, 117). Recently, however, it has been shown that in mice, high-dose intravenous administration of CpG ODN can cause upregulation of IDO expression in the spleen with potent IDO-induced immunosuppression (72, 114). This effect seemed to be mediated by autocrine and/or paracrine production of IFN-α since mice lacking the receptor for IFN-α did not induce IDO in response to CpG ODN (72). Therefore, although CpG ODN are potent immunostimulatory agents, under certain circumstances they can also induce immunosuppressive IDO expression. Blocking this unwanted IDO activity (for example, by coadministration of an IDO inhibitor) might enhance the efficacy of CpG ODN as an immunostimulatory agent in cancer.
Induction of IDO by IFNs and vaccine adjuvants
In addition to TLR ligands, IDO is known to be inducible by IFN-γ and IFN-α, one or both of which are frequently found at sites of inflammation (118). Vaccine adjuvants, which are critically important for enhancing responses to tumor and other vaccine antigens, typically function by inducing inflammation, and adjuvants often include TLR ligands (119). It has not yet been reported whether clinically relevant vaccine adjuvants might induce IDO. However, it has been shown that bacteria such as Mycobacterium bovis bacillus Calmette-Guérin and Listeria monocytogenes, both of which have been used as vaccine adjuvants, are also potent inducers of IDO in vivo (120, 121). Therefore, like CpG ODN, other vaccine adjuvants have the potential to induce counterregulatory IDO and might potentially show enhanced efficacy if IDO were blocked.
Induction of IDO by 4-1BB
The costimulatory molecule 4-1BB (also known as CD137) is a member of the TNF receptor superfamily with potent T cell–activating effects in certain mouse models of cancer (122). Agonistic monoclonal antibodies specific for 4-1BB have recently entered phase I clinical trials in solid tumors. Somewhat unexpectedly, however, systemic ligation of 4-1BB has been reported to induce IDO in vivo (123, 124). Treatment of mice with an agonistic 4-1BB–specific antibody was shown to suppress disease severity in autoimmune arthritis (124) and also in a model of uveitis (123), in both cases related to the collateral induction of IDO (mediated through elevated levels of IFN-γ). Therefore, it is possible that the antitumor efficacy of agents targeting 4-1BB might also be limited by counterregulatory induction of IDO.
Conclusion
Successful immunotherapy of cancer will require a combination of multiple immunomodulatory agents and strategies (125). Such combinations will need to be rationally designed, based on a mechanistic understanding of the goals to be accomplished and barriers to be overcome. Key steps include breaking preexisting tolerance to the tumor (especially removing the suppression mediated by Tregs); providing a suitable set of antigens (whether from the tumor itself or a vaccine); ensuring that these antigens are presented in an activating milieu (rather than the tolerizing milieu that seems to be the case in most tumor-draining lymph nodes); and providing sufficient adjuvant and immunostimulatory signals to drive a potent, curative antitumor immune response. As discussed in this Review, IDO might antagonize these desirable goals at a number of points. Therefore, inhibition of the IDO pathway might enhance the efficacy of various immunotherapeutic and chemotherapeutic strategies for the treatment of individuals with cancer.
Footnotes
Nonstandard abbreviations used: CpG ODN, oligodeoxynucleotide(s) containing one or more unmethylated CpG dinucleotides; CTLA4, cytotoxic T lymphocyte–associated antigen 4; GCN2, general control nonderepressible 2; IDO, indoleamine 2,3-dioxygenase; 1MT, 1-methyl-tryptophan.
Conflict of interest: D.H. Munn and A.L. Mellor hold intellectual property interests in the use of IDO and IDO inhibitors and receive income, equity ownership, and research support from NewLink Genetics.
Citation for this article: J. Clin. Invest. 117:1147–1154 (2007). doi:10.1172/JCI31178.
References
- 1.Boon T., van der Bruggen P. Human tumor antigens recognized by T lymphocytes. . J. Exp. Med. 1996;183:725–729. doi: 10.1084/jem.183.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lennerz V., et al. The response of autologous T cells to a human melanoma is dominated by mutated neoantigens. Proc. Natl. Acad. Sci. U. S. A. 2005;102:16013–16018. doi: 10.1073/pnas.0500090102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Bruggen P., van den Eynde B.J. Processing and presentation of tumor antigens and vaccination strategies. Curr. Opin. Immunol. 2006;18:98–104. doi: 10.1016/j.coi.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Ercolini A.M., et al. Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response. J. Exp. Med. 2005;201:1591–1602. doi: 10.1084/jem.20042167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ochsenbein A.F., et al. Immune surveillance against a solid tumor fails because of immunological ignorance. Proc. Natl. Acad. Sci. U. S. A. 1999;96:2233–2238. doi: 10.1073/pnas.96.5.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spiotto M.T., et al. Increasing tumor antigen expression overcomes “ignorance” to solid tumors via crosspresentation by bone marrow-derived stromal cells. Immunity. 2002;17:737–747. doi: 10.1016/s1074-7613(02)00480-6. [DOI] [PubMed] [Google Scholar]
- 7.Sotomayor E.M., et al. Cross-presentation of tumor antigens by bone marrow-derived antigen-presenting cells is the dominant mechanism in the induction of T-cell tolerance during B-cell lymphoma progression. Blood. 2001;98:1070–1077. doi: 10.1182/blood.v98.4.1070. [DOI] [PubMed] [Google Scholar]
- 8.Yu P., et al. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J. Exp. Med. 2005;201:779–791. doi: 10.1084/jem.20041684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou G., Drake C.G., Levitsky H.I. Amplification of tumor-specific regulatory T cells following therapeutic cancer vaccines. Blood. 2006;107:628–636. doi: 10.1182/blood-2005-07-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maksimow M., Miiluniemi M., Marttila-Ichihara F., Jalkanen S., Hanninen A. Antigen targeting to endosomal pathway in dendritic cell vaccination activates regulatory T cells and attenuates tumor immunity. Blood. 2006;108:1298–1305. doi: 10.1182/blood-2005-11-008615. [DOI] [PubMed] [Google Scholar]
- 11.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat. Rev. Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 12.Stone T.W., Darlington L.G. Endogenous kynurenines as targets for drug discovery and development. Nat. Rev. Drug Discov. 2002;1:609–620. doi: 10.1038/nrd870. [DOI] [PubMed] [Google Scholar]
- 13.Schrocksnadel K., Wirleitner B., Winkler C., Fuchs D. Monitoring tryptophan metabolism in chronic immune activation. Clin. Chim. Acta. 2006;364:82–90. doi: 10.1016/j.cca.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Huang A., et al. Serum tryptophan decrease correlates with immune activation and impaired quality of life in colorectal cancer. Br. J. Cancer. 2002;86:1691–1696. doi: 10.1038/sj.bjc.6600336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iweala O.I., Nagler C.R. Immune privilege in the gut: the establishment and maintenance of non-responsiveness to dietary antigens and commensal flora. Immunol. Rev. 2006;213:82–100. doi: 10.1111/j.1600-065X.2006.00431.x. [DOI] [PubMed] [Google Scholar]
- 16.Munn D.H., et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 17.Mellor A.L., et al. Prevention of T cell-driven complement activation and inflammation by tryptophan catabolism during pregnancy. Nat. Immunol. 2001;2:64–68. doi: 10.1038/83183. [DOI] [PubMed] [Google Scholar]
- 18.Muller A.J., Duhadaway J.B., Donover P.S., Sutanto-Ward E., Prendergast G.C. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat. Med. 2005;11:312–319. doi: 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]
- 19.Grohmann U., et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat. Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 20.Mellor A.L., et al. Cutting edge: Induced indoleamine 2,3 dioxygenase expression in dendritic cell subsets suppresses T cell clonal expansion. . J. Immunol. 2003;171:1652–1655. doi: 10.4049/jimmunol.171.4.1652. [DOI] [PubMed] [Google Scholar]
- 21.Chen X., et al. Indoleamine 2,3-dioxygenase (IDO) is involved in promoting the development of anterior chamber-associated immune deviation. Immunol. Lett. 2006;107:140–147. doi: 10.1016/j.imlet.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Gurtner G.J., Newberry R.D., Schloemann S.R., McDonald K.G., Stenson W.F. Inhibition of indoleamine 2,3-dioxygenase augments trinitrobenzene sulfonic acid colitis in mice. . Gastroenterology. 2003;125:1762–1773. doi: 10.1053/j.gastro.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 23.Kwidzinski E., et al. Indolamine 2,3-dioxygenase is expressed in the CNS and down-regulates autoimmune inflammation. FASEB J. 2005;19:1347–1349. doi: 10.1096/fj.04-3228fje. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi T., et al. Inhibition of experimental asthma by indoleamine 2,3-dioxygenase. J. Clin. Invest. 2004;114:270–279. doi: 10.1172/JCI200421275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swanson K.A., Zheng Y., Heidler K.M., Mizobuchi T., Wilkes D.S. CDIIc+ cells modulate pulmonary immune responses by production of indoleamine 2,3-dioxygenase. Am. J. Respir. Cell Mol. Biol. 2004;30:311–318. doi: 10.1165/rcmb.2003-0268OC. [DOI] [PubMed] [Google Scholar]
- 26.Liu H., Liu L., Fletcher B.S., Visner G.A. Sleeping Beauty-based gene therapy with indoleamine 2,3-dioxygenase inhibits lung allograft fibrosis. FASEB J. 2006;20:2384–2386. doi: 10.1096/fj.06-6228fje. [DOI] [PubMed] [Google Scholar]
- 27.Beutelspacher S.C., et al. Function of indoleamine 2,3-dioxygenase in corneal allograft rejection and prolongation of allograft survival by over-expression. Eur. J. Immunol. 2006;36:690–700. doi: 10.1002/eji.200535238. [DOI] [PubMed] [Google Scholar]
- 28.Uyttenhove C., et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat. Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 29.Munn D.H., et al. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J. Clin. Invest. 2004;114:280–290. doi: 10.1172/JCI200421583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grohmann U., et al. IL-6 inhibits the tolerogenic function of CD8alpha(+) dendritic cells expressing indoleamine 2,3-dioxygenase. J. Immunol. 2001;167:708–714. doi: 10.4049/jimmunol.167.2.708. [DOI] [PubMed] [Google Scholar]
- 31.Orabona C., et al. Toward the identification of a tolerogenic signature in IDO-competent dendritic cells. Blood. 2006;107:2846–2854. doi: 10.1182/blood-2005-10-4077. [DOI] [PubMed] [Google Scholar]
- 32.Munn D.H., et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 33.Munn D.H., Sharma M.D., Mellor A.L. Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J. Immunol. 2004;172:4100–4110. doi: 10.4049/jimmunol.172.7.4100. [DOI] [PubMed] [Google Scholar]
- 34.Grohmann U., et al. Functional plasticity of dendritic cell subsets as mediated by CD40 versus B7 activation. J. Immunol. 2003;171:2581–2587. doi: 10.4049/jimmunol.171.5.2581. [DOI] [PubMed] [Google Scholar]
- 35.Braun D., Longman R.S., Albert M.L. A two step induction of indoleamine 2,3 dioxygenase (IDO) activity during dendritic cell maturation. Blood. 2005;106:2375–2381. doi: 10.1182/blood-2005-03-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Bergwelt-Baildon M.S., et al. CD25 and indoleamine 2,3-dioxygenase are up-regulated by prostaglandin E2 and expressed by tumor-associated dendritic cells in vivo: additional mechanisms of T-cell inhibition. Blood. 2006;108:228–237. doi: 10.1182/blood-2005-08-3507. [DOI] [PubMed] [Google Scholar]
- 37.Taylor M.W., Feng G. Relationship between interferon-γ, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991;5:2516–2522. [PubMed] [Google Scholar]
- 38.Fallarino F., et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9:1069–1077. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- 39.Frumento G., et al. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J. Exp. Med. 2002;196:459–468. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terness P., et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J. Exp. Med. 2002;196:447–457. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weber W.P., et al. Differential effects of the tryptophan metabolite 3-hydroxyanthranilic acid on the proliferation of human CD8+ T cells induced by TCR triggering or homeostatic cytokines. Eur. J. Immunol. 2006;36:296–304. doi: 10.1002/eji.200535616. [DOI] [PubMed] [Google Scholar]
- 42.Chiesa M.D., et al. The tryptophan catabolite L-kynurenine inhibits the surface expression of NKp46- and NKG2D-activating receptors and regulates NK-cell function. Blood. 2006;108:4118–4125. doi: 10.1182/blood-2006-03-006700. [DOI] [PubMed] [Google Scholar]
- 43.Belladonna M.L., et al. Kynurenine pathway enzymes in dendritic cells initiate tolerogenesis in the absence of functional IDO. J. Immunol. 2006;177:130–137. doi: 10.4049/jimmunol.177.1.130. [DOI] [PubMed] [Google Scholar]
- 44.Bauer T.M., et al. Studying the immunosuppressive role of indoleamine 2,3-dioxygenase: tryptophan metabolites suppress rat allogeneic T-cell responses in vitro and in vivo. Transpl. Int. 2005;18:95–100. doi: 10.1111/j.1432-2277.2004.00031.x. [DOI] [PubMed] [Google Scholar]
- 45.Platten M., et al. Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science. 2005;310:850–855. doi: 10.1126/science.1117634. [DOI] [PubMed] [Google Scholar]
- 46.Wang J., et al. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J. Biol. Chem. 2006;281:22021–22028. doi: 10.1074/jbc.M603503200. [DOI] [PubMed] [Google Scholar]
- 47.Bubnoff D., et al. Fc-gamma RI induces the tryptophan degradation pathway involved in regulating T cell responses. J. Immunol. 2002;169:1810–1816. doi: 10.4049/jimmunol.169.4.1810. [DOI] [PubMed] [Google Scholar]
- 48.Munn D.H., et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 49.Fallarino F., et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. . J. Immunol. 2006;176:6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 50.Dong J., Qiu H., Garcia-Barrio M., Anderson J., Hinnebusch A.G. Uncharged tRNA activates GCN2 by displacing the protein kinase moiety from a bipartite tRNA-binding domain. Mol. Cell. 2000;6:269–279. doi: 10.1016/s1097-2765(00)00028-9. [DOI] [PubMed] [Google Scholar]
- 51.Harding H.P., et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 52.Wek R.C., Jiang H.Y., Anthony T.G. Coping with stress: eIF2 kinases and translational control. Biochem. Soc. Trans. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- 53.Staveley-O’Carroll K., et al. Induction of antigen-specific T cell anergy: an early event in the course of tumor progression. Proc. Natl. Acad. Sci. U. S. A. 1998;95:1178–1183. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cuenca A., et al. Extra-lymphatic solid tumor growth is not immunologically ignored and results in early induction of antigen-specific T-cell anergy: dominant role of cross-tolerance to tumor antigens. . Cancer Res. 2003;63:9007–9015. [PubMed] [Google Scholar]
- 55.Gajewski T.F., et al. Immune resistance orchestrated by the tumor microenvironment. Immunol. Rev. 2006;213:131–145. doi: 10.1111/j.1600-065X.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 56.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat. Rev. Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 57.Huang A.Y., et al. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 58.Yu P., Spiotto M.T., Lee Y., Schreiber H., Fu Y.X. Complementary role of CD4+ T cells and secondary lymphoid tissues for cross-presentation of tumor antigen to CD8+ T cells. J. Exp. Med. 2003;197:985–995. doi: 10.1084/jem.20021804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Mierlo G., et al. Activation of dendritic cells that cross-present tumor-derived antigen licenses CD8+ CTL to cause tumor eradication. . J. Immunol. 2004;173:6753–6759. doi: 10.4049/jimmunol.173.11.6753. [DOI] [PubMed] [Google Scholar]
- 60.Nowak A.K., et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J. Immunol. 2003;170:4905–4913. doi: 10.4049/jimmunol.170.10.4905. [DOI] [PubMed] [Google Scholar]
- 61.Hargadon K.M., et al. Incomplete differentiation of antigen-specific CD8 T cells in tumor-draining lymph nodes. J. Immunol. 2006;177:6081–6090. doi: 10.4049/jimmunol.177.9.6081. [DOI] [PubMed] [Google Scholar]
- 62.Itano A.A., Jenkins M.K. Antigen presentation to naive CD4 T cells in the lymph node. Nat. Immunol. 2003;4:733–739. doi: 10.1038/ni957. [DOI] [PubMed] [Google Scholar]
- 63.Samy E.T., Parker L.A., Sharp C.P., Tung K.S. Continuous control of autoimmune disease by antigen-dependent polyclonal CD4+CD25+ regulatory T cells in the regional lymph node. J. Exp. Med. 2005;202:771–781. doi: 10.1084/jem.20041033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Green E.A., Choi Y., Flavell R.A. Pancreatic lymph node-derived CD4(+)CD25(+) Treg cells: highly potent regulators of diabetes that require TRANCE-RANK signals. Immunity. 2002;16:183–191. doi: 10.1016/s1074-7613(02)00279-0. [DOI] [PubMed] [Google Scholar]
- 65.Wolvers D.A., et al. Intranasally induced immunological tolerance is determined by characteristics of the draining lymph nodes: studies with OVA and human cartilage gp-39. J. Immunol. 1999;162:1994–1998. [PubMed] [Google Scholar]
- 66.Kraal G., Samsom J.N., Mebius R.E. The importance of regional lymph nodes for mucosal tolerance. Immunol. Rev. 2006;213:119–130. doi: 10.1111/j.1600-065X.2006.00429.x. [DOI] [PubMed] [Google Scholar]
- 67.Munn D.H., Mellor A.L. The tumor-draining lymph node as an immune-privileged site. Immunol. Rev. 2006;213:146–158. doi: 10.1111/j.1600-065X.2006.00444.x. [DOI] [PubMed] [Google Scholar]
- 68.Cochran A.J., et al. Tumour-induced immune modulation of sentinel lymph nodes. Nat. Rev. Immunol. 2006;6:659–670. doi: 10.1038/nri1919. [DOI] [PubMed] [Google Scholar]
- 69.Lee J.R., et al. Pattern of recruitment of immunoregulatory antigen presenting cells in malignant melanoma. Lab. Invest. 2003;83:1457–1466. doi: 10.1097/01.lab.0000090158.68852.d1. [DOI] [PubMed] [Google Scholar]
- 70.Lee J.H., et al. Quantitative analysis of melanoma-induced cytokine-mediated immunosuppression in melanoma sentinel nodes. Clin. Cancer Res. 2005;11:107–112. [PubMed] [Google Scholar]
- 71.Baban B., et al. A minor population of splenic dendritic cells expressing CD19 mediates IDO-dependent T cell suppression via type 1 interferon-signaling following B7 ligation. Int. Immunol. 2005;17:909–919. doi: 10.1093/intimm/dxh271. [DOI] [PubMed] [Google Scholar]
- 72.Mellor A.L., et al. Cutting edge: CpG oligonucleotides induce splenic CD19+ dendritic cells to acquire potent indoleamine 2,3-dioxygenase-dependent T cell regulatory functions via IFN type 1 signaling. J. Immunol. 2005;175:5601–5605. doi: 10.4049/jimmunol.175.9.5601. [DOI] [PubMed] [Google Scholar]
- 73.Okamoto A., et al. Indoleamine 2,3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin. Cancer Res. 2005;11:6030–6039. doi: 10.1158/1078-0432.CCR-04-2671. [DOI] [PubMed] [Google Scholar]
- 74.Ino K., et al. Indoleamine 2,3-dioxygenase is a novel prognostic indicator for endometrial cancer. . Br. J. Cancer. 2006;95:1555–1561. doi: 10.1038/sj.bjc.6603477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brandacher G., et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin. Cancer Res. 2006;12:1144–1151. doi: 10.1158/1078-0432.CCR-05-1966. [DOI] [PubMed] [Google Scholar]
- 76.Dunn G.P., Koebel C.M., Schreiber R.D. Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 77.Nishikawa H., et al. Accelerated chemically induced tumor development mediated by CD4+CD25+ regulatory T cells in wild-type hosts. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9253–9257. doi: 10.1073/pnas.0503852102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sutmuller R.P.M., et al. Synergism of cytoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25+ regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoractive cytotoxic T lymphocyte responses. J. Exp. Med. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang Y., Huang C.T., Huang X., Pardoll D.M. Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nat. Immunol. 2004;5:508–515. doi: 10.1038/ni1059. [DOI] [PubMed] [Google Scholar]
- 80.Fallarino F., et al. Modulation of tryptophan catabolism by regulatory T cells. Nat. Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 81.Finger E.B., Bluestone J.A. When ligand becomes receptor-tolerance via B7 signaling on DCs. Nat. Immunol. 2002;3:1056–1057. doi: 10.1038/ni1102-1056. [DOI] [PubMed] [Google Scholar]
- 82.Hryniewicz A., et al. CTLA-4 blockade decreases TGF-{beta}, indoleamine 2,3- dioxygenase, and viral RNA expression in tissues of SIVmac251-infected macaques. Blood. 2006;108:3834–3842. doi: 10.1182/blood-2006-04-010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hiura T., et al. Both regulatory T cells and antitumor effector T cells are primed in the same draining lymph nodes during tumor progression. J. Immunol. 2005;175:5058–5066. doi: 10.4049/jimmunol.175.8.5058. [DOI] [PubMed] [Google Scholar]
- 84.Valzasina B., Piconese S., Guiducci C., Colombo M.P. Tumor-induced expansion of regulatory T cells by conversion of CD4+CD25– lymphocytes is thymus and proliferation independent. Cancer Res. 2006;66:4488–4495. doi: 10.1158/0008-5472.CAN-05-4217. [DOI] [PubMed] [Google Scholar]
- 85.Viguier M., et al. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J. Immunol. 2004;173:1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 86.Curti A., et al. Modulation of tryptophan catabolism by human leukemic cells results in the conversion of CD25– into CD25+ T regulatory cells. Blood. 2007;109:2871–2877. doi: 10.1182/blood-2006-07-036863. [DOI] [PubMed] [Google Scholar]
- 87.Nowak A.K., Robinson B.W., Lake R.A. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res. 2003;63:4490–4496. [PubMed] [Google Scholar]
- 88.Broomfield S., et al. Partial, but not complete, tumor-debulking surgery promotes protective antitumor memory when combined with chemotherapy and adjuvant immunotherapy. Cancer Res. 2005;65:7580–7584. doi: 10.1158/0008-5472.CAN-05-0328. [DOI] [PubMed] [Google Scholar]
- 89.Dummer W., et al. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J. Clin. Invest. 2002;110:185–192. doi: 10.1172/JCI200215175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.King C., Ilic A., Koelsch K., Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–277. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 91.Wang L.X., et al. Interleukin-7-dependent expansion and persistence of melanoma-specific T cells in lymphodepleted mice lead to tumor regression and editing. Cancer Res. 2005;65:10569–10577. doi: 10.1158/0008-5472.CAN-05-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brown I.E., Blank C., Kline J., Kacha A.K., Gajewski T.F. Homeostatic proliferation as an isolated variable reverses CD8+ T Cell anergy and promotes tumor rejection. J. Immunol. 2006;177:4521–4529. doi: 10.4049/jimmunol.177.7.4521. [DOI] [PubMed] [Google Scholar]
- 93.Awwad M., North R.J. Cyclophosphamide (Cy)-facilitated adoptive immunotherapy of a Cy-resistant tumour. Evidence that Cy permits the expression of adoptive T-cell mediated immunity by removing suppressor T cells rather than by reducing tumour burden. Immunology. 1988;65:87–92. [PMC free article] [PubMed] [Google Scholar]
- 94.Ghiringhelli F., et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur. J. Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 95.Lutsiak M.E., et al. Inhibition of CD4+25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 96.Beyer M., et al. Reduced frequencies and suppressive function of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood. 2005;106:2018–2025. doi: 10.1182/blood-2005-02-0642. [DOI] [PubMed] [Google Scholar]
- 97.Machiels J.P., et al. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689–3697. [PubMed] [Google Scholar]
- 98.Weigel B.J., Rodeberg D.A., Krieg A.M., Blazar B.R. CpG oligodeoxynucleotides potentiate the antitumor effects of chemotherapy or tumor resection in an orthotopic murine model of rhabdomyosarcoma. Clin. Cancer Res. 2003;9:3105–3114. [PubMed] [Google Scholar]
- 99.Gattinoni L., et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J. Exp. Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Taieb J., et al. Chemoimmunotherapy of tumors: cyclophosphamide synergizes with exosome based vaccines. J. Immunol. 2006;176:2722–2729. doi: 10.4049/jimmunol.176.5.2722. [DOI] [PubMed] [Google Scholar]
- 101.Dudley M.E., et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morgan R.A., et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hou D.Y., et al. Inhibition of IDO in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with anti-tumor responses. Cancer Res. 2007;67:792–801. doi: 10.1158/0008-5472.CAN-06-2925. [DOI] [PubMed] [Google Scholar]
- 104.Podsypanina K., Li Y., Varmus H.E. Evolution of somatic mutations in mammary tumors in transgenic mice is influenced by the inherited genotype. BMC Med. 2004;2:24–34. doi: 10.1186/1741-7015-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Peterson A.C., et al. Evaluation of functionalized tryptophan derivatives and related compounds as competitive inhibitors of indoleamine 2,3–dioxygenase. Med. Chem. Res. 1994;3:531–544. [Google Scholar]
- 106.Rutella S., et al. Hepatocyte growth factor favors monocyte differentiation into regulatory interleukin (IL)-10++IL-12low/neg accessory cells with dendritic-cell features. Blood. 2006;108:218–227. doi: 10.1182/blood-2005-08-3141. [DOI] [PubMed] [Google Scholar]
- 107.Zhu L., et al. Synovial autoreactive T cells in rheumatoid arthritis resist IDO-mediated inhibition. . J. Immunol. 2006;177:8226–8233. doi: 10.4049/jimmunol.177.11.8226. [DOI] [PubMed] [Google Scholar]
- 108.Agaugue S., Perrin-Cocon L., Coutant F., Andre P., Lotteau V. 1-methyl-tryptophan can interfere with TLR signaling in dendritic cells independently of IDO activity. J. Immunol. 2006;177:2061–2071. doi: 10.4049/jimmunol.177.4.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hayashi T., et al. Enhancement of innate immunity against Mycobacterium avium infection by immunostimulatory DNA is mediated by indoleamine 2,3-dioxygenase. Infect. Immun. 2001;69:6156–6164. doi: 10.1128/IAI.69.10.6156-6164.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Barcelo-Batllori S., et al. Proteomic analysis of cytokine induced proteins in human intestinal epithelial cells: implications for inflammatory bowel diseases. Proteomics. 2002;2:551–560. doi: 10.1002/1615-9861(200205)2:5<551::AID-PROT551>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 111.Odemuyiwa S.O., et al. Cutting edge: human eosinophils regulate T cell subset selection through indoleamine 2,3-dioxygenase. J. Immunol. 2004;173:5909–5913. doi: 10.4049/jimmunol.173.10.5909. [DOI] [PubMed] [Google Scholar]
- 112.Beutelspacher S.C., et al. Expression of indoleamine 2,3-dioxygenase (IDO) by endothelial cells: implications for the control of alloresponses. Am. J. Transplant. . 2006;6:1320–1330. doi: 10.1111/j.1600-6143.2006.01324.x. [DOI] [PubMed] [Google Scholar]
- 113.Yoshida R., Urade Y., Nakata K., Watanabe Y., Hayashi O. Specific induction of indoleamine 2,3-dioxygenase by bacterial lipopolysaccharide in the mouse lung. Arch. Biochem. Biophys. 1981;212:629–637. doi: 10.1016/0003-9861(81)90406-9. [DOI] [PubMed] [Google Scholar]
- 114.Wingender G., et al. Systemic application of CpG-rich DNA suppresses adaptive T cell immunity via induction of IDO. Eur. J. Immunol. 2006;36:12–20. doi: 10.1002/eji.200535602. [DOI] [PubMed] [Google Scholar]
- 115.Klinman D.M. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat. Rev. Immunol. 2004;4:249–258. doi: 10.1038/nri1329. [DOI] [PubMed] [Google Scholar]
- 116.Speiser D.E., et al. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J. Clin. Invest. 2005;115:739–746. doi: 10.1172/JCI200523373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Leichman G., et al. CPG 7909, a TLR9 agonist, added to first line taxane/platinum for advanced non-small cell lung cancer, a randomized, controlled phase II study. J. Clin. Oncol. 2005;23(Suppl.):7039. [Google Scholar]
- 118.Mellor A., Munn D.H. IDO expression by dendritic cells: tolerance and tryptophan catabolism. . Nat. Rev. Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 119.Bendelac A., Medzhitov R. Adjuvants of immunity: harnessing innate immunity to promote adaptive immunity. J. Exp. Med. 2002;195:F19–F23. doi: 10.1084/jem.20020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Moreau M., et al. Bacille Calmette-Guerin inoculation induces chronic activation of peripheral and brain indoleamine 2,3-dioxygenase in mice. . J. Infect. Dis. 2005;192:537–544. doi: 10.1086/431603. [DOI] [PubMed] [Google Scholar]
- 121.Popov A., et al. Indoleamine 2,3-dioxygenase-expressing dendritic cells form suppurative granulomas following Listeria monocytogenes infection. J. Clin. Invest. 2006;116:3160–3170. doi: 10.1172/JCI28996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nam K.O., Kang W.J., Kwon B.S., Kim S.J., Lee H.W. The therapeutic potential of 4-1BB (CD137) in cancer. Curr. Cancer Drug Targets. 2005;5:357–363. doi: 10.2174/1568009054629681. [DOI] [PubMed] [Google Scholar]
- 123.Choi B.K., Asai T., Vinay D.S., Kim Y.H., Kwon B.S. 4-1BB-mediated amelioration of experimental autoimmune uveoretinitis is caused by indoleamine 2,3-dioxygenase-dependent mechanisms. . Cytokine. 2006;34:233–242. doi: 10.1016/j.cyto.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 124.Seo S.K., et al. 4-1BB-mediated immunotherapy of rheumatoid arthritis. Nat. Med. 2004;10:1088–1094. doi: 10.1038/nm1107. [DOI] [PubMed] [Google Scholar]
- 125.Pure E., Allison J.P., Schreiber R.D. Breaking down the barriers to cancer immunotherapy. Nat. Immunol. 2005;6:1207–1210. doi: 10.1038/ni1205-1207. [DOI] [PubMed] [Google Scholar]