Abstract
Metformin is among the most widely prescribed drugs for the treatment of type 2 diabetes. Organic cation transporter 1 (OCT1) plays a role in the hepatic uptake of metformin, but its role in the therapeutic effects of the drug, which involve activation of AMP-activated protein kinase (AMPK), is unknown. Recent studies have shown that human OCT1 is highly polymorphic. We investigated whether OCT1 plays a role in the action of metformin and whether individuals with OCT1 polymorphisms have reduced response to the drug. In mouse hepatocytes, deletion of Oct1 resulted in a reduction in the effects of metformin on AMPK phosphorylation and gluconeogenesis. In Oct1-deficient mice the glucose-lowering effects of metformin were completely abolished. Seven nonsynonymous polymorphisms of OCT1 that exhibited reduced uptake of metformin were identified. Notably, OCT1-420del (allele frequency of about 20% in white Americans), previously shown to have normal activity for model substrates, had reduced activity for metformin. In clinical studies, the effects of metformin in glucose tolerance tests were significantly lower in individuals carrying reduced function polymorphisms of OCT1. Collectively, the data indicate that OCT1 is important for metformin therapeutic action and that genetic variation in OCT1 may contribute to variation in response to the drug.
Introduction
Metabolic syndrome and its pathological sequela, type 2 diabetes, have become major health problems in the world. The biguanide metformin is widely used as a first-line therapy for the treatment of type 2 diabetes (1). In addition, the drug has recently been implicated in the treatment and/or prevention of fatty liver diseases and polycystic ovary syndrome (2, 3). Metformin ameliorates hyperglycemia by reducing gastrointestinal glucose absorption and hepatic glucose production and by improving glucose utilization (1). The molecular mechanisms underlying metformin action appear to be related to its activation (phosphorylation) of the so-called energy sensor AMP-activated protein kinase (AMPK), which suppresses glucagon-stimulated glucose production and causes an increase in glucose uptake in muscle and in hepatic cells (4, 5). The activation of AMPK may also be responsible for the improvement of lipid metabolism by metformin (4). Recently, serine-threonine kinase 11 (STK11/LKB1), which phosphorylates AMPK, has also been reported to be involved in metformin effects (6, 7).
However, despite the extensive clinical use and the recent research progress, the mechanisms underlying the therapeutic effects of metformin are still not well known. Metformin has been well characterized in vitro as a substrate of organic cation transporters (OCTs), including the liver-specific OCT1 and its paralog, OCT2, a transporter expressed in abundance in the kidney (8–11). Compared with wild-type mice, Oct1–/– mice have reduced metformin distribution to the liver (11); however, it is not known whether this reduced uptake corresponds to a reduction in the therapeutic effects of metformin that occur following AMPK activation, including the lowering of blood glucose levels.
Genetic polymorphisms in drug transporter genes have been increasingly recognized as a possible mechanism accounting for variation in drug response (12). In previous studies, we and others showed that human OCT1 is highly polymorphic in ethnically diverse populations (13–15). Using model substrates, it was shown that a number of nonsynonymous polymorphisms of OCT1 exhibit reduced activity in cellular assays. However, the clinical significance of the OCT1 variants has not been investigated. Since response to metformin is clinically variable (16, 17), it is possible that polymorphisms in OCT1 contribute to this variation.
In this study we tested the hypothesis that the cellular uptake of metformin represents the first step in its activation of AMPK. In particular, as the liver is a primary target of metformin action (1), we hypothesized that OCT1 is required for the therapeutic effects of metformin. Furthermore, we examined the effects of nonsynonymous OCT1 polymorphisms on metformin uptake and response in cellular assays. Finally, the clinical effects of metformin in oral glucose tolerance tests (OGTTs) were compared in individuals with and without reduced-function variants of OCT1.
Results
OCT activity is a determinant of metformin response in cell lines.
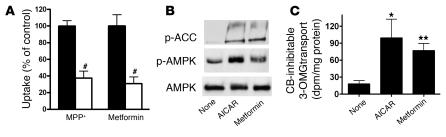
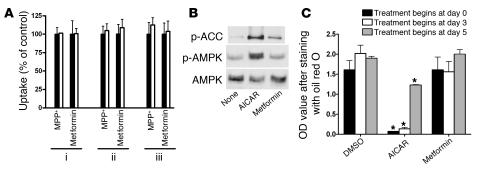
To understand whether OCT1 plays a critical role in defining metformin’s pharmacological effects, we first studied the effects of metformin in cells with conserved AMPK signaling pathways but different levels of OCT activity (Figures 1 and 2). 5-Aminoimidazole-4-carboxamine-1-β- d-ribofuranoside (AICAR), an AMPK activator, stimulated AMPK phosphorylation and glucose uptake in rat hepatocyte–derived Clone 9 cells (5). These cells retain OCT activity as demonstrated by the expression of Oct1 gene (data not shown) and the uptake of the typical substrate 1-methy-4-phenylpyridinium (MPP+) and metformin with and without an OCT inhibitor (Figure 1A). In contrast, 3T3-L1 adipocytes, an established model for studying AMPK signaling pathways (18), exhibited negligible Oct expression, which was apparent only in highly differentiated 3T3-L1 cells (data not shown), and little OCT-mediated uptake (Figure 2A). Although the cell-permeable AICAR stimulated the phosphorylation of AMPK and acetyl-CoA carboxylase (ACC), an AMPK target, and glucose uptake in both cell lines (5, 18), metformin caused markedly different responses. Metformin stimulated the phosphorylation of AMPK and ACC and glucose uptake in Clone 9 but not in 3T3-L1 cells (Figure 1, B and C, Figure 2B, and data not shown). Furthermore, whereas AICAR inhibited lipid accumulation during 3T3-L1 differentiation as assessed by oil red O staining (19), no such effect was observed for metformin (Figure 2C).
Figure 1. OCT activity and metformin responses in Clone 9 cells.
(A) Clone 9 cells exhibit OCT activity, as demonstrated by significantly reduced uptake of the typical OCT substrate MPP+ (1 μM) and metformin (250 μM) in the presence of the OCT inhibitor quinidine (100 μM; white bars) versus those without quinidine (control; black bars). The uptake times were 2 minutes for MPP+ and 10 minutes for metformin. The percentage uptake of the control was used to normalize the results for the 2 compounds in the same figure. #P < 0.001 versus respective controls (2-tailed Student’s t test). (B) Metformin (2 mM) stimulated the phosphorylation of AMPK and ACC in Clone 9 cells. The AMPK activator AICAR (2 mM) was used as a positive control. Cell extracts were detected with polyclonal antibodies against phospho-ACC (Ser79), phospho-AMPKα (Thr172), and AMPKα. (C) Metformin increased 3-OMG transport in Clone 9 cells. The cells were treated with metformin (2 mM) or AICAR (2 mM; positive control) for 2 hours before initiation of 3-OMG transport. 3-OMG transport was measured as described in Methods. CB, cytochalasin B. *P < 0.01, **P < 0.05 versus no treatment (ANOVA and Dunnett’s procedure).
Figure 2. No OCT activity and metformin responses in 3T3-L1 cells.
(A) No OCT activity was detected in 3T3-L1 cells of various differential stages. Uptake studies were done as described for Figure 1A. 3T3-L1 cells were differentiated as described in Methods. The cells for uptake experiments were: (i) preadipocytes; (ii) 3T3-L1 cells differentiated for 5 days; and (iii) 3T3-L1 cells differentiated for 10 days. (B) Metformin (2 mM) had little effect on the phosphorylation of AMPK and ACC in 3T3-L1 cells. The AMPK activator AICAR (2 mM) was used as a positive control. Cell extracts were detected with polyclonal antibodies against phospho-ACC (Ser79), phospho-AMPKα (Thr172), and AMPKα. (C) Metformin (2 mM) treatment during differentiation did not affect lipid accumulation in 3T3-L1 cells, in contrast to the significant effects of AICAR treatment (2 mM). The cellular lipids were stained with oil red O, and lipid content was determined by measuring the OD of the dye extracted with isopropyl alcohol. *P < 0.01 versus 0.05% DMSO (2-tailed Student’s t test).
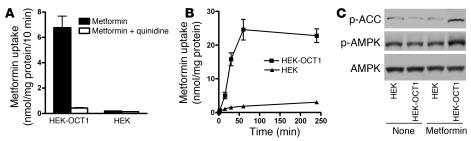
Since differences in metformin responses between Clone 9 and 3T3-L1 cells may have been due to differences between the cell types unrelated to OCT activity, we compared metformin-stimulated AMPK activation in cells transfected with human OCT1 versus control (empty vector–transfected) cells. The accumulation of metformin was time dependent and substantially increased in cells stably transfected with human OCT1 (HEK-OCT1 cells) (Figure 3, A and B). AMPK activation by metformin was significantly greater in HEK-OCT1 than in empty vector–transfected cells (Supplemental Figure 1; supplemental material available online with this article; doi:10.1172/JCI30558DS1). When cells were exposed for 1 hour to metformin (250 μM), the phosphorylation of AMPK and ACC were apparent in HEK-OCT1 cells, with little activation in control cells (Figure 3C). The data support the hypothesis that OCT1 modulates metformin-stimulated AMPK phosphorylation.
Figure 3. Overexpressing human OCT1 in HEK293 cells increases metformin uptake and metformin-stimulated AMPK phosphorylation.
(A) The uptake of metformin in HEK293 cells was markedly increased by stable overexpression of human OCT1 in the cells. The uptake experiments were performed as described in Methods and in Figure 1A. “HEK” represents empty vector–transfected cells. (B) The uptake of metformin in the HEK293 cells overexpressing human OCT1 was time dependent. (C) The phosphorylation of AMPK and ACC by metformin in HEK293 cells was markedly increased by stably overexpressing human OCT1 in the cells. The cells were treated with metformin (250 μM) for 1 hour. Cell extracts were detected with polyclonal antibodies against phospho-ACC (Ser79), phospho-AMPKα (Thr172), and AMPKα.
Oct1 deletion results in reduced metformin uptake and response in primary mouse hepatocytes.
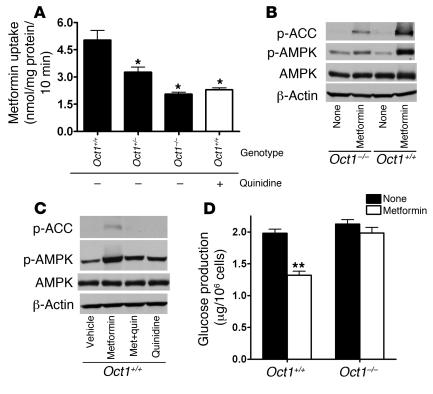
In an effort to understand the role of OCT1 in metformin action in the liver, a key target for metformin (1), we used gene targeting to construct an Oct1-knockout (Oct1–/–) mouse (Supplemental Figure 2). Consistent with the study of Jonker et al. (20), our Oct1–/– mice appeared to be healthy. As expected, metformin uptake in primary mouse hepatocytes was significantly lower (3.4-fold; P < 0.0001) in Oct1–/– cells compared with those in cells with a functional Oct1 allele (Oct1+/– and Oct1+/+ hepatocytes) (Figure 4A). Importantly, phosphorylation of AMPK and ACC by metformin was substantially reduced in Oct1–/– hepatocytes in comparison with those in wild-type hepatocytes (Figure 4B). Quinidine, an OCT inhibitor, decreased the phosphorylation of AMPK and ACC by metformin in wild-type hepatocytes (Figure 4C). Downstream, metformin-stimulated AMPK phosphorylation results in a decrease in hepatic gluconeogenesis (4, 7). Metformin (1 mM) significantly suppressed glucagon-stimulated glucose production in hepatocytes from wild-type mice (30% suppression; P < 0.001) but not in hepatocytes from Oct1–/– mice (Figure 4D). Collectively, these data are consistent with those from the cell lines and suggest that the effect of metformin on AMPK and ACC in the hepatocyte is modulated by OCT1. Thus, OCT1 appears to play a key role in determining one of the major pharmacologic effects of metformin, inhibition of hepatic gluconeogenesis.
Figure 4. Oct1 deletion results in reduced metformin uptake and response in primary hepatocytes from mice.
(A) Metformin uptake was lower in the primary hepatocytes isolated from Oct1-knockout (Oct1–/–) mice than in those with a normal Oct1 allele (Oct1+/+ and Oct1+/–). The uptake of metformin (250 μM) was performed for 10 minutes in the presence or absence of 100 μM quinidine, where indicated. *P < 0.01 versus Oct1+/– without quinidine (ANOVA and Dunnett’s procedure). (B) Metformin resulted in less phosphorylation of AMPK and ACC in Oct1–/– hepatocytes than in Oct1+/+ hepatocytes. The cellular extracts from primary hepatocytes treated with or without metformin (250 μM) for 4.5 hours were detected with polyclonal antibodies against phospho-ACC (Ser 79), phospho-AMPKα (Thr172), AMPKα, and β-actin. (C) Treatment with the OCT inhibitor quinidine reduced the stimulation of AMPK phosphorylation and thus ACC phosphorylation by metformin in Oct1+/+ hepatocytes. Where indicated, 100 μM quinidine was added 30 minutes before metformin (250 μM) treatment (Met + quin). (D) Metformin suppressed glucagon-stimulated glucose production in Oct1+/+ hepatocytes, with no effect in Oct1–/– hepatocytes. Metformin (1 mM) was added 2 hours before glucose measurement. The primary hepatocytes were isolated and cultured as described in Methods. **P < 0.001 versus no treatment (2-tailed Student’s test).
Oct1 deletion results in reduced hepatic accumulation and therapeutic response of metformin in mice.
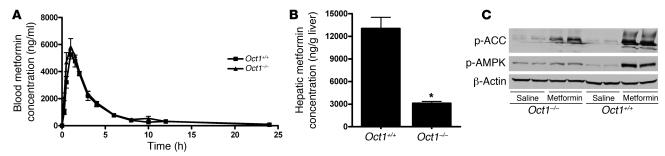
Next, we examined the role of OCT1 in metformin disposition and glucose-lowering effects in vivo. Our results were similar to those of the previous report of metformin pharmacokinetics following intravenous doses (10, 11): we observed that following a single oral dose (15 mg/kg), the plasma concentrations of metformin were similar in Oct1–/– and wild-type mice (Figure 5A) but that the hepatic accumulation was significantly greater in wild-type mice (4.2-fold; P < 0.001; 1 hour after dosing) than in Oct1–/– mice (Figure 5B). No accumulation difference was measured in other major organs (Supplemental Figure 3). Importantly, after metformin treatment, phosphorylation of both AMPK and ACC was substantially reduced in livers from Oct1–/– mice compared with those from wild-type mice (Figure 5C).
Figure 5. Oct1 deletion results in reduced hepatic metformin accumulation and phosphorylation of AMPK and ACC in mice receiving oral doses of metformin.
(A) The pharmacokinetics of metformin was similar in age-matched Oct1+/+ mice and Oct1–/– mice after an oral dose. Shown here are blood metformin concentration–time profiles. The mice (n = 4 per group) were given an oral dose of metformin (15 mg/kg containing 0.2 mCi/kg of [14C]metformin), approximating the single dose of 1,000 mg in humans. The radioactivity in blood was determined and converted to mass amounts. Data represent mean ± SD. (B) Hepatic metformin accumulation after an oral dose was much higher for Oct1+/+ mice than for age-matched Oct1–/– mice. The mice (n = 4 per group) were sacrificed 1 hour after the oral dose, and the livers were removed immediately. The radioactivity determined in liver homogenates was converted to mass amounts. Data represent mean ± SD. *P < 0.001 versus Oct1+/+ (2-tailed Student’s t test). (C) OCT1 was required for metformin to fully stimulate hepatic AMPK phosphorylation and ACC phosphorylation in mice. A daily dose of metformin (50 mg/kg) or saline was administered i.p. for 3 consecutive days to 10-week-old male mice. The mice were sacrificed 1 hour after the i.p. administration on the third day. Liver extracts were detected with polyclonal antibodies against phospho-ACC (Ser79), phospho-AMPKα (Thr172), and β-actin.
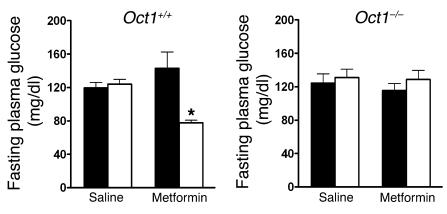
The primary end-therapeutic effect of metformin in the treatment of diabetes is to lower blood glucose levels. To increase fasting blood glucose levels, we fed Oct1–/– and Oct1+/+ mice a high-fat diet for 8 weeks. Then mice were treated with either saline or metformin for 5 days. Although no differences were observed in baseline fasting blood glucose levels between Oct1–/– and Oct1+/+ mice on high-fat diets, we observed that metformin significantly reduced fasting plasma glucose levels by more than 30% in wild-type mice fed the high-fat diet (P = 0.012) but not in the Oct1–/– mice on the same diet (Figure 6). These data suggest that in vivo, OCT1 serves a critical function in metformin’s primary therapeutic effect of lowering fasting plasma glucose levels.
Figure 6. OCT1 is required for metformin to lower fasting plasma glucose levels in mice.
The 6-week-old Oct1+/+ mice and Oct1–/– mice (n = 5–8 per group) were administered a high-fat diet for 8 weeks, and 18-hour fasting plasma glucose concentrations were measured before (black bars; day 0) and after (white bars) 5 days of i.p. treatment with saline or metformin (50 mg/kg each day). Data represent mean ± SD. *P = 0.012 versus day 0 (2-tailed Student’s t test).
OCT1 polymorphisms modulate metformin uptake and response in cells.
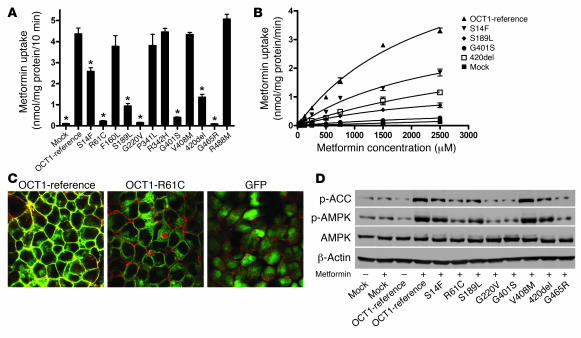
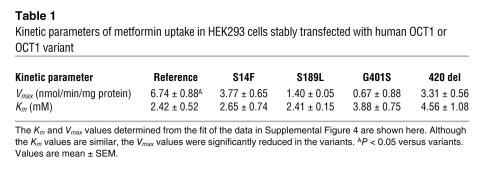
Previously, we and others showed that human OCT1 is a highly polymorphic gene (13–15). To determine whether OCT1 polymorphisms modulate metformin response, we measured the uptake of metformin in stable cell lines expressing empty vector, OCT1-reference, and 12 OCT1 nonsynonymous variants (Figure 7A). Compared with OCT1-reference, 7 OCT1 variants exhibited significantly reduced or lost metformin uptake, despite similar levels of mRNA (Supplemental Figure 4). Kinetic studies in cells expressing 4 of the reduced-function variants indicated that the decrease in uptake was probably due to reduced Vmax values (Figure 7B and Table 1). In a previous report, we showed that OCT1-G465R has reduced expression on the plasma membrane (13). In this study, GFP fusion proteins for OCT1-reference and OCT1-R61C (a very common reduced-function nonsynonymous polymorphism of OCT1) were constructed and expressed by stable transfection in HEK cells. Confocal microscopy revealed that although OCT1-reference showed robust localization to the plasma membrane, OCT1-R61C showed a more diffuse pattern of localization, with some plasma membrane staining as well as marked cytosolic retention of the variant protein (Figure 7C). Follow-up studies in this laboratory are currently underway examining the mechanisms responsible for the reduced function of the other less common variants. Notably, the phosphorylation of AMPK and ACC by metformin was reduced in cells expressing the nonfunctional or reduced-function variants compared with those expressing OCT1-reference and OCT1-V408M, a variant with normal metformin uptake (Figure 7D and Supplemental Figure 5). These data from cells suggest that OCT1 polymorphisms modulate responses to metformin. Of significance, polymorphisms of OCT1 with reduced metformin uptake are common in human populations (13–15). For example, the allele frequencies of OCT1-420del are 19% and 5% in white and African Americans, respectively, and that of OCT1-R61C is 7.2% in white individuals (13).
Figure 7. OCT1 genetic variants are associated with different accumulation rates and responses to metformin in stably transfected HEK293 cells.
(A) Uptake of [14C]metformin by cell lines stably expressing human OCT1 and its variants. Cells expressing OCT1 and its variants were incubated with [14C]metformin (250 μM) for 10 minutes. Seven OCT1 variants exhibited reduced metformin uptake as compared with OCT1-reference. Data are expressed as mean ± SD for samples analyzed in quadruplicate. *P < 0.001 compared with the reference (2-tailed Student’s t test). (B) Metformin kinetics in cell lines expressing reduced function variants of OCT1. Four of the reduced function variants shown in A had enough activity to allow us to perform kinetic studies with metformin. The metformin uptake data at 8 different concentrations are plotted. The variants had significantly different Vmax values, with a similar Michaelis-Menten constant (Km) (Table 1). (C) OCT1-R61C tagged with GFP exhibits reduced membrane and enhanced cytoplasmic localization. GFP fusion constructs were generated for OCT1-reference and OCT1-R61C, which is common in human populations (13), and used to generate stable cell lines using Flp-In-293 cells. The plasma membrane was stained using Alexa Fluor 594 conjugated to wheat germ agglutinin, and cells were visualized by confocal microscopy. Original magnification, ×100. (D) Metformin-stimulated AMPK phosphorylation and ACC phosphorylation in cell lines stably overexpressing human OCT1 and its variants. The cells were treated with metformin (1 mM) for 1 hour, washed with blank medium, and then incubated for 5 hours before harvest. Immunoblots were performed against phospho-ACC (Ser79), phospho-AMPKα (Thr172), AMPKα, and β-actin.
Table 1 .
Kinetic parameters of metformin uptake in HEK293 cells stably transfected with human OCT1 or OCT1 variant
OCT1 polymorphisms affect the response to metformin in healthy volunteers.
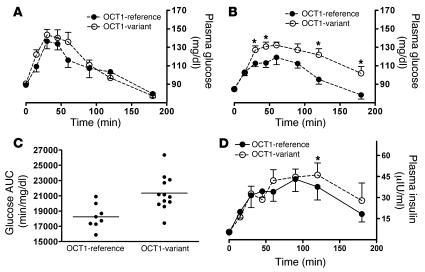
To extend the cellular studies further, we conducted a genotype-to-phenotype clinical study in healthy volunteers with different OCT1 genotypes. Although the glucose-lowering effect of metformin is not apparent in nondiabetic subjects (21), this effect can be detected in healthy subjects after plasma glucose levels are increased by administering oral glucose or conducting an OGTT (22). We observed similar plasma glucose levels and areas under the glucose concentration–time curve (AUCs) after OGTT in volunteers carrying only reference OCT1 alleles and those carrying a reduced-function polymorphism of OCT1 (AUC: 19,800 ± 1,480 versus 19,800 ± 2,520 min/mg/dl; P = 0.955; Figure 8A). However, after metformin treatment, volunteers carrying the OCT1 polymorphisms had significantly higher plasma glucose levels for most of the sampling time points during the 180-minute OGTT than those carrying only reference OCT1 alleles (Figure 8B); and thus AUC was significantly greater for the volunteers carrying the OCT1 polymorphisms as compared with those carrying only reference alleles (18,200 ± 1,600 versus 21,300 ± 2,290 min/mg/dl; P = 0.004; Figure 8C). When we assessed differences in glucose AUC with and without metformin for each individual and compared the differences between individuals with reference alleles and those with the polymorphic alleles, we also observed a significant difference (P = 0.001). In particular, metformin produced a significant decrease in the maximal glucose concentration during OGTT in the individuals with only OCT1 reference alleles, whereas the improvement was not significant in the individuals with the variants (Supplemental Table 1). Consistent with the difference in glucose levels after metformin treatment, insulin levels in individuals with the variants were significantly higher 2 hours after glucose administration in comparison with those in individuals with only OCT1 reference alleles (Figure 8D; P < 0.05). These results are consistent with the data from the mice, further supporting our finding that OCT1 is critical for the therapeutic response of metformin and suggesting that genetic variation in OCT1 may cause variation in response to metformin. A more pronounced effect of OCT1 genotypes on metformin therapy may be expected among diabetic patients, and this is being investigated.
Figure 8. OCT1 genetic variants are associated with different responses to metformin in healthy human volunteers.
(A) The time course of plasma glucose concentrations for a baseline OGTT without metformin treatment in healthy subjects having only reference OCT1 alleles (n = 8) and those having at least 1 reduced-function OCT1 allele (n = 12). The data are expressed as mean ± SEM. (B) The time course of plasma glucose concentrations for OGTT after metformin treatment in the same healthy subjects represented in A. The data are expressed as mean ± SEM; *P < 0.05 compared with volunteers with only reference OCT1 alleles (unpaired Student’s t test). (C) The glucose exposure with OGTT (AUC) after metformin treatment for healthy subjects represented in B. The horizontal lines represent mean values for the 2 groups. The mean value for volunteers with only reference OCT1 alleles is significantly lower than that for the variant group. P = 0.004 (unpaired Student’s t test). (D) The time course of insulin levels during the OGTT after metformin administration in the same healthy individuals represented in A. The data are expressed as mean ± SEM; *P < 0.05 compared with individuals with only OCT1-reference alleles (unpaired 1-tailed Student’s t test).
Discussion
In this study, we performed a comprehensive analysis of the role of OCT1 in response to metformin. We began our studies with continuous cell lines from different tissues and transfected cells to investigate whether the pharmacological activity of metformin corresponds to the activity of OCTs. We extended these studies to studies in primary hepatocytes and in vivo in Oct1+/+ and Oct1–/– mice. Finally, we performed cellular and clinical studies to determine the effect of genetic polymorphisms of OCT1 in the response to metformin. We observed the following: (a) In the cell lines, by controlling intracellular concentrations of metformin, OCT1 was an important determinant of metformin action. (b) In primary cultures of hepatocytes from mice, eliminating functional Oct1 reduced the response to metformin, and in vivo in mice, OCT1 was required for metformin to therapeutically lower blood glucose levels. (c) In humans, OCT1 polymorphisms modulated cellular and clinical response to metformin. Collectively, our studies suggest that OCT1 mediates the first step in the response pathway of metformin and that genetic variation in OCT1 may modulate response to metformin in humans (Figure 9).
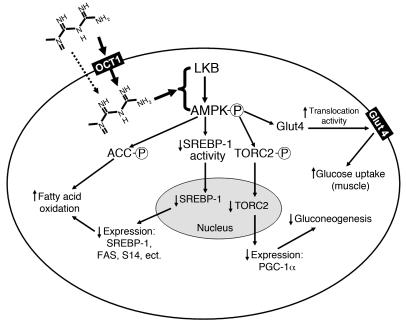
Figure 9. Mechanism of metformin action in cells.
By controlling the intracellular concentrations, OCT1 is a direct determinant of metformin pharmacological effects in the liver (bold arrow). Passive diffusion and other transporters may account for small portion of hepatic uptake of metformin (dashed arrow). Other transporters may control metformin uptake into other tissues, such as skeletal muscle. Factors such as genetic variation in transporter genes may alter transporter activity and thus metformin response. LKB, alias of serine-threonine kinase 11 (STK11); PGC-1α, peroxisome proliferator activated receptor γ coactivator 1 α; TORC2, target of rapamycin complex 2.
Our results have important implications for the tissue-specific effects of metformin. Although AMPK is ubiquitously expressed (23), the in vivo effects of metformin have been primarily ascribed to decreased hepatic gluconeogenesis and increased glucose uptake in skeletal muscle, both of which involve AMPK activation (4, 7, 24). Although direct evidence of colocalization of AMPK and OCTs in a specific cell is not available, we observed that the activation of AMPK by metformin was enhanced in cell lines (or transfected cells) that exhibited OCT activity, suggesting that the tissue-specific action of metformin may be related to expression of influx transporters such as OCTs that can deliver metformin intracellularly. Our results differ from those of Huypens et al., who observed that metformin enhanced AMPK phosphorylation in 3T3-L1 cells (25). This discrepancy may be related to different experimental conditions. Huypens et al. used high metformin concentrations and prolonged exposure times, which may have enhanced entry of metformin into the cells through passive diffusion, resulting in sufficiently high intracellular drug concentrations for AMPK activation (25). It should also be noted that there was significant metformin uptake and metformin-independent AMPK activation in Oct1–/– primary hepatocytes (Figure 4). Therefore, while OCT1 may play the major role in metformin uptake and response in the liver, other mechanisms, such as passive diffusion and other transporters, may contribute to response to metformin in the liver and in other tissues.
Our studies in Oct1+/+ and Oct –/– mice (Figures 4–6) clearly demonstrated that OCT1 is a major determinant of the hepatic effects of metformin. This transporter-mediated tissue-specific targeting of metformin may restrict the potential for unwanted effects in other tissues. Targeting influx transporters in the liver and skeletal muscle may be important in the design of other antidiabetic agents to treat type 2 diabetes. Further, our studies demonstrate that OCT1 plays a role in the primary therapeutic effect of metformin in lowering fasting blood glucose levels, suggesting that these effects may be mediated centrally in the liver. Previously, Wang et al. observed that OCT1 mediated the major adverse effect of metformin, lactic acidosis, in the liver of mice (26). We and Wang et al. (11) did not observe a significant difference in levels of metformin in skeletal muscle between Oct1–/– and Oct1+/+ mice, suggesting that the uptake of metformin in skeletal muscle may involve another transporter.
Clinically, there is enormous variation in response to metformin, and the drug is generally combined with other agents such as sulfonylureas to treat diabetes. Data from a few clinical trials indicate that greater than 36% of patients receiving metformin monotherapy do not achieve acceptable control of fasting glucose levels (16, 17). Our data suggest that genetic variation in OCT1 may contribute to variation in response to metformin. In cellular studies, we observed that 7 of the 12 polymorphisms of OCT1 exhibited reduced transport of metformin (Figure 7). It is noteworthy that 2 of the 7 variants, 420del and R61C, are common polymorphisms of OCT1, with allele frequencies of 19% and 7.2%, respectively, in individuals of European descent (13). Previous studies from this laboratory using the model organic cation MPP+ demonstrated that 4 of these 7 variants also had reduced activity (13). S14F was previously shown to exhibit an increased uptake of MPP+, whereas it displayed a reduced uptake of metformin in this study related to a reduction in its Vmax for metformin (Figure 7B and Table 1). Two other OCT1 variants, S189L and 420del, exhibited normal uptake of MPP+ previously (13), whereas in this study both variants also exhibited a reduced uptake of metformin, related to a decrease in their Vmax values (Figure 7B and Table 1). These data underscore the findings in the metaanalysis of Urban et al., who showed that transporter polymorphisms may interact differently with different substrates (27).
Our data showed that the OCT1 variants and reference have similar levels of mRNA transcripts in the stable cell lines (Supplemental Figure 4), suggesting that posttranscriptional changes account for the reduced function of the variants. In our previous report, OCT1-G465R was found to have a dramatically reduced expression level on the plasma membrane and an enhanced level in the cytosol (13). Similarly, in this study, we found that OCT1-R61C had a reduced plasma membrane and enhanced cytosolic presence in comparison to the reference OCT1 (Figure 7C). However, there was still significant protein expressed on the membrane for this particular variant, consistent with a reduction in function rather than a loss of function. Studies are underway to investigate the mechanisms responsible for the less common reduced-function nonsynonymous variants and for the common amino acid deletion variant, 420del.
Our clinical studies were focused on 4 of the OCT1 variants, R61C, G410S, 420del, and G465R, all of which exhibited reduced function in the cellular assays. Although no effect of OCT1 genotype on baseline OGTT was observed, significant effects of OCT1 genotype on OGTT were observed after metformin treatment (Figure 8). These data strongly suggest that polymorphisms in OCT1 may contribute to reduced therapeutic response to metformin clinically. Interestingly, an increase in glucose half-life in individuals with the OCT1 variants was observed following metformin treatment. This may be related to dynamic differences in metformin levels in the intestine between individuals with OCT1 variants and those without the variants, which may delay and decrease glucose absorption (28). Further studies are required to explore this interesting phenomenon. Based on the findings from this study, clinical studies in diabetic patients are planned. These further studies may provide a basis for personalizing selection or dosing of metformin based on OCT1 genotype to achieve a maximal benefit for patients.
It is noteworthy that variation in the renal clearance of metformin also has a strong genetic component (29). In a previous study, genetic variation in OCT2, an OCT1 paralog expressed in abundance in the kidney, was found to alter metformin uptake kinetics (30). Further clinical studies are thus warranted to examine metformin disposition and response among individuals with different OCT2 genotypes. In addition, our results for metformin may be extended to other drugs that rely on transporters for disposition and/or targeting. For example, oxaliplatin, an important anticancer drug, has recently been well characterized as a substrate of both OCT1 and OCT2 (31). Patients with different OCT1 or OCT2 genotypes may respond differently to oxaliplatin chemotherapy.
In conclusion, the present study demonstrated that an uptake transporter, OCT1, is required for the antidiabetic efficacy of metformin. The study provides proof of concept that genetic variation in OCT1 may be associated with variation in response to metformin.
Methods
Cell lines and transfection.
Clone 9 cells and 3T3-L1 cells were obtained from ATCC. HEK cells (Flp-In-293) were from Invitrogen. The cells were maintained in DMEM of high glucose supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin (UCSF Cell Culture Facility). HEK293 Flp-In-293 cells were transfected with pcDNA5/FRT vector (Invitrogen) containing the reference (HEK-OCT1), mutant human OCT1 cDNA inserts, or empty vector using Lipofectamine 2000 (Invitrogen) or FuGENE 6 (Roche Diagnostics) following the manufacturers’ protocols. The OCT1 variants were identified in a previous study (13) and constructed by site-directed mutagenesis from the reference OCT1 cDNA that was cloned into the pcDNA5/FRT vector. EGFP gene was subcloned from the pEGFP-C1 vector (BD Biosciences — Clontech) into the pcDNA5/FRT vector to make the GFP-tagged clone, and the OCT1-R61C variant was then constructed by site-directed mutagenesis (Stratagene). The variants were confirmed through DNA sequencing. After initial transfection, the stable clones were selected and maintained with 75 μg/ml of hygromycin B (Invitrogen). All the cells were grown at 37°C in a humidified atmosphere with 5% CO2/95% air.
Generation of Oct1–/– mice.
Our strategy results in the complete deletion of exons 3–5 and partial deletion of exons 2 and 6 from the murine Oct1 gene (Supplemental Figure 2). The deleted region is replaced with an IRES-LacZ expression cassette and a positive selection cassette containing the neomycin phophotransferase gene driven by the PGK promoter. 5′ and 3′ homology arms (3 kb and 3.5 kb, respectively) were cloned by proofreading PCR from the E14.1 ES cell line and placed on either side of the IRES-lacZ expression cassette and positive selection cassette to generate the targeting construct. Homologous recombination in neomycin-resistant ES cells was confirmed by Southern blotting of SpeI-digested genomic DNA using a 3′ external probe that detects 8-kb and 6-kb bands at the wild-type and targeted locus, respectively. Approximately 1 in 60 G418-resistant clones had undergone homologous recombination. Homologous recombination at the 5′ end was confirmed in these ES cell clones by Southern blot analysis, and homologous recombination of both ends was reconfirmed by PCR using primers external to the targeting construct. Gene targeting was performed in E14.1 ES cells. Three targeted clones were injected into C57BL/6J-derived blastocysts. Male chimaeras were crossed with C57BL/6J females to produce N1F0 offspring, which were subsequently intercrossed to generate (C57BL/6J × 129Ola) N1F1 mice used in initial testing. The mutant mice were crossed with wild-type FBV/N mice for 4 generations to acquire the FVB/N background in this study.
All animals were housed in a virus-free facility on a 12-hour light/12-hour dark cycle. We fed the mice either standard mouse food or a high-fat diet (diet D12492; Research Diets Inc.). All experiments on mice were approved by the Institutional Animal Care and Use Committee of UCSF.
Primary mouse hepatocytes.
Primary hepatocytes were isolated from wild-type and Oct1–/– mice by the UCSF Liver Center using the standard collagenase method (32). The cells were plated in Williams E medium supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 0.1% bovine albumin, 0.1 μM dexamethasone, 2 mM l-glutamine, 1 X ITS (100 X Insulin-Transferrin-Selenium; Invitrogen) at a density of 1.5 × 105 cells/cm2 on collagen-coated 6- or 12-well plates (BD). After attachment (2–3 hours), hepatocytes were maintained in the completed medium with 0.25 mg/ml Matrigel (BD Biosciences) for 16 hours followed by regular medium change and drug treatment as described below.
Drug uptake in cells.
Clone 9 cells and 3T3-L1 cells were grown on regular plastic 12- and 24-well plates, respectively, and HEK293 cells were grown on poly- d-lysine–coated 24-well plates to at least 90% confluence. Primary hepatocytes were plated on collagen-coated 12-well plates at a density of 1.5 × 105 cells/cm2. The cells were washed once with room-temperature PBS and then incubated in the uptake buffer (MPP+ in PBS or metformin in serum- and antibiotic-free culture media) containing 1 μM MPP+ (0.1 μM [3H]MPP+ [72 mCi/mmol; PerkinElmer] and 0.9 μM MPP+ [Sigma-Aldrich]) or various concentrations of metformin [35 μM [14C]metformin [26 mCi/mmol; Moravek Biochemicals and Radiochemicals) and unlabeled metformin [Sigma-Aldrich]). The uptake was performed at room temperature for 2 minutes (MPP+) or 10 minutes (metformin), and then the cells were washed 3 times with ice-cold PBS. The cells were lysed with 0.1 N NaOH and 0.1% SDS, and the lysate was used for scintillation counting (Beckman Coulter) and for the BCA protein assay (Pierce).
Kinetic studies were performed in cells stably expressing reference OCT1 and 4 polymorphisms. These 4 variants were selected for kinetic studies because they exhibited a reduced uptake of metformin, and our goal was to identify the kinetic mechanisms responsible for the reduced uptake. Further, the variants had sufficiently high activities of metformin uptake to obtain accurate kinetic parameters. To obtain the Km and Vmax in the kinetic studies, the data were fit to the Michaelis-Menten equation: V = Vmax × S/(Km+S), where Vmax is the maximum transport rate, Km is the Michaelis-Menten constant at which the transport rate (V) is one-half the Vmax, and S is the concentration of substrate. GraphPad Prism (version 4.03; GraphPad Software) was used to fit the data.
Glucose uptake in cells.
Clone 9 cells and 3T3-L1 cells were grown on 12-well plates to confluence. Two days after confluence, differentiation was initiated in 3T3-L1 cells by adding a medium containing 10 μg/ml insulin, 0.5 mM 1-methyl-3-isobutylxanthine (IMBX), and 1 μM dexamethasone (33). After 48 hours, the medium was switched back to regular growth medium with 10 μg/ml insulin. The cells were considered satisfactory for studies when at least 90% were differentiated as adipocytes. Twenty-four hours before initiation of the glucose uptake experiments, the medium was changed to serum-free DMEM. The cells were incubated in the medium containing AICAR or metformin for 2 hours before the initiation of glucose uptake. The uptake substrate consisted of 3-O-methyl- d-[1-3H]glucose ([3H]3-OMG, 4.0 Ci/mmol; GE HealthCare) plus unlabeled 3-OMG (Sigma-Aldrich). The rate of cytochalasin B–inhibitable 3-OMG uptake was measured as described previously, with minor modifications (34). After the uptake was terminated by removal of the medium, cells were washed 3 times with ice-cold PBS containing 0.1 mM phloretin. As describe above, the cells were then lysed, and the lysate was used for scintillation counting and protein determination.
Lipid accumulation in 3T3-L1 cells.
The 3T3-L1 cells were grown on 6-well plates and were differentiated as described above. AICAR and metformin were added to the medium before, 3 days after, or 5 days after the initiation of differentiation. The intracytoplasmic lipids of differentiated 3T3-L1 cells were stained with oil red O, and the dye was extracted with isopropyl alcohol and quantitated spectrophotometrically, as previously described (33).
Hepatocyte glucose production.
Glucose production in primary mouse hepatocytes was measured by modifying a previously described method (4). Hepatocytes on collagen-coated 6-well plates (1.5 × 105 cell/cm2) were incubated in medium containing Matrigel for 16 hours and then were maintained in the regular medium overnight before the glucose production experiments. For the glucose production experiments, the hepatocytes were incubated in bicarbonate-buffered saline medium containing 10 mM l-lactate, 1 mM pyruvate, and 0.3 μM glucagon (Sigma-Aldrich), with or without metformin (1 mM). The glucose concentration in the medium was measured with the glucose oxidase kit from Sigma-Aldrich.
Animal experiments.
Age-matched Oct1+/+ and Oct1–/– mice were used in this study. For measurement of tissue distribution of metformin, male mice were fasted for 16 hours, then given an oral gavage dose of 15 mg/kg metformin in saline with 0.2 μCi/g of [14C]metformin and sacrificed 1 hour later. The liver, kidney, heart, spleen, intestine, brain, and femoral muscle were removed immediately. All the tissues were weighed and homogenized with PBS. For the metformin pharmacokinetic study, age-matched male mice were fasted for 12 hours, then given an oral gavage dose of 15 mg/kg metformin in saline with 0.2 μCi/g of [14C]metformin and placed in metabolic cages for 24 hours. The food was readministered 4 hours after metformin treatment. Blood samples were collected at specific time points by tail bleeding into heparinized microhematocrit capillary tubes (Fisher Scientific). Urine and feces were collected from tubes attached to the cages. Metformin from tissue and fecal homogenates, blood, and urine were measured with a scintillation counter. The pharmacokinetic parameters were obtained by fitting the raw data using a noncompartmental model with WinNonlin (Pharsight).
To study the in vivo pharmacologic effects of metformin, 6-week-old mice were fed high-fat diets (Diet D12492; Research Diets Inc.) for 8 weeks. Mice were then injected i.p. with 0.9% sterile saline or metformin in 0.9% sterile saline in a manner similar to that described by Shaw et al. (7). Instead of 250 mg/kg metformin for 3 days, we treated the mice with 50 mg/kg metformin, a dosage approximating maximal doses used to treat diabetic patients, for 5 days. In brief, we collected blood at 12:00 pm (day 0) by tail bleeding from the mice fasted for 18 hours. Food was then resumed, and the mice were injected with metformin or saline at 10:00 am for the next 5 days (days 1–5). At 6:00 pm on day 4, the mice were again fasted. Blood was collected by tail bleeding at 12:00 pm on day 5. After blood collection, the animals were sacrificed and livers rapidly removed by freeze clamping. Plasma was isolated by centrifuging the capillary tubes in microhematocrit centrifuge (Thermo Electron Corp.), and glucose concentration was measured using the glucose oxidase assay kit (Sigma-Aldrich).
Clinical study.
The study protocol was reviewed and approved by the Committee on Human Research at UCSF. The subjects were selected from the participants recruited for a large project named SOPHIE (Study of Pharmacogenetics in Ethnically Diverse Populations). At the time of initial enrollment, SOPHIE participants consent to be recontacted about their willingness to participate in subsequent clinical pharmacogenetic research studies. Previously we had identified the ethnic-specific allele frequencies of common variants of OCT1 with reduced or no function (13). The current clinical study was initially based on these data and designed to assess the effects of OCT1-R61C, -G401S, and -G465R on metformin response. Because these 3 variants mainly occur in individuals with European ancestry, our initial recruitment was limited to healthy male or female European Americans from SOPHIE with wild-type OCT1 or with 1 of the 3 variants. However, during the cellular study, we identified another common OCT1 variant (420del) with reduced metformin but not MPP+ uptake (Figure 7A). All participants were then genotyped for this polymorphism, too. The characteristics and genotypes for the subjects are summarized in Supplemental Table 2.
After informed consent was obtained, healthy individuals with known OCT1 genotypes were recruited into this open-label study. Once enrolled, participants were advised to maintain stable activity levels (without periods of strenuous exercise) for 7 days before the formal study. Approximately 7 days prior to the study, subjects met with a dietitian to create a 3-day meal plan that maintained carbohydrate intake at 200–250 g/d. The volunteers recorded their food intake in a 3-day food diary. Consistent and adequate carbohydrate intake would reduce variation in the results of the OGTT, our primary determinant of metformin action in this study. After the initial 3-day diet-maintained period, subjects were admitted (day 0) to the General Clinical Research Center at San Francisco General Hospital, and they remained at this center for the duration of the study. On the following morning (day 1), a 3-hour OGTT (75 g glucose) was conducted. Subjects were then given a doses of 1,000 mg metformin in the evening, followed by a dose of 850 mg on the morning of day 2, two hours before a second OGTT was performed. After the second metformin dose, additional blood samples were collected to determine pharmacokinetics. The plasma glucose concentrations from OGTT before and after metformin treatment were compared in individuals who carry the decreased or nonfunctional OCT1 variants and those who carry the reference alleles.
Subcellular localization studies.
The stable cells transfected with GFP-containing vectors described above were seeded at 3 × 105 cells per well on 12-mm poly- d-lysine–coated glass coverslips (BD Biosciences — Discovery Labware) in 24-well plates. Cells were stained using the Image-IT LIVE labeling kit (Invitrogen) and fixed in 4% paraformaldehyde according to the manufacturer’s protocol. Coverslips were mounted in VECTASHIELD antifade solution (Vector Laboratories) on glass microscope slides and visualized by confocal microscopy using a Zeiss 510 laser scanning microscope.
Immunoblots.
Cultured cells were lysed at 4°C for 20 minutes in buffer containing 20 mM Tris, pH 7.4, 1% Triton X-100, 150 mM NaCl, 250 mM sucrose, 50 mM NaF, 2.5 mM Na3P2O4, 2 mM DTT, and 10 mM Na3VO4, with the protease inhibitors dissolved from Complete protease inhibitor cocktail tablet (Roche Applied Science). Liver samples were homogenized in ice-cold lysis buffer using a tissue homogenizer at 4°C for 20 minutes. After centrifugation for 20 minutes at 14,000 g at 4°C, the supernatants were removed for determination of protein content and separated on 10% SDS-PAGE gels. Forty micrograms of proteins from the supernatant were separated and transferred to nitrocellulose membranes. The membranes were blocked overnight at 4°C with Tris-buffered saline with 0.05% Tween 20 and 5% nonfat milk. Immunblotting was performed following standard procedures, and the signals were detected by chemiluminescence reagents (Amersham). Primary antibodies were directed against: AMPKα, AMPKα phosphorylated at Thr172, ACC phosphorylated at Ser79, and β-actin (all from Cell Signaling Technology).
RNA isolation and RT-PCR.
Three OCTs (OCT1, OCT2, and OCT3) have been cloned. Metformin is a substrate for OCT1 and OCT2 (10, 11, 35) but not for OCT3 (data not shown). mRNA transcripts of OCT1 and OCT2 in different cell lines were detected by RT-PCR. Cells were cultured in 6-well plates as described above. Total RNA was extracted using TRI zol (Invitrogen). To detect OCT1, we used a One-Step RT-PCR kit (Roche Applied Science). Thirty-five amplification cycles were used in the PCR. To detect OCT2, the first-strand cDNA was synthesized from 1 μg of total RNA using SuperScript III First-Strand Synthesis System kit (Invitrogen), and the resulting cDNAs were used for PCR with 40 amplification cycles. The mouse, rat, and human GAPDHs were used as the expression control for Clone 9 cells, 3T3-L1 cells, and HEK293 cells, respectively. Primers for PCR are provided in Supplemental Table 3. Real-time PCR was also performed to quantify the transcript levels of human OCT1 or its variants in stable HEK293 cells. cDNA (20 ng) was used in quantitative real-time PCR with the OCT1 probes from ABI and an ABI Prism 7700 machine (Applied Biosystems).
Statistics.
Unless otherwise indicated, the data are presented as mean ± SD and from a representative experiment performed in triplicate or quadruple. Unless otherwise indicated, all experiments were repeated at least twice. Student’s 1- or 2-tailed t test was applied to analyze data, when appropriate, as indicated in the figure legends. For multiple comparison tests, ANOVA was used followed by Dunnett’s procedure. A P value of less than 0.05 was considered significant.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH (GM36780 and GM61390). This work was also made possible in part by core services provided by the General Clinical Research Center (GCRC) at San Francisco General Hospital (SFGH), funded by the National Center for Research Resources (NIH MO1-RR00083-44), and by the UCSF Liver Center (NIH P30 DK26743). We thank Morrie Schambelan and all staff at GCRC at SFGH for their excellent assistance in our clinical study. We thank Jacquelyn Maher, Gene Lee, and Colleen Hefner at the Cell and Tissue Biology Core of UCSF Liver Center for their great help in isolating mouse primary hepatocytes. We thank Thomas J. Urban for his technical help. We thank Harma M. Ellens at GlaxoSmithKline for her advice and critical reading the manuscript. The data from this study will be deposited at PharmGKB (www.pharmgkb.org).
Footnotes
Nonstandard abbreviations used: ACC, acetyl-CoA carboxylase; AICAR, 5-aminoimidazole-4-carboxamine-1-β- d-ribofuranoside; AMPK, AMP-activated protein kinase; AUC, area under the glucose concentration–time curve; MPP+, 1-methy-4-phenylpyridinium; OCT, organic cation transporter; OGTT, oral glucose tolerance test; 3-OMG, 3-O-methyl- d-glucose.
Conflict of interest: S.A. Sheardown and L. Yue are employees of GlaxoSmithKline.
Citation for this article: J. Clin. Invest. 117:1422–1431 (2007). doi:10.1172/JCI30558
Yan Shu’s present address is: Department of Neurosurgery, Cedars-Sinai Medical Center, Los Angeles, California, USA.
See the related Commentary beginning on page false.
References
- 1.Kirpichnikov D., McFarlane S.I., Sowers J.R. Metformin: an update. Ann. Intern. Med. 2002;137:25–33. doi: 10.7326/0003-4819-137-1-200207020-00009. [DOI] [PubMed] [Google Scholar]
- 2.Lin H.Z., et al. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat. Med. 2000;6:998–1003. doi: 10.1038/79697. [DOI] [PubMed] [Google Scholar]
- 3.Baillargeon J.P., Iuorno M.J., Nestler J.E. Insulin sensitizers for polycystic ovary syndrome. Clin. Obstet. Gynecol. 2003;46:325–340. doi: 10.1097/00003081-200306000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Zhou G., et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 2001;108:1167–1174. doi: 10.1172/JCI200113505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbud W., et al. Stimulation of AMP-activated protein kinase (AMPK) is associated with enhancement of Glut1-mediated glucose transport. Arch. Biochem. Biophys. 2000;380:347–352. doi: 10.1006/abbi.2000.1935. [DOI] [PubMed] [Google Scholar]
- 6.Woods A., et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 7.Shaw R.J., et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L., et al. Cloning and functional expression of a human liver organic cation transporter. Mol. Pharmacol. 1997;51:913–921. doi: 10.1124/mol.51.6.913. [DOI] [PubMed] [Google Scholar]
- 9.Dresser M.J., Leabman M.K., Giacomini K.M. Transporters involved in the elimination of drugs in the kidney: organic anion transporters and organic cation transporters. J. Pharm. Sci. 2001;90:397–421. doi: 10.1002/1520-6017(200104)90:4<397::aid-jps1000>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 10.Dresser M.J., Xiao G., Leabman M.K., Gray A.T., Giacomini K.M. Interactions of n-tetraalkylammonium compounds and biguanides with a human renal organic cation transporter (hOCT2). Pharm. Res. 2002;19:1244–1247. doi: 10.1023/a:1019870831174. [DOI] [PubMed] [Google Scholar]
- 11.Wang D.S., et al. Involvement of organic cation transporter 1 in hepatic and intestinal distribution of metformin. J. Pharmacol. Exp. Ther. 2002;302:510–515. doi: 10.1124/jpet.102.034140. [DOI] [PubMed] [Google Scholar]
- 12.Leabman M.K., et al. Natural variation in human membrane transporter genes reveals evolutionary and functional constraints. Proc. Natl. Acad. Sci. U. S. A. 2003;100:5896–5901. doi: 10.1073/pnas.0730857100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shu Y., et al. Evolutionary conservation predicts function of variants of the human organic cation transporter, OCT1. Proc. Natl. Acad. Sci. U. S. A. 2003;100:5902–5907. doi: 10.1073/pnas.0730858100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerb R., et al. Identification of genetic variations of the human organic cation transporter hOCT1 and their functional consequences. Pharmacogenetics. 2002;12:591–595. doi: 10.1097/00008571-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Sakata T., et al. Novel single nucleotide polymorphisms of organic cation transporter 1 (SLC22A1) affecting transport functions. Biochem. Biophys. Res. Commun. 2004;313:789–793. doi: 10.1016/j.bbrc.2003.11.175. [DOI] [PubMed] [Google Scholar]
- 16.Hermann L.S., Schersten B., Melander A. Antihyperglycaemic efficacy, response prediction and dose-response relations of treatment with metformin and sulphonylurea, alone and in primary combination. Diabet. Med. 1994;11:953–960. doi: 10.1111/j.1464-5491.1994.tb00253.x. [DOI] [PubMed] [Google Scholar]
- 17.Hermann L.S., et al. Therapeutic comparison of metformin and sulfonylurea, alone and in various combinations. A double-blind controlled study. Diabetes Care. 1994;17:1100–1109. doi: 10.2337/diacare.17.10.1100. [DOI] [PubMed] [Google Scholar]
- 18.Guigas B., et al. 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside and metformin inhibit hepatic glucose phosphorylation by an AMP-activated protein kinase-independent effect on glucokinase translocation. Diabetes. 2006;55:865–874. doi: 10.2337/diabetes.55.04.06.db05-1178. [DOI] [PubMed] [Google Scholar]
- 19.Habinowski S.A., Witters L.A. The effects of AICAR on adipocyte differentiation of 3T3-L1 cells. Biochem. Biophys. Res. Commun. 2001;286:852–856. doi: 10.1006/bbrc.2001.5484. [DOI] [PubMed] [Google Scholar]
- 20.Jonker J.W., et al. Reduced hepatic uptake and intestinal excretion of organic cations in mice with a targeted disruption of the organic cation transporter 1 (Oct1 [Slc22a1]) gene. Mol. Cell. Biol. 2001;21:5471–5477. doi: 10.1128/MCB.21.16.5471-5477.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hermann L.S. Metformin: a review of its pharmacological properties and therapeutic use. Diabete. Metab. 1979;5:233–245. [PubMed] [Google Scholar]
- 22.Gyr N., Berger W., Fridrich R., Denes A., Stalder G.A. Effect of dimethylbiguanide on stomach emptying and on oral glucose tolerance [In German]. Schweiz. Med. Wochenschr. 1971;101:1876–1879. [PubMed] [Google Scholar]
- 23.Hardie D.G., Carling D., Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu. Rev. Biochem. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- 24.Fujii N., Aschenbach W.G., Musi N., Hirshman M.F., Goodyear L.J. Regulation of glucose transport by the AMP-activated protein kinase. Proc. Nutr. Soc. 2004;63:205–210. doi: 10.1079/PNS2004340. [DOI] [PubMed] [Google Scholar]
- 25.Huypens P., Quartier E., Pipeleers D., Van de Casteele M. Metformin reduces adiponectin protein expression and release in 3T3-L1 adipocytes involving activation of AMP activated protein kinase. Eur. J. Pharmacol. 2005;518:90–95. doi: 10.1016/j.ejphar.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 26.Wang D.S., et al. Involvement of organic cation transporter 1 in the lactic acidosis caused by metformin. Mol. Pharmacol. 2003;63:844–848. doi: 10.1124/mol.63.4.844. [DOI] [PubMed] [Google Scholar]
- 27.Urban T.J., et al. Functional genomics of membrane transporters in human populations. Genome Res. 2006;16:223–230. doi: 10.1101/gr.4356206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caspary W.F., Creutzfeldt W. Analysis of the inhibitory effect of biguanides on glucose absorption: inhibition of active sugar transport. Diabetologia. 1971;7:379–385. doi: 10.1007/BF01219474. [DOI] [PubMed] [Google Scholar]
- 29.Leabman M.K., Giacomini K.M. Estimating the contribution of genes and environment to variation in renal drug clearance. Pharmacogenetics. 2003;13:581–584. doi: 10.1097/00008571-200309000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Leabman M.K., et al. Polymorphisms in a human kidney xenobiotic transporter, OCT2, exhibit altered function. Pharmacogenetics. 2002;12:395–405. doi: 10.1097/00008571-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Zhang S., et al. Organic cation transporters are determinants of oxaliplatin cytotoxicity. Cancer Res. 2006;66:8847–8857. doi: 10.1158/0008-5472.CAN-06-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moldeus P., Hogberg J., Orrenius S. Isolation and use of liver cells. Methods Enzymol. 1978;52:60–71. doi: 10.1016/s0076-6879(78)52006-5. [DOI] [PubMed] [Google Scholar]
- 33.Ramirez-Zacarias J.L., Castro-Munozledo F., Kuri-Harcuch W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with oil red O. Histochemistry. 1992;97:493–497. doi: 10.1007/BF00316069. [DOI] [PubMed] [Google Scholar]
- 34.Mercado C.L., Loeb J.N., Ismail-Beigi F. Enhanced glucose transport in response to inhibition of respiration in Clone 9 cells. Am. J. Physiol. 1989;257:C19–C28. doi: 10.1152/ajpcell.1989.257.1.C19. [DOI] [PubMed] [Google Scholar]
- 35.Kimura N., et al. Metformin is a superior substrate for renal organic cation transporter OCT2 rather than hepatic OCT1. Drug Metab. Pharmacokinet. 2005;20:379–386.. doi: 10.2133/dmpk.20.379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.