Abstract
We hypothesize that changes in adrenal gene expression mediate the increased plasma corticosterone and steroidogenesis in rat pups exposed to hypoxia from birth. In the current study, rat pups (with their dams) were exposed to hypoxia from birth and compared to pups from normoxic dams fed ad libitum or pair fed to match the decreased maternal food intake that occurs during hypoxia. Microarray analysis was performed, followed by verification with real-time PCR. Furthermore, the expression of selected genes involved in adrenal function was analyzed by real-time PCR, regardless of microarray results. Hypoxia increased plasma ACTH and corticosterone, while food restriction had no effect. Microarray revealed that many of the genes affected by hypoxia encode proteins that require molecular oxygen (monooxygenases, oxidoreductases, and electron transport), whereas only a few genes known to be involved in adrenal steroidogenesis were affected. Interestingly, the expression of genes involved in mitochondrial function and intermediary metabolism were increased by hypoxia. Real-time PCR detected a small but significant increase in the expression of Cyp21a1 mRNA in the hypoxic adrenal. When decreased maternal food intake was controlled for, the effects of hypoxia were more pronounced, in that real-time PCR detected significant increases in the expression of StAR (244%), Cyp21a1 (208%), and Ldlr (233%). The present study revealed that increased plasma corticosterone in rat pups was due to hypoxia per se, and not as a result of decreased food intake by the hypoxic dam. Furthermore, hypoxia induced changes in gene expression that account for more productive and efficient steroidogenesis.
Keywords: Steroidogenesis, neonatal, mRNA, adrenal cortex
INTRODUCTION
Systemic hypoxia is a condition frequently encountered in the neonatal intensive care unit, is often a complication of preterm birth, and is associated with significant morbidity and mortality (9, 19). The ability to survive a hypoxic episode depends on coordinated physiological responses by a number of systems. These responses include, but are not limited to, adaptations in metabolic, hemodynamic, digestive, and cardiorespiratory function (8, 10,13, 17, 22). A coordinated endocrine response is also a critical component (23, 26). The ability of the neonatal hypothalamic-pituitary-adrenal (HPA) axis to mount a response to low oxygen is of paramount importance. A majority of preterm infants lack the capacity for an adrenocortical response to exogenous ACTH and physiological stressors, and this leads to enhanced inflammation and decreased pulmonary function requiring ventilation (15, 35).
In the neonatal rat, there is a unique developmental aspect to the maturation of the adrenocortical response to stressors such as hypoxia (30, 34). We have previously shown that hypoxia from birth to postnatal day (PD) seven significantly increased plasma corticosterone and adrenal sensitivity to ACTH in suckling rat pups (25, 26). This increase was largely independent of increased plasma ACTH concentrations and was partially attenuated by the administration of guanethidine, a post-ganglionic sympathetic nerve terminal blocker that prevents the release of norepinephrine from nerve terminals and affects adrenocortical responsiveness (27, 33). Furthermore, the increase in corticosterone occurred during the so-called stress hyporesponsive period of the neonatal rat (30, 34, 36). This period of relative suppression of the HPA axis is thought to play a protective role during a critical period of brain development.
The molecular and cellular aspects of hypoxia-induced increases in plasma corticosterone have also been explored. We have shown that hypoxia elicited small but significant increases in steroidogenic acute regulatory (StAR) protein expression (25). Other studies from our laboratory measured unique changes in the lipid and fatty acid profile of the hypoxic adrenal (6). Interestingly, hypoxia significantly increased the total concentration of cholesterol ester (CE) in the adrenal, with specific increases in CE-associated 20:4n6 (arachidonic acid) concentrations. Previous studies using semi-quantitative PCR revealed no changes in the expression of genes encoding proteins involved in the steroidogenic pathway (25, 26). It was therefore pertinent to address changes in gene expression using more advanced screening approaches.
The present study examined the effects of hypoxia from birth to PD7 on adrenal gene expression using microarray analysis. This approach was used to search for hypoxia-induced changes in the expression of novel genes not necessarily considered a component of the steroid synthetic pathway. Microarray results considered highly significant were verified with real-time PCR. In addition, expression of genes involved with the steroidogenic pathway was also analyzed by real-time PCR. Food intake of nursing dams was measured daily in order to examine the interaction between the anorectic effects of hypoxia and HPA axis responsiveness in pups. Nursing normoxic dams were pair-fed an amount of food that matched the intake of hypoxic dams. Plasma ACTH and corticosterone, as well as changes in adrenal gene expression, were measured in the three treatment groups (Normoxia, Hypoxia, and Normoxia Pair Fed) as functional endpoints of HPA axis activity. We hypothesized that hypoxia from birth would increase the expression of genes involved in steroidogenesis or the steps that lead up to steroidogenesis. We also hypothesized that maternal food restriction per se may contribute to changes in plasma ACTH or corticosterone.
METHODS
Animal Treatment
The Institutional Animal Care and Use Committee of Aurora Health Care approved all animal protocols. Timed-pregnant Sprague-Dawley rats (Harlan Sprague Dawley Inc., Indianapolis, IN; N=26) were obtained at 14 days gestation and maintained on a standard diet and water ad libitum in a controlled environment (0600-1800 lights on). As soon as a litter was completely delivered, the dam and her pups were immediately moved to an environment chamber and exposed to 21% (Normoxia; N=9) or 12% (Hypoxia; N=8) O2 as described previously (26, 28, 32). Litter size was normalized to 12 pups per litter (mixed sexes). Maternal food intake was measured for normoxic and hypoxic dams. Food equivalent to the average daily intake of hypoxic dams (N=4) was then given to a separate set of normoxic dams (Pair Fed; N=9). Pups were weighed on postnatal day (PD) 7. Experimentation was performed on the morning of PD7, at which time pups were decapitated and trunk blood was collected into EDTA. Blood from two pups was pooled for each sample. Plasma was separated and frozen at −20 °C until further analysis. Adrenal glands were quickly removed, frozen on dry ice, and pooled to form one sample for analysis (one litter per sample).
Total RNA for Microarray Analysis
Total RNA from pooled adrenal glands (N=4 pooled samples per Normoxia or Hypoxia) was isolated with TRIzol reagent (Invitrogen Life Technologies; Carlsbad, CA) and column purified (Qiagen; Valencia, CA) as described previously (17). RNA quality was assessed spectrophotometrically on the basis of the A260/A280 ratio. All RNA samples were checked for integrity of 18S and 28S RNA by gel electrophoresis.
Microarray Analysis
Changes in adrenal gene expression due to hypoxia were measured using microarray analysis, as described previously (16). Briefly, double-stranded DNA was synthesized from 10 μg total RNA using a Superscript cDNA Synthesis Kit (Invitrogen). Biotin-labeled cRNA was generated by transcription with T7 polymerase and purified on RNeasy affinity columns (Qiagen). Fragmented, biotinylated cRNA (10 μg), along with hybrid controls (Affymetrix; Santa Clara, CA), were hybridized to the Affymetrix Rat Genome U34A GeneChip array containing probes for 15,923 transcripts. Arrays were washed and stained and then scanned at 488 nm in a G2500A GeneArray Scanner (Agilent; Palo Alto, CA).
Microarray Data Quantification, Normalization, and Analysis
Evaluation of data from microarray analysis was performed as described in detail previously (16). Briefly, scanned images were quantified by GeneChip Operating Software 1.1 (Affymetrix). Signal intensities were used to determine overall expression level and a detection confidence score. Fold-change in expression was calculated from the average signal intensity of each group. A modified version of the false-discovery rate (FDR) method was used to assess significance of results (16). To identify genes significantly affected by hypoxia, genes with a FDR ≤ 0.25 and a minimum fold-change of ± 1.3 were selected. Functional categories used to sort genes according to biological function were derived from the Expression Analysis Systematic Explorer (EASE) software. More specific descriptions of gene function were obtained from the National Center for Biotechnology Information Entrez Gene Database (http://www.ncbi.nlm.nih.gov). The data comply with the MIAME standard and the accession numbers are GSM143946 through GSM143953.
Real-Time PCR Analysis
Total RNA for real-time PCR was isolated from a separate set of pooled adrenal tissue (one litter [24 adrenals] per pooled sample; N=4 pooled samples per treatment group) using the RNeasy Mini Protocol (Qiagen). The concentration of RNA was quantified using the absorbance value at 260 nm and the quality of the sample preparation was assessed using the A260/A280 ratio. All RNA preparations were diluted to a final concentration of 10 ng/μL. Real-time PCR was performed using the Taqman One-Step RT-PCR Protocol (Applied Biosystems, Inc. (ABI); Foster City, CA). Pre-made primers and probes were purchased from ABI (see Table 1) and expression of 18S RNA was used as an endogenous control. The final reaction volume of 25 uL consisted of 1X AmpliTaq Gold® DNA Polymerase mix, 1X RT enzyme mix containing MultiScribe™ Reverse Transcriptase and RNase Inhibitor, 1X primer/probe mix, and 50 ng of total RNA. Amplification and detection were performed with the ABI Prism 7300 Sequence Detection System with the following thermal cycler conditions: 48 °C for 30 min (RT), 95 °C for 10 min, and 40 cycles at 95 °C for 0.25 min and 60 °C for 1 min. Each measurement was carried out in triplicate or quadruplicate. Differences in gene expression, expressed as fold-change, were calculated using the 2−ΔΔCt method using 18S as the internal control.
Table 1.
TaqMan Gene Expression Assays Used for Real-Time PCR
| Gene | Symbol | Assay ID# |
|---|---|---|
| Cytochrome P450, subfamily 1B, polypeptide 1 | Cyp1b1 | Rn00564055_m1 |
| Guanosine monophosphate reductase | Gmpr | Rn00589361_m1 |
| Xanthine dehydrogenase | Xdh | Rn00567654_m1 |
| Cadherin 1 | Cdh1 | Rn00580109_m1 |
| Phospholipase A2, group IVA (cytosolic, Ca-dependent) | Pla2g4a | Rn00591916_m1 |
| Aldehyde oxidase | Aox1 | Rn00571242_m1 |
| Regulator of G-protein signaling protein 2 | Rgs2 | Rn00584932_m1 |
| Carnitine palmitoyltransferase 2 | Cpt2 | Rn00563995_m1 |
| Steroid 5 alpha-reductase 1 | Srd5a1 | Rn00567064_m1 |
| Enolase 1, alpha | Eno1 | Rn00820594_g1 |
| N-ethylmaleimide sensitive factor | Nsf | Rn00572694_m1 |
| Cytochrome P450, subfamily 11B, polypeptide 3 | Cyp11b3 | Rn00822066_g1 |
| Steroidogenic acute regulatory protein | StAR | Rn00580695_m1 |
| Cytochrome P450, subfamily 11A | Cyp11a | Rn00568733_m1 |
| Cytochrome P450, subfamily 21A, polypeptide 1 | Cyp21a1 | Rn00588996_g1 |
| 3-hydroxy-3-methylglutaryl-Coenzyme A reductase | Hmgcr | Rn00565598_m1 |
| Low density lipoprotein receptor | Ldlr | Rn00598438_m1 |
| Vascular endothelial growth factor | Vegf | Rn00582935_m1 |
| Hypoxia-inducible factor 1 alpha | Hif1a | Rn00577560_m1 |
Each Gene Expression Assay consisted of a mix of sequence specific forward/reverse primers and a FAM-labeled probe. Note: Primer/probe sets that cross an exon-exon boundary are designated “m1” at the end of the Assay ID#. Primer/probe sets designated “g1” cross an exon-exon boundary, but may still detect genomic DNA.
Hormone Measurements
Plasma ACTH and corticosterone were measured using radioimmunoassays (MP Biomedicals, Inc.; Orangeburg, NJ) as described previously (25-27).
Statistics
Data for food intake measurements were analyzed by two-way repeated measures analysis of variance (ANOVA) with p<0.05 considered significant. Plasma hormone data were analyzed with one-way ANOVA with p<0.05 considered significant. The delta Ct values obtained from real-time PCR, those used for calculation of fold-change differences (expressed here as percent of control), were also used for statistical analyses. PCR data were analyzed with one-way ANOVA with p<0.05 considered significant. All post hoc analyses were performed by Student-Newman-Keuls method for multiple comparisons (SigmaStat 2.03). Explanation of statistical analyses used for data generated by microarray may be found above.
RESULTS
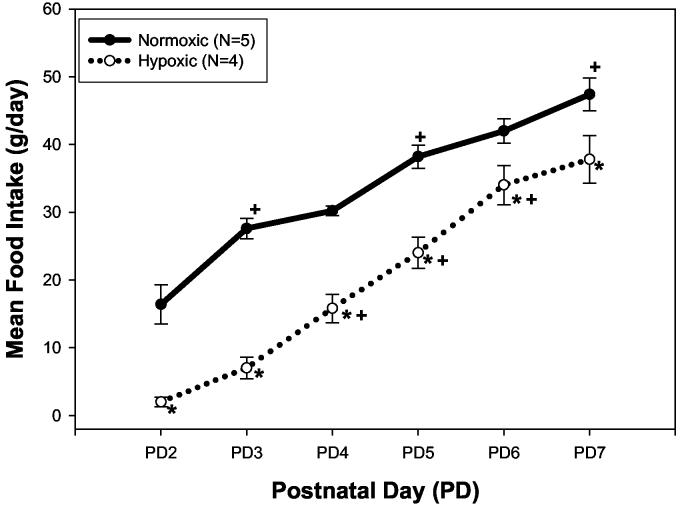
Figure 1 depicts the mean daily food intake (g) of nursing dams exposed to 21% O2 (Normoxia; N=5) or 12% O2 (Hypoxia; N=4). Hypoxia significantly decreased food intake at each time point, when compared to control (p<0.05). Interestingly, hypoxic dams significantly increased their daily food consumption during the study period, more so than that of the normoxic dams. As a result, hypoxic dam food intake approached that of normoxic dams by the end of the study.
Figure 1.
Mean food intake (g/day) of nursing dams exposed to normoxia (21% O2) or hypoxia (12% O2) from delivery to postnatal (PD) 7. Dams were fed ad libitum and individual daily food intake was recorded. Data are reported as mean ± SEM for each group at each time point (Normoxia N=5; Hypoxia N=4). * Indicates a significant difference from Normoxia at the indicated time point with p<0.05. + Indicates a significant increase in food intake from the previous time point within the specified treatment group with p<0.05.
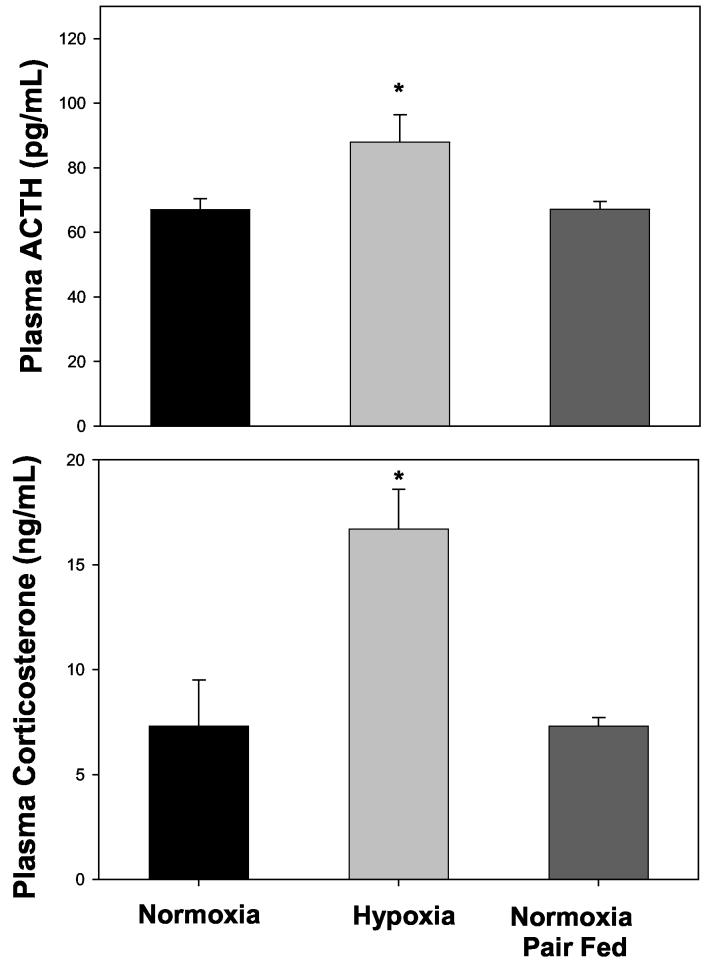
Figure 2 shows plasma ACTH (upper panel) and corticosterone (lower panel) concentrations in pups sacrificed at PD7. Hypoxia from birth significantly increased plasma ACTH when compared to the normoxic control (p=0.025). Normoxic pups reared by dams pair-fed to reduce daily food intake to match hypoxic dams did not exhibit any change in plasma ACTH concentrations (p>0.05). Plasma corticosterone in hypoxic pups was significantly increased when compared to control (p=0.006). There was no effect of decreased maternal food intake on plasma corticosterone concentrations in normoxic pups (p>0.05).
Figure 2.
Plasma ACTH (pg/mL) and corticosterone (ng/mL) in control pups (Normoxia; N=10), in pups exposed to 12% O2 (Hypoxia; N=8), and in pups reared by dams pair-fed to match hypoxic dam food intake (Normoxia Pair Fed; N=22). Pups were sacrificed at PD7 and blood from two pups was pooled as one sample. * Indicates a significant difference from Normoxia with p<0.05.
Table 2 lists selected results from the microarray analysis of adrenal gene expression in pups exposed to hypoxia from birth with food available ad libitum (as compared to Normoxia). Genes were selected based on the putative role(s) their products may play in increasing adrenal steroid production. Table 2A lists genes that exhibited a significant increase in expression following hypoxia. Many of the genes increased by hypoxia encode proteins having oxidoreductase activity, including Cyp1b1, Gmpr, Xdh, Srd5a1, Cox4b, Dbh, Fmo1, and G6pdx. Other genes that displayed increased expression encode proteins involved in the glycolytic pathway (Eno1 and Aldoc), electrochemical-driven transport (Slc18a2 and Slc7a1), lipid metabolism (Cpt2 and Vldlr), and intracellular signaling (Pde10a and Ass). Hypoxia also increased the expression of genes associated with neural/adrenomedullary function (Nefh, Reln, Anp32a, Uts2, Npy, and Dbh).
Table 2.
Microarray analysis of adrenal gene expression in the 7-day-old rat: effects of hypoxia from birth.
| A. Increased by Hypoxia | ||||
|---|---|---|---|---|
| Gene | Symbol | FDR | Fold Change | Function |
| Aldolase C, fructose-biphosphate | Aldoc | 0.0037 | 4.6 | Glycolytic enzyme |
| Solute carrier family 18, member 2 | Slc18a2 | 0.0037 | 2.0 | Monoamine transporter |
| Cytochrome P450, subfamily 1B, polypeptide 1 | Cyp1b1 | 0.0037 | 1.7 | Aromatic hydrocarbon metabolism |
| Neurofilament, heavy polypeptide | Nefh | 0.0037 | 2.6 | Intermediate filament protein |
| Guanosine monophosphate reductase | Gmpr | 0.0057 | 2.0 | Guanine nucleotide metabolism |
| N-ethylmaleimide sensitive factor | Nsf | 0.0078 | 1.9 | Synaptic AMPA receptor modulator |
| Arginosuccinate synthetase | Ass | 0.0095 | 2.2 | Arginine biosynthesis |
| Solute carrier family 7, member 1 | Slc7a1 | 0.0116 | 1.6 | Cationic amino acid transport |
| Reelin | Reln | 0.0212 | 1.5 | Regulates neuronal cell migration |
| Phosphodiesterase 10A | Pde10a | 0.0212 | 1.8 | cAMP metabolism |
| Interleukin 18 | Il18 | 0.0219 | 1.6 | Pro-inflammatory cytokine |
| Acidic nuclear phosphoprotein 32 family, a | Anp32a | 0.0415 | 2.5 | Neuronal differentiation |
| Neuropeptide Y | Npy | 0.0463 | 1.5 | Neurotransmitter |
| Xanthine dehydrogenase | Xdh | 0.0486 | 1.6 | Purine metabolism |
| Glutamate oxaloacetate transaminase 1 | Got1 | 0.0491 | 1.6 | Intermediary metabolism |
| Guanine deaminase | Gda | 0.0604 | 1.7 | Purine metabolism |
| Carnitine palmitoyltransferase 2 | Cpt2 | 0.0908 | 1.5 | Mitochondrial fatty acid transport |
| Hydroxyacyl glutathione hydrolase | Hagh | 0.0908 | 1.5 | Intermediary metabolism |
| Urotensin 2 | Uts2 | 0.1056 | 2.2 | Neuropeptide |
| VGF nerve growth factor inducible | Vgf | 0.1126 | 1.9 | Growth factor |
| Cytochrome c oxidase, subunit 4b | Cox4b | 0.1204 | 1.6 | Oxidative phosphorylation |
| Steroid 5 alpha-reductase 1 | Srd5a1 | 0.1204 | 1.4 | Steroid metabolism |
| Dopamine beta hydroxylase | Dbh | 0.1204 | 1.4 | Norepinephine biosynthesis |
| Flavin-containing monooxygenase 1 | Fmo1 | 0.1204 | 1.5 | Monoxygenase activity; electron transport |
| Enolase 1, alpha | Eno1 | 0.1204 | 1.5 | Glycolytic enzyme |
| Very low density lipoprotein receptor | Vldlr | 0.1344 | 1.5 | Lipid import |
| Glucose-6-phosphate dehydrogenase | G6pdx | 0.2410 | 1.3 | Intermediary metabolism |
| B. Decreased by Hypoxia | ||||
|---|---|---|---|---|
| Gene | Symbol | FDR | Fold Change | Function |
| Cadherin 1 | Cdh1 | 0.0010 | −2.1 | Adhesion molecule |
| Phospholipase A2, group IVA (cytosolic) | Pla2g4a | 0.0012 | −1.8 | Cell signaling |
| Aldehyde oxidase | Aox1 | 0.0038 | −1.6 | Omega-oxidation of fatty acids |
| Neurofascin | Nfasc | 0.0039 | −3.5 | Adhesion molecule; synapse formation |
| Cytochrome P450, subfamily 2F, polypeptide 1 | Cyp2f1 | 0.0078 | −5.7 | Aromatic hydrocarbon metabolism |
| Cytochrome P450, subfamily 11B, polypeptide 3 | Cyp11b3 | 0.0491 | −1.6 | Steroid synthesis (neonatal only?) |
| Mevalonate kinase | Mvk | 0.0758 | −2.2 | Cholesterol biosynthesis |
| Aquaporin 9 | Aqp9 | 0.0854 | −3.5 | Solute channel |
| Regulator of G-protein signaling protein 2 | Rgs2 | 0.0873 | −1.5 | GTPase activating protein |
| Cathepsin C | Ctsc | 0.0908 | −2.3 | Dipeptidyl aminopeptidase |
| Hypocretin (orexin) receptor 2 | Hcrtr2 | 0.0908 | −1.7 | G-protein coupled receptor |
| Nestin | Nes | 0.0953 | −1.6 | Intermediate filament protein |
| Plasminogen activator, urokinase receptor | Plaur | 0.1204 | −3.4 | Tumor progression; neuronal differentiation |
| 3-alpha-hydroxysteroid dehydrogenase | LOC191574 | 0.1204 | −1.3 | Steroid metabolism |
| Thymosin, beta 10 | Tmsb10 | 0.1204 | −1.5 | Inhibits actin polymerization |
| Uncoupling protein 1 | Ucp1 | 0.1250 | −3.2 | Thermogenesis; uncouples oxidative phosphorylation |
| Hydroxysteroid 11-beta dehydrogenase 2 | Hsd11b2 | 0.1363 | −1.4 | Steroid Metabolism |
| Cytochrome P450 4F6 | Cyp4f6 | 0.1424 | −1.5 | Leukotriene metabolism |
| Jun B proto-oncogene | Junb | 0.1487 | −1.5 | Transcription factor |
| Cytochrome c oxidase subunit VIII-H(heart/muscle) | Cox8h | 0.1502 | −7.9 | Electron transport/ oxidative phosphorylation |
| Cholinergic receptor, muscarinic 3 | Chrm3 | 0.1502 | −1.7 | Phospholipase C activator |
| PAK-interacting exchange factor beta | Pak3bp | 0.1564 | −7.3 | Mediates neuronal growth; guanine nucleotide exchange factor |
| Hypocretin receptor 1 | Hcrtr1 | 0.1846 | −1.5 | G-protein coupled receptor |
| Potassium inwardly-rectifying channel | Kcnk4 | 0.2137 | −3.6 | Activated by PLA2 metabolites; putative oxygen sensor |
| Cholinergic receptor, nicotinic, alpha polypeptide 4 | Chrna4 | 0.2248 | −3.3 | Calcium-sensing subunit |
| Solute carrier family 6, member 5 | Slc6a5 | 0.2257 | −3.1 | Glycine transporter; neuronal |
| Cytochrome b5, outer mitochondrial membrane | Omb5 | 0.2380 | −1.6 | Steroid metabolism |
Gene expression was either increased or decreased by exposure to hypoxia from birth to PD7. Functional designations were assigned based on information obtained from Expression Analysis Systematic Explorer (EASE) software and the National Center for Biotechnology Information database (http://www.ncbi.nlm.nih.gov). All genes listed exhibited at least a ±1.3 fold-change difference in expression (compared to normoxic control) and an FDR ≤ 0.25. Pooled adrenal glands were used for analyses (N=4 pooled samples/treatment group).
Table 2B lists significant decreases in adrenal gene expression in pups exposed to hypoxia from birth. The expression of genes encoding proteins with oxidoreductase activity (Cyp2f1, Cyp11b3, Hsd11b2, Cyp4f6, and Cox8h) was decreased. Interestingly, the expression of Cox8h (cytochrome c oxidase subunit; mitochondrial) was decreased nearly eight-fold, the largest fold-change measured. The expression of Ucp1 and Omb5 was also decreased by hypoxia. These genes encode mitochondrial proteins involved in oxidative phosphorylation and steroid metabolism, respectively. Other examples of decreased expression include Mvk (cholesterol biosynthesis) and Pla2g4a and Rgs2 (intracellular signaling), as well as that of Cdh1, Nfasc, Nes, and Tmsb10 (cellular adhesion and cytoskeleton-associated proteins).
Table 3 lists real-time PCR results, expressed as a percent of control (Normoxia). Calculated delta Ct values are also included in Table 3. Real-time PCR was initially used to verify selected microarray results. Twelve genes were selected based on their possible contribution to the functional endpoint of the study (i.e. increased plasma corticosterone). Three of the twelve PCR results did not agree with those indicated by microarray (Gmpr, Rgs2, and Cpt2). This discordance was confirmed by a second PCR assay for each of the three genes. The other nine results from real-time PCR showed trends that agreed with the microarray; however, only two of them reached statistical significance (Cyp1b1 and Nsf).
Table 3.
Real-time PCR analysis of adrenal gene expression: verification of selected microarray results.
| Hypoxia vs. Normoxia (food ad libitum) | Mean Delta Ct | |||
|---|---|---|---|---|
| Gene | Symbol | Percent of Normoxia | Normoxia | Hypoxia |
| Cytochrome P450, subfamily 1B, polypeptide 1 | Cyp1b1 | 197* | 13.53 ± 0.15 | 12.55 ± 0.04 |
| Guanosine monophosphate reductase | Gmpr | 67 1 | 15.73 ± 0.20 | 16.34 ± 0.14 |
| Xanthine dehydrogenase | Xdh | 137 | 19.77 ± 0.16 | 19.33 ± 0.22 |
| Cadherin 1 | Cdh1 | 62 | 19.58 ± 0.34 | 20.27 ± 0.31 |
| Phospholipase A2, group IVA (cytosolic) | Pla2g4a | 73 | 18.85 ± 0.28 | 19.32 ± 0.09 |
| Aldehyde oxidase | Aox1 | 84 | 18.94 ± 0.12 | 19.19 ± 0.28 |
| Regulator of G-protein signaling protein 2 | Rgs2 | 100 1 | 16.16 ± 0.11 | 16.14 ± 0.11 |
| Carnitine palmitoyltransferase 2 | Cpt2 | 94 1 | 12.76 ± 0.05 | 12.84 ± 0.11 |
| Steroid 5 alpha-reductase 1 | Srd5a1 | 130 | 15.29 ± 0.16 | 14.91 ± 0.12 |
| Enolase 1, alpha | Eno1 | 135 | 11.63 ± 0.12 | 11.20 ± 0.16 |
| N-ethylmaleimide sensitive factor | Nsf | 130* | 18.03 ± 0.06 | 17.65 ± 0.11 |
| Cytochrome P450, subfamily 11B, polypeptide 3 | Cyp11b3 | 67 | 9.75 ± 0.14 | 10.30 ± 0.25 |
Whole adrenal glands were pooled (24 glands or one litter/sample) and total RNA was extracted according to the manufacturer's protocol. Genes picked for verification with real-time PCR were chosen based on their possible significance in adrenal function. Real-time PCR results that did not agree with microarray results were re-assayed. Data are presented as percent of control (Normoxia).
Significant difference from Normoxia with p<0.05.
Result did not agree with microarray result (see Discussion). N=4 per treatment group.
Note: an increase in the Delta Ct value represents a decrease in mRNA expression.
The expression of genes that encode proteins involved in adrenal function or the cellular response to hypoxia was also assayed by real-time PCR (Table 4), even though these genes were not considered significant by microarray analysis. Interestingly, hypoxia from birth had a tendency to increase the expression of StAR, Cyp11a, Ldlr, and Vegf (p>0.05). The only statistically significant changes in expression were for Cyp21a1 and Cyp1b1 (p<0.05). The effects of maternal food restriction per se (Normoxia vs. Normoxia Pair Fed) on genes involved in adrenal function included significant decreases in StAR, Cyp11a, Cyp1b1, and Cyp21a1 (p<0.05). Comparison of PCR results between Hypoxia and Normoxia Pair Fed treatments showed that the expression of StAR, Cyp21a1, Cyp1b1, and Ldlr were significantly increased (p<0.05). These comparisons provide a more detailed description of the effects of hypoxia per se by accounting for decreased maternal food intake associated with hypoxic exposure.
Table 4.
Real-time PCR analysis of adrenal gene expression: analysis of genes not meeting microarray cutoff criteria.
| Mean Delta Ct | |||||||
|---|---|---|---|---|---|---|---|
| Gene | Symbol | Hypoxia vs. Normoxia (food ad libitum) | Normoxia Pair Fed vs. Normoxia (food ad libitum) | Hypoxia vs Normoxia Pair Fed | Normoxia (ad libitum) | Hypoxia | Normoxia Pair Fed |
| Cytochrome P450, subfamily 1B, polypeptide 1 | Cyp1b1 | 158* | 50 | 313* | 11.52 ± 0.28 | 10.87 ± 0.32 | 12.51 ± 0.21 |
| Steroidogenic acute regulatory protein | StAR | 148 | 60* | 244* | 8.29 ± 0.05 | 7.72 ± 0.21 | 9.02 ± 0.29 |
| Cytochrome P450, subfamily 11A | Cyp11a | 117 | 72* | 164 | 9.09 ± 0.14 | 8.86 ± 0.18 | 9.57 ± 0.17 |
| Cytochrome P450, subfamily 21A, polypeptide 1 | Cyp21a1 | 139* | 67* | 208* | 9.55 ± 0.09 | 9.07 ± 0.02 | 10.12 ± 0.12 |
| Low density lipoprotein receptor | Ldlr | 153 | 58 | 233* | 20.24 ± 0.16 | 19.82 ± 0.21 | 21.03 ± 0.30 |
| Vascular endothelial growth factor | Vegf | 117 | 86 | 135 | 15.74 ± 0.03 | 15.52 ± 0.16 | 15.95 ± 0.10 |
| Hypoxia-inducible factor 1 alpha | Hif1a | 100 | 83 | 120 | 12.59 ± 0.08 | 12.58 ± 0.19 | 12.86 ± 0.15 |
Whole adrenal glands were pooled (24 glands/sample) and total RNA was extracted according to the manufacturer's protocol. Data are presented as percent of control (Normoxia or Normoxia Pair Fed). Genes assayed for these real-time PCR analyses were chosen based on their known roles in the steroidogenic pathway or in the cellular response to hypoxia.
Significant difference from Normoxia or Normoxia Pair Fed with p<0.05. N=4 per treatment group.
Note: an increase in the Delta Ct value represents a decrease in mRNA expression.
DISCUSSION
The present study analyzed the effects of hypoxia from birth on gene expression in the adrenal gland of the neonatal rat. Microarray analysis revealed changes in the expression of many genes encoding proteins that utilize oxygen, play a role intermediary metabolism and oxidative phosphorylation, and/or participate in adrenomedullary function. While microarray analysis did not reveal significant effects of hypoxia on steroidogenic gene expression when compard to normoxia (food ad libitum), real-time PCR detected a significant increase in Cyp21a1 expression and a tendency towards increased expression of StAR, Cyp11a, and Ldlr. Maternal food restriction in normoxic dams, used to account for the anorectic effects of hypoxia, had no effect on plasma ACTH or corticosterone concentrations in suckling pups. Comparisons between Hypoxia and Normoxia Pair Fed groups showed that, when normalized for maternal food intake, hypoxia had a more profound stimulatory effect on StAR, Cyp21a1, and Ldlr expression.
There was a small but significant increase in plasma ACTH concentrations in hypoxic pups in the current study. We have previously shown either no or only a small increase in ACTH using our experimental model (25-27). We are confident that the large increase in plasma corticosterone is due to increased adrenal sensitivity to ACTH, at least partly mediated by the sympathetic nervous system (27). In addition, changes in adrenal gene expression described below were probably not mediated by small increases in plasma ACTH, since similar changes were not observed with pharmacological doses of ACTH in neonatal rat pups (16). Therefore, the effects described next appear to be primarily mediated by hypoxia.
Microarray Results
In an effort to further the understanding of hypoxia-induced increases in plasma corticosterone, a broad approach (i.e. microarray) was used to analyze changes in adrenal gene expression. A similar approach has been used to study the effects of chronic ACTH treatment in rat pups (16). None of the genes that encode proteins of the corticosterone synthetic pathway were significantly affected by hypoxia according to this method. Chronic ACTH treatment in rat pups was also without effect on these genes (16). Two genes whose products are involved in further metabolism of corticosterone, Srd5a1 (steroid 5α-reductase) and LOC191574 (3αHSD), were significantly increased and decreased by hypoxia, respectively. An increase in plasma concentrations of 5α-dihydrocorticosterone (5αTHB; the product of steroid 5α-reductase) could influence physiological responses to hypoxia, since it has been shown that 5αTHB binds to and activates the glucocorticoid receptor (GR) (20). A decrease in the expression of Hsd11b2 (11βHSD2) was also detected, and may indicate decreased conversion of corticosterone to its inactive metabolite in the adrenal cortex.
Hypoxia affected the expression of a number of genes encoding proteins that take part in intermediary metabolism and oxidative phosphorylation (microarray data). Expression of Aldoc (Aldolase C) and Eno1 (Enolase 1α), enzymes involved in the glycolytic pathway, was significantly increased. In addition, the expression levels of Got1 (glutamate oxaloacetate transaminase 1), Cpt2 (carnitine palmitoytransferase 2), Fmo1 (flavin-containing monooxygenase 1), and Cox4b (cytochrome oxidase c, subunit 4b), all involved in mitochondrial respiration, were increased by hypoxia. These findings suggest that hypoxia may increase substrate available for mitochondrial respiration, thus fostering the concomitant increase in steroid production. Recent studies provided evidence that Leydig cell steroidogenesis requires actively respiring mitochondria that maintain the mitochondrial membrane potential (Δψm) (1).
Interestingly, the expression of Ucp1 (uncoupling protein 1) was significantly decreased in response to hypoxia. Ucp1 dissipates the proton gradient required for mitochondrial ATP synthesis and is involved in thermogenesis, but is normally associated with mitochondria of adipose, heart, and muscle tissue (11, 21). Our detection of Ucp1 mRNA in the neonatal rat adrenal may be a novel finding. A decrease in Ucp1 expression, along with the changes noted above, suggests that neonatal adrenal mitochondria may operate more efficiently when exposed to a moderately low oxygen environment. This phenomenon could augment steroid production as proposed above. An argument against this mitochondrial theory would be the nearly eight-fold decrease in Cox8h (cytochrome c oxidase, subunit VIII-H); however, interpretation of these findings must be done with caution. It is difficult to assign cause and effect when dealing with a multitude of changes in gene expression as in the present study.
Since whole adrenal glands were pooled for microarray and real-time PCR analyses, a brief discussion of genes linked to adrenal neural and adrenomedullary function is pertinent. It is also pertinent since we have previously shown that hypoxia-induced increases in plasma corticosterone were attenuated with guanethidine administration, which blocks post-ganglionic sympathetic innervation of the adrenal cortex (27, 33). Subsequent studies failed to measure any hypoxia-induced changes in plasma catecholamine concentrations, although there was a tendency towards increased plasma epinephrine (5). Hypoxia increased the expression of Dbh (dopamine β-hydroxylase), Nsf (N-ethylmaleimide sensitive fusion protein), and Slc18a2 (solute carrier family 18, member 2) in the present study. Dbh encodes the enzyme responsible for the conversion of dopamine to norepinephrine (NE), and an increase in its expression may indicate hypoxia-induced adrenal NE synthesis. The proteins encoded by Slc18a2 and Nsf function in vesicular monoamine transport, a prerequisite to the exocytic pathway involved in monoamine release (2, 7, 12, 29). Taken together with our previous studies with guanethidine (27), these findings may indicate increased adrenocortical sympathetic input and/or increased chromaffin cell catecholamine production during hypoxia. These changes, in concert with increases in steroidogenic gene expression, may contribute to the stimulation of corticosterone production. An interesting possibility is that increased corticosterone during hypoxia may alter adrenomedullary gene expression and function via paracrine effects (37). Additional studies will be required to evaluate this possible mechanism.
Real-Time PCR vs. Microarray- Concordant Results
A dozen genes that were significantly affected by hypoxia, according to microarray results, were chosen for confirmation with real-time PCR. Genes were selected based on involvement in intra- and inter-cellular pathways that may have an influence on adrenal steroid production (Table 3). We confirmed that hypoxia significantly increased the expression of Cyp1b1 and Nsf (N-ethylmaleimide sensitive factor). Cyp1b1 is probably not involved directly in steroidogenesis (3); however, it may be involved in O2 metabolism as it does alter the metabolism of aromatic hydrocarbons. The stimulation of Cyp1b1 expression during hypoxia overrides the normal developmental suppression of the expression of this gene, as shown previously (3). There was a tendency for increased expression of Xdh, Srd5a1, and Eno1 using real-time PCR, but the differences were not considered significant. There was also a tendency for decreased expression of Cdh1, Pla2g4a, Aox1, and Cyp11b3. While agreeing with the trends measured using microarray, these PCR results did not reach statistical significance.
Real-Time PCR vs. Microarray- Discordant Results
Comparison of microarray and real-time PCR results revealed discordance for three of the twelve genes selected (Gmpr, Rgs2, and Cpt2). In the case of Rgs2, microarray results indicated a decrease in expression (−1.5-fold) while real-time PCR revealed no change. The expression level of Cpt2 was increased by hypoxia according to the microarray, but it was decreased when assayed using real-time PCR. In the third case, microarray detected a significant increase in Gmpr expression while real-time PCR results indicated a decrease, albeit not significant. Real-time PCR were verified by running a second assay for each gene. The discordance of these results may be explained by the decreased sensitivity of the microarray per se, or by the relatively low expression levels of these genes.
Expression of Steroidogenic Genes and Functional Correlates
Using real-time PCR, a more sensitive method of detection, we measured hypoxia-induced changes in the expression of genes involved in adrenocortical function. Hypoxia from birth to PD7, compared to Normoxia (food ad libitum), tended to increase the expression of StAR, Cyp11a, and Ldlr. The only significant increases in expression were with Cyp21a1 and Cyp1b1. It is possible that the measured increase in Cyp21a1 mRNA expression was due to the detection of genomic DNA (see Table 1). These results corroborate the finding of increased plasma corticosterone concentrations and increased sensitivity to ACTH (25, 27), and indicate that increased steroid production due to hypoxia is at least partly mediated by changes in adrenal gene expression. We have previously shown that zona fasciculata cells from 7-day-old pups exposed to hypoxia from birth exhibit increased corticosterone production (compared to cells from normoxic pups) when studied in vitro (24). These increases were probably driven by increased early pathway activity (i.e. pregnenolone production) (26). This fits with the current findings of increased expression of Ldlr, StAR, and Cyp11a mRNA, as these genes encode proteins involved in cholesterol uptake, transport into mitochondria, and metabolism to pregnenolone, respectively.
Expression of Hypoxia-Responsive Genes
Hypoxia had no effect on the expression of Hif1a (hypoxia-inducible factor 1α) or Vegf (vascular endothelial growth factor), although the expression of Vegf tended to be increased by hypoxia. The fact that Hif1a mRNA expression was not altered by hypoxia does not indicate that HIF-1α protein expression also remained unaffected. It is the protein itself that is stabilized in response to acute decreases in oxygen concentration (31), and the transcriptional effects of this stabilization may normalize at some point during the hypoxic treatment used in the present study. The Vegf gene is a target of the HIF-1α protein (31) and expression of Vegf mRNA would be expected to increase in hypoxic tissues in order to maximize perfusion. Hypoxia has been shown to increase adrenal blood flow in dogs (4) and the present results with Vegf expression, though not statistically significant, may support this phenomenon.
Hypoxia and Maternal Food Restriction
Our laboratory has consistently shown that hypoxia from birth to PD7 attenuates weight gain in rat pups (5, 23, 27). The nature of our experimental model necessitates placing the dam in the hypoxic chamber with her pups. Therefore, examination of the effects of hypoxia on the dam is necessary. It was not surprising to find that hypoxia-induced anorexia was most profound at the onset of the hypoxic exposure (PD1-PD2). Hypoxic dam food intake remained significantly lower than normoxic controls throughout the study, even though hypoxic dams increased their food intake daily from PD3-6. Pups of normoxic dams pair-fed to match hypoxic dam intake did not exhibit any changes in plasma ACTH or corticosterone. Body weights of these pups were also no different from those reared by dams fed ad libitum. This confirmed that the effects of hypoxia on the neonatal HPA axis were not due to reduced maternal food intake.
When the anorexic effect of hypoxia on the dam was accounted for (Hypoxia vs. Normoxia Pair Fed) using the real-time PCR results, the magnitude of the increases in gene expression became greater and more genes (StAR, Cyp21a1, Ldlr, and Cyp1b1) were significantly different. This phenomenon suggests that hypoxia-induced decreases in maternal food intake did not play a major role in the modulation of adrenal function in pups. Pups of pair fed dams did not exhibit elevated plasma corticosterone concentrations, the functional endpoint of adrenocortical function. Maternal food restriction in lactating dams has been shown to decrease the plasma concentration of corticosterone-binding globulin (CBG) in pups, and was thought to play a reinforcing role in the suppression of HPA axis activity in the pups (18). We have not found any changes in CBG concentrations in pups using our experimental model of neonatal hypoxia (25).
Summary
The present study used microarray and real-time PCR analyses to examine changes in adrenal gene expression in the hypoxic neonatal rat. We also measured the effect of hypoxia on maternal food intake, accounting for the anorectic effects of hypoxia. Hypoxia from birth to seven days of age significantly increased plasma corticosterone and elicited a small but significant increase in plasma ACTH. Matching normoxic dam food intake to that of hypoxic dams had no effect on plasma ACTH or corticosterone concentrations in seven-day-old pups. Real-time PCR analysis measured a significant increase in the expression of Cyp21a1 in the hypoxic adrenal. The effects of hypoxia became more pronounced when accounting for hypoxia-induced anorexia (e.g. significant increases in StAR, Cyp21a1, and Ldlr expression). Microarray results revealed significant changes in the expression of genes involved in both adrenocortical and adrenomedullary function. We postulate that hypoxia induces shifts in adrenal cellular function such as mitochondrial respiration and vesicular trafficking that, together, act in concert with augmented cholesterol uptake to increase sensitivity to ACTH and corticosterone output.
Acknowledgements
The authors would like to thank Barbara M. Jankowski and Peter J. Homar for their expert technical assistance.
The authors would also like to thank Dr. Mingyu Liang for his expert advice.
This study was supported in part by NIH Grant DK54685 to HR, NIH Grant DK55793 to EPW, and Aurora St. Luke's Medical Center.
REFERENCES
- 1.Allen JA, Shankara T, Janus P, Buck S, Diemer T, Held Hales K, Hales DB. Energized, polarized, and actively respiring mitochondria are required for acute Leydig cell steroidogenesis. Endocrinology. 2006;147:3924–3935. doi: 10.1210/en.2005-1204. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee A, Barry VA, DasGupta BR, Martin TFJ. N-ethylmaleimide-sensitive factor acts at a prefusion ATP-dependent step in Ca2+-activated exocytosis. J Biol Chem. 1996;271:20223–20226. doi: 10.1074/jbc.271.34.20223. [DOI] [PubMed] [Google Scholar]
- 3.Brake PB, Arai M, As-Sanie S, Jefcoate CR, Widmaier EP. Developmental expression and regulation of adrenocortical cytochrome P4501B1 in the rat. Endocrinology. 1999;140:1672–1680. doi: 10.1210/endo.140.4.6628. [DOI] [PubMed] [Google Scholar]
- 4.Breslow MJ, Ball TD, Miller CF, Raff H, Traystman RJ. Adrenal blood flow and secretory relationships during hypoxia in anesthetized dogs. Am J Physiol Heart Circ Physiol. 1989;257:H1458–H1465. doi: 10.1152/ajpheart.1989.257.5.H1458. [DOI] [PubMed] [Google Scholar]
- 5.Bruder ED, Henderson LM, Raff H. Adrenal lipid profiles of chemically sympathectomized normoxic and hypoxic neonatal rats. Horm Metab Res. 2006;38:807–811. doi: 10.1055/s-2006-956183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruder ED, Lee PC, Raff H. Metabolomic analysis of adrenal lipids during hypoxia in the neonatal rat: implications in steroidogenesis. Am J Physiol Endocrinol Metab. 2004;286:E697–E703. doi: 10.1152/ajpendo.00502.2003. [DOI] [PubMed] [Google Scholar]
- 7.Burgoyne RD, Williams G. NSF and SNAP are present on adrenal chromaffin granules. FEBS Letters. 1997;414:349–352. doi: 10.1016/s0014-5793(97)01031-4. [DOI] [PubMed] [Google Scholar]
- 8.Chiodi H, Whitmore S. Lipid metabolism in suckling rats with fatty liver induced by hypoxia. Experientia. 1974;30:463–465. doi: 10.1007/BF01926294. [DOI] [PubMed] [Google Scholar]
- 9.Frankel L, Stevenson DK. Metabolic emergencies of the newborn: hypoxemia and hypoglycemia. Compr Ther. 1987;13:14–19. [PubMed] [Google Scholar]
- 10.Friedman AH, Fahey JT. The transition from fetal to neonatal circulation: normal responses and implications for infants with heart disease. Semin Perinatol. 1993;17:106–121. [PubMed] [Google Scholar]
- 11.Gnaiger E, Mendez G, Hand SC. High phosphorylation efficiency and depression of uncoupled respiration in mitochondria under hypoxia. Proc Nat Acad Sci. 2000;97:11080–11085. doi: 10.1073/pnas.97.20.11080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanson SR, Mezey E, Hoffman BJ. Ontogeny of vesicular monoamine transporter mRNAs VMAT1 and VMAT2. II. Expression in neural crest derivatives and their target sites in the rat. Brain Res Develop Brain Res. 1998;110:159–174. doi: 10.1016/s0165-3806(98)00103-5. [DOI] [PubMed] [Google Scholar]
- 13.Harris RJ. Plasma nonesterified fatty acid and blood glucose levels in healthy and hypoxemic newborn infants. Fetal Neonatal Med. 1974;84:578–584. doi: 10.1016/s0022-3476(74)80685-2. [DOI] [PubMed] [Google Scholar]
- 14.Henry JP, Sagne C, Bedet C, Gasnier B. The vesicular monoamine transporter: from chromaffin granule to brain. Neurochem Internat. 1998;32:227–246. doi: 10.1016/s0197-0186(97)00092-2. [DOI] [PubMed] [Google Scholar]
- 15.Hingre RV, Gross SJ, Hingre KS, Mayes DM, Richman RA. Adrenal steroidogenesis in very low birth weight preterm infants. J Clin Endocrinol Metab. 1994;78:266–270. doi: 10.1210/jcem.78.2.8106610. [DOI] [PubMed] [Google Scholar]
- 16.Lee JJ, Widmaier EP. Gene array analysis of the effects of chronic adrenocorticotropic hormone in vivo on immature rat adrenal glands. Steroid Biochem Mol Biol. 2005;96:31–44. doi: 10.1016/j.jsbmb.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 17.Lee PC, Jelinek B, Struve M, Bruder ED, Raff H. Effect of neonatal hypoxia on the development of hepatic lipase in the rat. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1341–R1347. doi: 10.1152/ajpregu.2000.279.4.R1341. [DOI] [PubMed] [Google Scholar]
- 18.Leonhardt M, Lesage J, Dufourny L, Dickes-Coopman A, Montel V, Dupouy JP. Perinatal maternal food restriction induces alterations in hypothalamo-pituitary-adrenal axis activity and in plasma corticosterone-binding globulin capacity of weaning rat pups. Neuroendocrinology. 2002;75:45–54. doi: 10.1159/000048220. [DOI] [PubMed] [Google Scholar]
- 19.Low JA, Froese AB, Galbraith RS, Smith JT, Sauerbrei EE, Derrick EJ. The association between preterm newborn hypotension and hypoxemia and outcome during the first year. Acta Paediatr. 1993;82:433–437. doi: 10.1111/j.1651-2227.1993.tb12717.x. [DOI] [PubMed] [Google Scholar]
- 20.McInnes KJ, Kenyon CJ, Chapman KE, Livingstone DEW, MacDonald LJ, Walker BR, Andrew R. 5α-reduced glucocorticoids, novel endogenous activators of the glucocorticoid receptor. J Biol Chem. 2004;279:22908–22912. doi: 10.1074/jbc.M402822200. [DOI] [PubMed] [Google Scholar]
- 21.Morsicot A, Rabelo R, Bianco AC. Corticosterone inhibits uncoupling protein gene expression in brown adipose tissue. Am J Physiol Endocrinol Metab. 1993;265:E81–E87. doi: 10.1152/ajpendo.1993.265.1.E81. [DOI] [PubMed] [Google Scholar]
- 22.Mortola JP. How newborn mammals cope with hypoxia. Respir Physiol. 1999;116:95–103. doi: 10.1016/s0034-5687(99)00038-9. [DOI] [PubMed] [Google Scholar]
- 23.Raff H, Bruder ED, Jankowski BM, Colman RJ. Effect of neonatal hypoxia on leptin, insulin, growth hormone, and body composition in the rat. Horm Metab Res. 2001;33:151–155. doi: 10.1055/s-2001-14929. [DOI] [PubMed] [Google Scholar]
- 24.Raff H, Bruder ED, Jankowski BM, Goodfriend TL. Neonatal hypoxic hyperlipidemia in the rat: effects on aldosterone and corticosterone synthesis in vitro. Am J Physiol Regul Integr Comp Physiol. 2000;278:R663–R668. doi: 10.1152/ajpregu.2000.278.3.R663. [DOI] [PubMed] [Google Scholar]
- 25.Raff H, Hong JJ, Oaks MK, Widmaier EP. Adrenocortical response to ACTH in neonatal rats: effect of hypoxia from birth on corticosterone, StAR, and PBR. Am J Physiol Regul Integr Comp Physiol. 2003;284:R78–R85. doi: 10.1152/ajpregu.00501.2002. [DOI] [PubMed] [Google Scholar]
- 26.Raff H, Jankowski BM, Bruder ED, Engeland WC, Oaks MK. The effect of hypoxia from birth on the regulation of aldosterone in the 7-day-old rat: plasma hormones, steroidogenesis in vitro, and steroidogenic enzyme messenger ribonucleic acid. Endocrinology. 1999;140:3147–3153. doi: 10.1210/endo.140.7.6794. [DOI] [PubMed] [Google Scholar]
- 27.Raff H, Lee JJ, Widmaier EP, Oaks MK, Engeland WC. Basal and ACTH-stimulated corticosterone in the neonatal rat exposed to hypoxia from birth: modulation by chemical sympathectomy. Endocrinology. 2004;145:79–86. doi: 10.1210/en.2003-1130. [DOI] [PubMed] [Google Scholar]
- 28.Raff H, Sandri RB, Segerson TP. Renin, ACTH, and adrenocortical function during hypoxia and hemorrhage in conscious rats. Am J Physiol Regul Integr Comp Physiol. 1986;250:R240–R244. doi: 10.1152/ajpregu.1986.250.2.R240. [DOI] [PubMed] [Google Scholar]
- 29.Sabban EL, Nankova BB, Serova LI, Kvetnansky R, Liu X. Molecular regulation of gene expression of catecholamine biosynthetic enzymes by stress: sympathetic ganglia versus adrenal medulla. Ann NY Acad Sci. 2004;1018:370–377. doi: 10.1196/annals.1296.046. [DOI] [PubMed] [Google Scholar]
- 30.Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res Rev. 1986;11:65–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- 31.Semenza GL. Hypoxia-inducible factor 1: control of oxygen homeostasis in health and disease. Pediatr Res. 49(5):614–6177. doi: 10.1203/00006450-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Thomas T, Marshall JM. A study on rats of the effects of chronic hypoxia from birth on respiratory and cardiovascular responses evoked by acute hypoxia. J Physiol. 1995;487:513–525. doi: 10.1113/jphysiol.1995.sp020896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker CD. Chemical sympathectomy and maternal separation affect neonatal stress responses and adrenal sensitivity to ACTH. Am J Physiol Regul Integr Comp Physiol. 1995;268:R1281–R1288. doi: 10.1152/ajpregu.1995.268.5.R1281. [DOI] [PubMed] [Google Scholar]
- 34.Walker CD, Scribner KA, Cascio CS, Dallman MF. The pituitary adrenocortical system of neonatal rats is responsive to stress throughout development in a time-dependent and stressor-specific fashion. Endocrinology. 1991;128:1385–1395. doi: 10.1210/endo-128-3-1385. [DOI] [PubMed] [Google Scholar]
- 35.Watterberg KL, Gerdes JS, Cook KL. Impaired glucocorticoid synthesis in premature infants developing chronic lung disease. Pediatr Res. 2001;50:190–195. doi: 10.1203/00006450-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Zilz A, Li H, Castello R, Papadopoulos V, Widmaier EP. Developmental expression of the peripheral-type benzodiazepine receptor and the advent of steroidogenesis in rat adrenal glands. Endocrinology. 1999;140:859–864. doi: 10.1210/endo.140.2.6475. [DOI] [PubMed] [Google Scholar]
- 37.Zuckerman-Levin N, Tiosano D, Eisenhofer G, Bornstein S, Hochberg Z. The importance of adrenocortical glucocorticoids for the adrenomedullary and physiological response to stress: a study in isolated glucocorticoid deficiency. J Clin Endocrinol Metab. 2001;86:5920–5924. doi: 10.1210/jcem.86.12.8106. [DOI] [PubMed] [Google Scholar]