Abstract
Background
Recent studies have demonstrated that aging is associated with reduced tolerance to ischemia and that the aged (not senescent) female heart has greater susceptibility to ischemia as compared with the aged male heart. Previously, we have shown that ischemia can be modulated with cardioplegia in the male heart; however, efficacy in the female heart was unknown.
Methods
In this study, male and female mature (15 to 20 weeks) aged (>32 months) rabbit hearts (n = 134) were subjected to Langendorff perfusion. Control hearts were perfused for 180 minutes. Global ischemia hearts received 30 minutes of equilibrium, 30 minutes of global ischemia, and 120 minutes of reperfusion. Cardioplegia ± diazoxide was infused separately, 5 minutes before global ischemia.
Results
Global ischemia significantly decreased post-ischemic functional recovery and significantly increased infarct size in the mature and aged male and female heart (p < 0.05 versus control). The effects of global ischemia were significantly exacerbated (p < 0.05) in the aged heart as compared with the mature heart. Cardioplegia ± diazoxide significantly increased postischemic functional recovery and significantly decreased infarct size in mature male and female hearts, but these effects were significantly decreased in the aged heart (p < 0.05) and in the aged female as compared with the aged male heart.
Conclusions
Postischemic functional recovery and infarct size are affected by age but not by gender. The cardioprotection afforded by cardioplegia is affected by age and gender with a strong age-by-gender interaction for end-diastolic pressure and infarct size. Our results indicate that currently optimized cardioplegia protocols effective in the male heart are not as efficacious in the aged female heart.
The efficacy of cardioplegia has been shown to vary with age. However, few data are available on the effects of gender [1–3]. In the United States and Canada, the mean age of patients requiring coronary artery bypass surgery is 68 years, with approximately 44% of these patients being female [1, 2, 4, 5]. Analysis of preoperative risk variables contributing to postoperative morbidity and mortality indicates that patients aged 70 years or older are at significantly greater risk than those less than 60 years of age, and census-based projections indicate that both mean age and the proportion of females requiring cardiac surgery will increase in the coming years [4, 5].
Recent studies have shown that women have a significantly greater risk potential as compared with men [4, 5], and that women have worse outcomes after cardiac surgery [4, 5]. The Society of Thoracic Surgeons National Cardiac Surgery Database provides evidence that women undergoing coronary artery bypass grafting surgery have a significantly higher operative mortality rate (4.5%) compared with men (2.6%; p < 0.0001) [4, 5]. Multivariate analysis also shows that women have higher mortality rates than equally matched men in low-risk and medium-risk groups [1, 6]. It is only among very high risk patients that sex is not found to be an independent predictor of adverse outcomes [1, 6]. Not only is postoperative mortality higher among women, but also postoperative morbidity and long-term survival are generally less favorable in women compared with men undergoing coronary artery bypass grafting surgery [6]. These data suggest that current cardioprotective procedures are inadequate, and further investigation is required to identify mechanisms to reduce morbidity and mortality in the aged female patient.
In previous reports, we have shown that magnesium-supplemented potassium (K/Mg) cardioplegia is superior to high-potassium cardioplegia in the mature and aged male rabbit heart [7–10]. We have also shown that the cardioprotection afforded by K/Mg cardioplegia is modulated by mitochondrial adenosine triphosphate (ATP)–sensitive potassium (mitoKATP) channels and that the addition of diazoxide, a mitoKATP channel opener, to K/Mg cardioplegia (K/Mg+DZX) to allow for the early or enhanced opening of mitoKATP channels significantly enhances the cardioprotection afforded by K/Mg cardioplegia alone [7–10]. In this paper, we investigate the effects of cardioplegia in the mature (sexually mature) and aged (not senescent) male and female heart and provide comparative analysis to determine if cardioplegia protocols effective in the male heart provided similar cardioprotection in the female heart.
Material and Methods
Animals
Male (n = 38) and female (n = 28 )mature (sexually mature, 15 to 20 weeks; weight, 3 to 4 kg) and male (n = 40) and female (n = 28 ) aged (not senescent, older than 32 months; weight, 5 to 6 kg) New Zealand white rabbits were obtained from Millbrook Farm, Amherst, Massachusetts. The classification of mature and aged is derived from normative rabbit data meeting accepted criteria for aging (Laboratory Animal Medicine, 2nd edition [11]). All experiments were approved by the Beth Israel Deaconess Medical Center Animal Care and Use Committee and the Harvard Medical Area Standing Committee on Animals and conformed to the US National Institutes of Health “Guide for the Care and Use of Laboratory Animals” (NIH publication no. 5377-3, 1996). All research was performed in accordance with the American Physiological Society “Guiding Principles in the Care and Use of Animals.”
Langendorff Perfusion
All rabbits were anesthetized with pentobarbital sodium (Nembutal, 100 mg/kg) and heparin (200 U/kg) intravenously through the marginal ear vein [7, 10]. Langendorff retrograde perfusion was performed as previously described [7, 10]. Hearts were paced through the right atrium so that heart rate was maintained at 180 ± 3 beats per minute throughout the experiment using a Medtronic Model 5330 stimulator (Medtronic, Minneapolis, Minnesota). Hemodynamic variables were acquired using the PO-NE-MAH digital data acquisition system (Gould, Valley View, Ohio), with an Acquire Plus processor board, and left ventricular pressure analysis software [7, 10].
Experimental Protocol
Hearts (n = 134) consisting of mature male (n = 10 each group), mature female (n = 7 each group), aged male (n = 10 each group) and aged female (n = 7 each group) were perfused for 30 minutes to establish equilibrium hemodynamics (Fig 1). Equilibrium was achieved when left ventricular end-diastolic pressure (LVEDP) and left ventricular peak developed pressure (LVPDP) were maintained at the same level for three continuous measurement periods timed 5 minutes apart. Control hearts were perfused without global ischemia at 37°C for 180 minutes. Global ischemia hearts were subjected to 30 minutes of global ischemia and 120 minutes of reperfusion. Global ischemia was achieved by cross-clamping the perfusion line. The K/Mg hearts received normothermic (37°C) cardioplegia (K+ 20 mmol/L, Mg2+ 20 mmol/L in Krebs-Ringer solution) for 5 minutes before ischemia. The K/Mg + DZX hearts (n = 6 to 10 in each group) received normothermic K/Mg cardioplegia containing 50 μ M diazoxide for 5 minutes before ischemia [7, 10]. Diazoxide was dissolved in dimethyl sulfoxide (DMSO; Fisher Scientific, Fair Lawn, New Jersey) before being added into Krebs solutions. The final concentration of DMSO was less than 0.1%. The DMSO was added to control and global ischemia hearts at the same concentration [7, 10].
Fig 1.
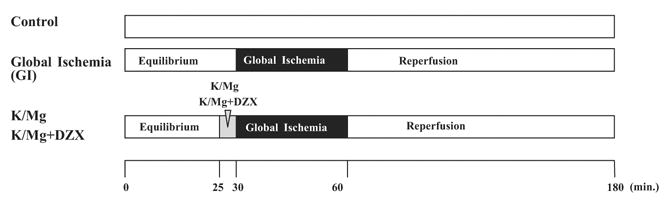
Experimental protocol: Hearts were perfused for 30 minutes to establish equilibrium function and paced at 180 beats per minute. Control hearts were perfused without global ischemia (GI) at 37°C for 180 minutes. Global ischemia hearts were subjected to 30 minutes of global ischemia and 120 minutes of reperfusion. Global ischemia was achieved by cross-clamping the perfusion line. The magnesium-supplemented potassium (K/Mg) hearts received K/Mg cardioplegia (K+20 mmol/L, Mg2+20 mmol/L in Krebs-Ringer solution) for 5 minutes before ischemia. The K/Mg plus diazoxide (K/Mg+DZX) hearts received K/Mg cardioplegia with 50 μM diazoxide for 5 minutes before ischemia. Hemodynamic variables were acquired using the PO-NE-MAH digital data acquisition system. After 120 minutes’ reperfusion, infarct size was determined by 1% triphenyl tetrazolium chloride analysis (TTC; Sigma Chemical Co, St. Louis, MO).
Measurement of Infarct Size
Infarct sizes was determined using 1% triphenyl tetrazolium chloride (TTC; Sigma Chemical Co, St. Louis, MO) and expressed as a percentage of left ventricular mass for each heart as previously described [7].
Statistical Analysis
Statistical analysis was performed using SAS (version 8.02) software package (SAS Institute, Cary, North Carolina). The mean ± SD is shown in the Table, and mean ± SEM shown in the figures. Area under the curves (AUC) above baseline (end of equilibrium) were calculated using the trapezoidal rule for LVPDP and LVEDP. Standard t tests were used for comparing global ischemia results with the control group, using the Satterthwaite correction if variances were not homogeneous (p < 0.05 using an F-test). The same approach was used for comparisons between male and female animals within an age and treatment group. Because there were significant differences in the variability between control and global ischemia groups, two separate analysis of variances (ANOVA) were performed, one for the comparison of control, K/Mg, and K/Mg+DZX, and one for the comparisons of global ischemia, K/Mg, and K/Mg+DZX. Post hoc pairwise testing was performed using a Bonferroni correction when the overall analysis of variance was significant. All p values for the between group comparisons were reported adjusted for the three comparisons within each analysis of variance. Area under the curve, the area above end of equilibrium, was calculated using the trapezoidal rule for LVPDP and LVEDP. A two-by-two ANOVA with factors for age, gender, and age-by-gender interaction was performed. Results presented always include the age-by-gender interaction. Statistical significance was claimed at p less than 0.05.
Results
Equilibrium Hemodynamics
No significant difference in LVPDP or LVEDP was observed within or between groups of mature and aged, male and female hearts at the end of equilibrium (p = 1.000 for all groups).
Effect of Global Ischemia, K/Mg Cardioplegia, and K/Mg+DZX Cardioplegia on Postischemic Functional Recovery and Infarct Size in the Mature Male and Female Heart
Global ischemia significantly decreased LVPDP and significantly increased LVEDP during reperfusion in both the mature male and mature female heart (p < 0.0001 versus control for each; Fig 2A, 2B). The LVPDP was significantly decreased in the mature male global ischemia heart as compared with the mature female global ischemia heart (p < 0.0001; Fig 2A, Table 1). No significant difference in LVEDP was observed between mature male and mature female global ischemia hearts (p = 0.341; Fig 2B, Table 1).
Fig 2.
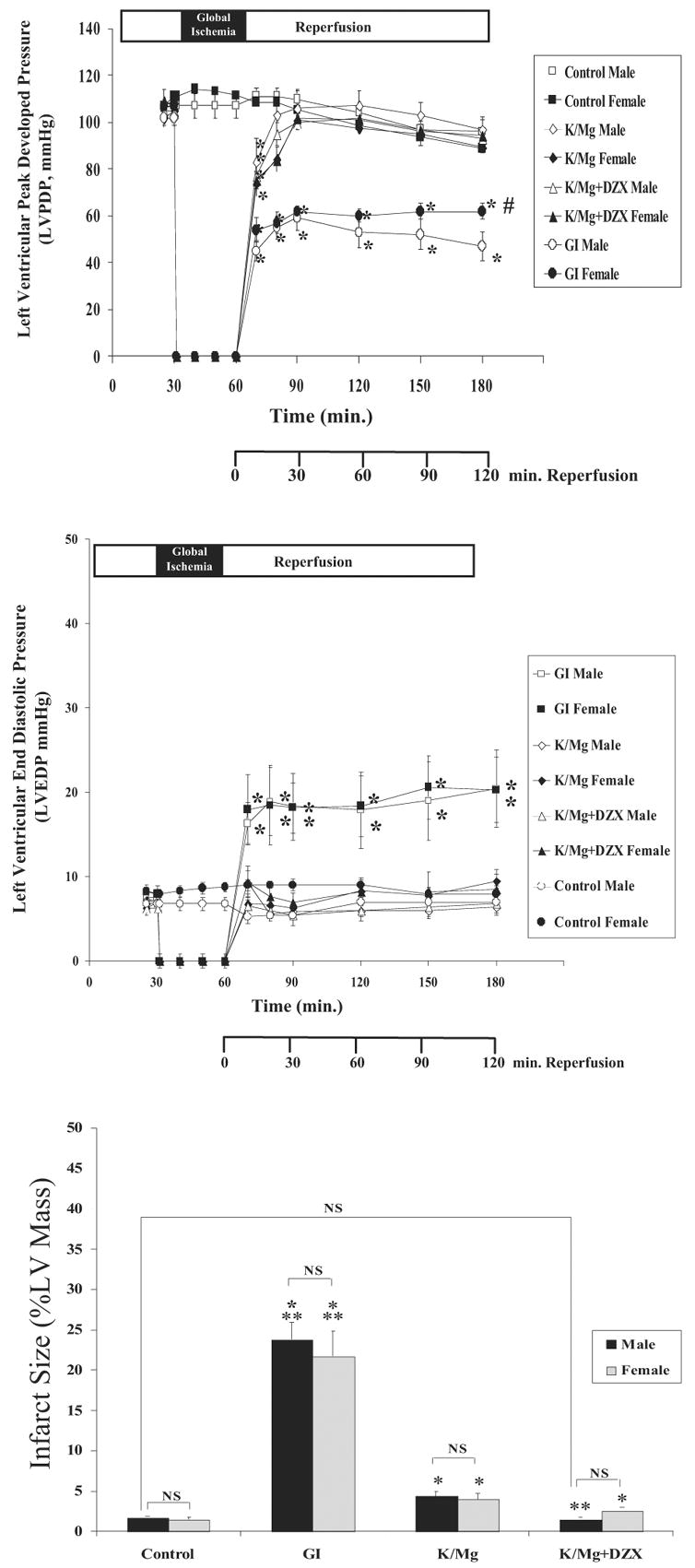
Mature male and female (A) left ventricular peak developed pressure (LVPDP); (B) left ventricular end-diastolic pressure (LVEDP) during equilibrium, global ischemia and reperfusion; and (C) infarct size (% LV mass) following 120 min reperfusion. Statistical significance is shown as *p < 0.05 versus control; #p < 0.05 versus male; and **p < 0.05 versus K/Mg. NS = not significant. All results are shown as the mean ± SEM for n = 6 to 10 for each group.
Table 1.
LVPDP, LVEDP, and Infarct Size in Mature and Aged Male and Female Hearts: Effects of Age, Gender, and Age by Gender on Global Ischemia (GI) and the Cardioprotection Afforded by K/Mg and K/Mg + DZX Cardioplegia
| LVPDP (mm Hg)
|
Probability
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mature Male | Mature Female | Aged Male | Aged Female | Age | Gender | Age × Gender | Mature Male vs Female | Aged Male vs Female | |
| Control | |||||||||
| Mean ± SD (n) | 112.5 ± 8.2 (10) | 114.7 ± 4.8 (7) | 94.6 ± 12.7 (10) | 95.6 ± 9.0 (7) | < 0.0001 | 0.6361 | 0.8514 | 0.5336 | 0.8642 |
| Mean AUC ± SD | − 0.4 ± 8.5 | 2.7 ± 5.6 | − 3.5 ± 6.9 | − 2.5 ± 6.7 | 0.1146 | 0.4102 | 0.6826 | 0.4104 | 0.7591 |
| GI | |||||||||
| Mean ± SD (n) | 48.3 ± 5.0 (10) | 61.7 ± 4.0 (7) | 44.1 ± 14.2 (10) | 42.3 ± 13.9 (7) | 0.0066 | 0.1221 | 0.0453 | < 0.0001 | 0.7969 |
| Mean AUC ± SD | − 51.3 ± 4.7 | − 45.2 ± 5.3 | − 56.6 ± 15.8 | − 62.8 ± 16.5 | 0.0167 | 0.9878 | 0.1485 | 0.0246 | 0.4454 |
| K/Mg | |||||||||
| Mean ± SD (n) | 98.8 ± 9.3 (10) | 89.1 ± 6.1 (7) | 83.1 ± 10.7 (10) | 77.6 ± 9.0 (7) | 0.0002 | 0.0266 | 0.5294 | 0.0330 | 0.2818 |
| Mean AUC ± SD | − 4.8 ± 15.8 | − 14.0 ± 11.4 | − 32.9 ± 14.8 | − 30.9 ± 6.9 | < 0.0001 | 0.4558 | 0.2382 | 0.2154 | 0.7499 |
| K/Mg ± DZX | |||||||||
| Mean ± SD (n) | 93.6 ± 9.0 (10) | 92.1 ± 6.4 (7) | 88.5 ± 9.4 (10) | 76.9 ± 10.2 (7) | 0.0059 | 0.0432 | 0.1143 | 0.7314 | 0.0276 |
| Mean AUC ± SD | − 7.9 ± 14.9 | − 18.5 ± 9.6 | − 28.1 ± 8.4 | − 31.6 ± 5.9 | < 0.0001 | 0.0680 | 0.3415 | 0.1248 | 0.3560 |
| LVEDP (mm Hg)
|
Probability
|
||||||||
| Mature Male | Mature Female | Aged Male | Aged Female | Age | Gender | Age × Gender | Mature Male vs Female | Aged Male vs Female | |
|
| |||||||||
| Control | |||||||||
| Mean ± SD (n) | 8.1 ± 0.74 (10) | 8.6 ± 4.3 (7) | 8.0 ± 0.7 (10) | 7.7 ± 0.8 (7) | 0.5562 | 0.8958 | 0.5941 | 0.7822 | 0.4229 |
| Mean AUC ± SD | − 0.1 ± 0.9 | − 0.1 ± 3.3 | 0.2 ± 1.9 | − 0.4 ± 0.7 | 0.9313 | 0.6909 | 0.6512 | 0.9781 | 0.4032 |
| GI | |||||||||
| Mean ± SD (n) | 20.0 ± 2.4 (10) | 21.3 ± 2.8 (7) | 35.3 ± 9.0 (10) | 43.0 ± 10.8 (7) | < 0.0001 | 0.0806 | 0.2067 | 0.3270 | 0.1299 |
| Mean AUC ± SD | 10.0 ± 2.4 | 11.9 ± 2.4 | 21.6 ± 7.9 | 31.5 ± 13.4 | < 0.0001 | 0.0338 | 0.1380 | 0.1404 | 0.0736 |
| K/Mg | |||||||||
| Mean ± SD (n) | 6.8 ± 1.4 (10) | 9.3 ± 2.6 (7) | 8.9 ± 2.2 (10) | 16.1 ± 4.2 (7) | 0.0002 | < 0.0001 | 0.0172 | 0.0249 | 0.0003 |
| Mean AUC ± SD | − 0.7 ± 2.0 | 0.4 ± 0.7 | 0.1 ± 1.8 | 4.4 ± 4.4 | 0.0223 | 0.0046 | 0.0868 | 0.1595 | 0.0456 |
| K/Mg ± DZX | |||||||||
| Mean ± SD (n) | 7.0 ± 3.8 (10) | 10.3 ± 1.1 (7) | 8.3 ± 1.4 (10) | 20.7 ± 6.3 (7) | 0.0005 | < 0.0001 | 0.0013 | 0.0334 | 0.0018 |
| Mean AUC ± SD | − 1.8 ± 3.7 | 0.8 ± 1.0 | 0.0 ± 1.6 | 8.7 ± 4.9 | 0.0006 | < 0.0001 | 0.0101 | 0.0785 | 0.0028 |
| Infarct Size (% LV Mass)
|
Probability
|
||||||||
| Mature Male | Mature Female | Aged Male | Aged Female | Age | Gender | Age × Gender | Mature Male vs Female | Aged Male vs Female | |
|
| |||||||||
| Control | |||||||||
| Mean ± SD (n) | 1.7 ± 0.4 (10) | 1.4 ± 0.7 (7) | 1.9 ± 0.5 (10) | 1.9 ± 0.7 (7) | 0.1286 | 0.3497 | 0.5059 | 0.2363 | 0.8567 |
| GI | |||||||||
| Mean ± SD (n) | 22.1 ± 5.4 (10) | 21.6 ± 6.4 (7) | 34.2 ± 9.7 (10) | 39.6 ± 9.9 (7) | < 0.0001 | 0.3895 | 0.3030 | 0.8668 | 0.2809 |
| K/Mg | |||||||||
| Mean ± SD (n) | 3.5 ± 1.6 (10) | 3.9 ± 0.9 (7) | 7.7 ± 2.8 (10) | 8.8 ± 2.5 (7) | < 0.0001 | 0.3178 | 0.6576 | 0.5327 | 0.4218 |
| K/Mg ± DZX | |||||||||
| Mean ± SD (n) | 1.7 ± 1.0 (10) | 2.6 ± 0.9 (7) | 3.7 ± 1.3 (10) | 9.6 ± 3.2 (7) | < 0.0001 | < 0.0001 | 0.0003 | 0.0750 | 0.0023 |
Results are shown following 180 min perfusion for Control and following 30 min equilibrium, 30 min global ischemia and 120 min reperfusion for GI, K/Mg, and K/Mg + DZX hearts. All results are shown as mean (SD). Area under the curve above equilibrium were calculated using the trapezoidal rule for LVPDP and LVEDP. The number for each group is shown as n. The p values are shown first for a 2 × 2 ANOVA with factors for age, gender and age × gender. The p values for specific comparisons between male and female animals, within an age group, were calculated using a t-test (with Satterthwaite correction, when variances in the two groups were not homogeneous). Significant differences at p < 0.05 are shown in bold face type.
AUC = area under the curve; DZX = diazoxide; K/Mg = magnesium supplemented potassium; LVEDP = left ventricular end-diastolic pressure; LVPDP = left ventricular peak developed pressure.
Global ischemia significantly decreased LVPDP (p < 0.0001 versus control) and significantly increased LVEDP (p < 0.001 versus control) in mature male and mature female hearts. The LVPDP was significantly decreased after 180 minutes of reperfusion in mature male global ischemia hearts as compared with female global ischemia hearts (p < 0.0001; Fig 2A, Table 1). There was no significant difference in LVEDP after 180 minutes of reperfusion between mature male global ischemia hearts and mature female global ischemia hearts (p = 0.3270; Fig 2B, Table 1).
In both mature male and mature female hearts, K/Mg and K/Mgt+DZX cardioplegia significantly increased LVPDP (p < 0.001 for each versus global ischemia; p = 1.000 for each versus control; Fig 2A, 2B) and significantly decreased LVEDP (p < 0.001 for each versus global ischemia; p = 1.000 for each versus control; Fig 2A, 2B) during reperfusion. A significant difference was observed for both LVPDP and LVEDP between mature male and mature female K/Mg hearts (p = 0.0330 and 0.0249 respectively; Table 1). The LVPDP was significantly different between mature male and mature female K/Mg+DZX hearts (p = 0.0334, Table 1).
Infarct size was significantly increased (p < 0.0.0001 for each versus control) in both mature male and mature female global ischemia hearts (Fig 2C). Both K/Mg and K/Mg+DZX significantly decreased infarct size (p < 0.001 for each versus global ischemia) in mature male and mature female hearts.
Infarct size was significantly decreased by K/Mg+DZX in mature male hearts such that there was no significant difference as compared with control (p = 1.000; Fig 2C, Table 1); however, in mature female K/Mg+DZX hearts, infarct size was significantly increased as compared with control (p = 0.042; Fig 2C, Table 1).
Effect of Global Ischemia, K/Mg Cardioplegia, and K/Mg+DZX Cardioplegia on Postischemic Functional Recovery and Infarct Size in the Aged Male and Female Heart
Global ischemia significantly decreased LVPDP (p < 0.0001 versus control) and significantly increased LVEDP (p < 0.0001 versus control) during reperfusion in both the aged male and aged female heart (Fig 3A, 3B). No significant difference in LVPDP or LVEDP was observed between aged male and aged female global ischemia hearts (p = 0.7969 and p = 0.1299, respectively; Table 1).
Fig 3.
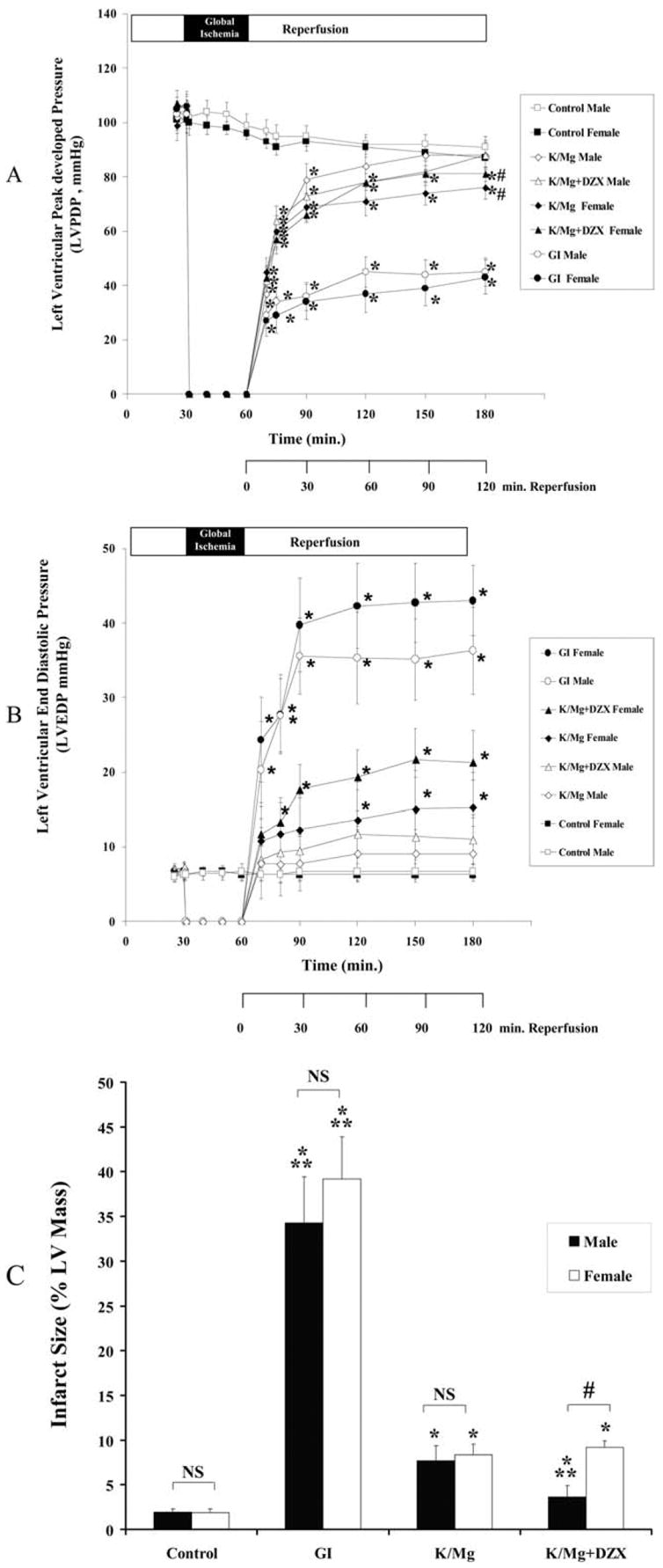
Aged male and female (A) left ventricular peak developed pressure (LVPDP); (B) left ventricular end-diastolic pressure (LVEDP) during equilibrium, global ischemia and reperfusion; and (C) infarct size (% LV mass) following 120 min reperfusion. Statistical significance is shown as *p < 0.05 versus control; #p < 0.05 versus male; and **p < 0.05 versus K/Mg. NS = not significant. All results are shown as the mean ± SEM for n = 6 to 10 for each group.
In contrast to mature male and female hearts, a significant difference was observed for LVEDP and LVPDP (AUC, p < 0.001 versus control for each) was observed in aged male and aged female K/Mg and K/Mg+DZX hearts.
Infarct size was significantly increased (p < 0.001 for each versus control) in both aged male and aged female global ischemia hearts (Fig 3C). Both K/Mg and K/Mg + DZX significantly decreased infarct size (p < 0.001 for each versus global ischemia) in aged male and aged female hearts. No significant difference in infarct size was observed between aged male and aged female K/Mg hearts (p = 0.4218; Fig 2C, Table 1). However, a significant difference in infarct size reduction was observed between aged male and aged female K/Mg + DZX hearts (p = 0.0023; Fig 3C, Table 1). Infarct size in aged male K/Mg+DZX hearts was not significantly different as compared with control (p = 0.117); however, infarct size in aged female K/Mg+DZX hearts was significantly increased (p < 0.001) as compared with control (Fig 3).
Effects of Age, Gender, and Age and Gender on Postischemic Functional Recovery and Infarct Size After Global Ischemia: Effects on Cardioplegia
Results were analyzed using models that test for age and gender effects (and their interaction), first separately within each treatment and then for an overall analysis attempting to determine whether age, gender, or their combination interacts with treatment.
Our results indicate that postischemic functional recovery (LVPDP and LVEDP) after global ischemia is significantly affected by age but not gender (Table 1) with a small combined age-by-gender interaction in LVPDP (p = 0.0453; Table 1). Infarct size in global ischemia hearts was significantly affected by age but not gender (Table 1). The overall model for global ischemia indicates a significant age but not a gender effect on postischemic functional recovery and infarct size.
Analysis of age and gender and age-by-gender effects on the cardioprotection afforded by K/Mg and K/Mg+DZX cardioplegia indicate that LVPDP is significantly affected by age and gender but there is no age-by-gender interaction with either K/Mg or K/Mg+DZX cardioplegia (Table 1). However, LVEDP was found to be significantly affected by age and gender with combined age-by-gender interaction in K/Mg and K/Mg+DZX hearts (Table 1). Infarct size in K/Mg hearts was affected by age but not by gender or age-by-gender (Table 1). In contrast, both age, gender, and age-by-gender were found to significantly modulate infarct size in K/Mg+DZX hearts (Table 1). The overall model for K/Mg and K/Mg+DZX cardioplegia is an age and gender effect with a strong age-by-gender interaction for LVEDP and for infarct size with K/Mg+DZX cardioplegia (Table 1).
Comment
The differential response between men and women to cardiac surgery has been empirically noted; however, the majority of studies have utilized a male model to provide a paradigm for cardioprotective protocols. The use of males has been justified in that changes in hormonal levels may have complicated or masked results. While providing a strong paradigm, it is now imperative that these cardioprotective methodologies be verified in the aging female population.
In the present study, we have used a model of male and female aging. This model incorporates the sexually mature heart and the aged, not senescent heart and was chosen because previous studies in animal models have shown that age-related ischemic intolerance develops well before senescence, being primarily evident by middle age [3, 12, 13]. These studies have demonstrated an apparent nadir in ischemic tolerance in middle-aged to aged hearts, with a subsequent modest improvement with senescence [3, 12, 13]. This pattern is consistent with empirical clinical observations and with prior studies showing an improvement in the senescent as compared with the aged heart and may be related to selection of a subpopulation resistant to myocardial injury [1–5, 7, 12, 13]. Vaccarino and associates [14] have reported that in coronary artery bypass graft surgery patients between 50 and 60 years of age, women experienced an 86% higher risk of in-hospital death than men; however, gender differences in in-hospital mortality were less marked in the older-age subgroups.
Our results demonstrate that the aged myocardium is less tolerant of surgically induced ischemia than the mature myocardium. Our results also demonstrate that the cardioprotection afforded by cardioplegia is affected by age and gender, with cardioprotection afforded by cardioplegia being significantly decreased in the aged as compared with the mature heart and significantly decreased in the aged female as compared with the aged male heart. Furthermore, our results demonstrate that the opening of the mitochondrial ATP-sensitive potassium (mitoKATP) channels is significantly less effective in limiting infarct size in aged females as compared to mature and aged males.
Our data are in agreement with previous reports showing that age increases ischemic injury and indicate that the cardioprotection afforded by cardioplegia is affected by age and gender with a strong age-by-gender interaction for end-diastolic pressure and infarct size [15, 16]. The infarct limiting effects of diazoxide shown to occur in the mature male and female heart (p < 0.001 versus global ischemia) are less apparent in the aged male heart and not apparent in the aged female heart. These data are in agreement with the recent findings of Willems and colleagues [3] who have shown that aging in mice is associated with reduced tolerance to ischemia and that aged (not senescent) female mouse hearts have greater susceptibility to ischemia as compared with aged male hearts.
The mechanisms contributing to age- and gender-related differences in cardioprotection have yet to be fully elucidated. Presently available data indicate that premenopausal women have a greater resistance to ischemic injury and that this resistance is lost after menopause [17–19]. These data would suggest that estrogen may play an important role in modulating ischemic injury in females. In our model, we have used mature and aged rabbits. The rabbit is an inducible ovulator and estrogen levels are low in the adult rabbit [11]. Estradiol levels in rabbits of 38 months are undetectable as they are in diestrus, until induced to ovulate [11]. Our data would suggest that estradiol levels may play an important role in cardioprotection. Support for this hypothesis comes from studies demonstrating that supplemental 17β-estradiol, the active form of estradiol, enhances cardioprotection [20, 21]. Hale and coworkers [20] have previously demonstrated that supplemental 17β-estradiol significantly decreased necrosis in rabbits after ischemia and reperfusion.
In previous reports, we have shown using specific openers and blockers of sarcolemmal and mitoKATP channels that the mechanism of action of K/Mg cardioplegia is modulated mitoKATP channels and that the early or enhanced opening of mitoKATP channels with diazoxide significantly enhances the infarct-limiting effects of K/Mg cardioplegia, in agreement with studies by others [8–10, 22, 24]. In the present study, we show that the addition of diazoxide to K/Mg cardioplegia (K/Mg+DZX) failed to enhance infarct-limiting effects in the aged female as compared with the aged male. This gender-related difference may be related to alterations in mitoKATP channel sensitivity. Previous studies have shown that the mechanism of action of 17β-estradiol is modulated by the mitoKATP channels [18, 21]. It has also been shown that the infarct size–limiting effects of 17β-estradiol are abolished by 5-hydroxydecanoate, a mitoKATP channel blocker [21, 24, 25]. In recent studies, we have observed that the effects of diazoxide on mitochondrial oxygen consumption and mitochondrial calcium accumulation varied with age and with gender [26]. Whether these changes are related to hormonal changes or other factors remains at the level of speculation, and more involved studies are required.
It is important to note that our results have been obtained in an isolated perfused heart and require further studies using an in-situ blood perfused model and further human analysis. This being acknowledged, the clinical import of our data remains and provides impetus for further studies into the observed differences occurring in the aged male and female patient undergoing cardiac surgery.
At present only K/Mg cardioplegia is used clinically as Deaconess Surgical Association cardioplegia and is used in both male and female patients. The use of diazoxide has been shown in the in-situ pig heart model to be effective and does not induce hypotension if used at 50 μmol/L in the first administration of cardioplegia only [8, 9]. Our data indicate that for the mature and aged male heart, the addition of diazoxide would be of value in reducing infarct size; however, in the aged female heart, the use of diazoxide is not efficacious.
In conclusion, our data show that currently optimized cardioplegia protocols effective in the mature and aged male heart are not as efficacious in the mature and aged female, and that these differences are exacerbated in the aged female heart. Further studies in the aged female patient are required to develop age- and gender-specific cardioprotective protocols to limit morbidity and mortality after cardiac operations performed in a technically adequate manner.
Footnotes
This study was supported by the National Institutes of Health (HL29077).
References
- 1.Edwards FH, Carey JS, Grover FL, Bero JW, Hartz RS. Impact of gender on coronary bypass operative mortality. Ann Thorac Surg. 1998;66:125–31. doi: 10.1016/s0003-4975(98)00358-0. [DOI] [PubMed] [Google Scholar]
- 2.Koch CG, Khandwala F, Nussmeier N, Blackstone EH. Gender and outcomes after coronary artery bypass grafting: a propensity matched comparison. J Thorac Cardiovasc Surg. 2003;126:2032–43. doi: 10.1016/s0022-5223(03)00950-4. [DOI] [PubMed] [Google Scholar]
- 3.Willems L, Zatta A, Holmgren K, Ashton KJ, Headrick JP. Age-related changes in ischemic tolerance in male and female mouse hearts. J Mol Cell Cardiol. 2005;38:245–56. doi: 10.1016/j.yjmcc.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Butterworth J, Prielipp JR, Cerese J, Livingston J, Burnett D. Female gender associates with increased duration of intubation and length of stay after coronary artery surgery. CABG clinical benchmarking database participants. Anesthesiology. 2000;92:414–24. doi: 10.1097/00000542-200002000-00023. [DOI] [PubMed] [Google Scholar]
- 5.Abramov D, Tamariz MG, Sever JY, et al. The influence of gender on the outcome of coronary artery bypass surgery. Ann Thorac Surg. 2000;70:800–5. doi: 10.1016/s0003-4975(00)01563-0. [DOI] [PubMed] [Google Scholar]
- 6.Bridgman P, Aronovitz MA, Kakkar R, et al. Gender-specific patterns of left ventricular and myocyte remodeling following myocardial infarction in mice deficient in the angiotensin II type 1a receptor. Am J Physiol Heart Circ Physiol. 2005;289:H586–92. doi: 10.1152/ajpheart.00474.2004. [DOI] [PubMed] [Google Scholar]
- 7.Toyoda Y, Levitsky S, McCully JD. Opening of mitochondrial ATP-sensitive potassium channels enhances cardioplegic protection. Ann Thorac Surg. 2001;71:1281–9. doi: 10.1016/s0003-4975(00)02667-9. [DOI] [PubMed] [Google Scholar]
- 8.McCully JD, Wakiyama H, Cowan DB, Federman M, Levitsky S. Diazoxide amelioration of myocardial injury and mitochondrial damage during cardiac surgery. Ann Thorac Surg. 2002;74:2138–46. doi: 10.1016/s0003-4975(02)04348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCully JD, Levitsky S. Mitochondrial ATP-sensitive potassium channels in surgical cardioprotection. Arch Biochem Biophys. 2003;420:237–45. doi: 10.1016/j.abb.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Rousou AJ, Ericsson M, Federman M, Levitsky S, McCully JD. Diazoxide and cardioplegia ameliorate ischemia/ reperfusion cell death through the modulation of mitochondrial volume and calcium accumulation and mitochondrial respiratory control index. Am J Physiol Heart Circ Physiol. 2004;287:H1967–76. doi: 10.1152/ajpheart.00338.2004. [DOI] [PubMed] [Google Scholar]
- 11.Suckow MA, Brammer DW, Rush HG, Chrisp CE. Biology and diseases of rabbits. In: Fox JG, Anderson LC, Loew FM, Quimby FW, editors. Laboratory animal medicine. 2. Toronto: Academic Press; 2002. pp. 329–64. [Google Scholar]
- 12.Regitz-Zagrosek V, Lehmkuhl E, Lehmkuhl HB, Hetzer R. Gender aspects in heart failure. Pathophysiology and medical therapy. Arch Mal Coeur Vaiss. 2004;97:899–908. [PubMed] [Google Scholar]
- 13.Boucher F, Tanguy S, Toufektsian MC, Besse S, Tressallet N, Favier A. Age-dependent changes in myocardial susceptibility to zero flow ischemia and reperfusion in isolated perfused rat hearts: relation to antioxidant status. Mech Ageing Dev. 1998;103:301–16. doi: 10.1016/s0047-6374(98)00050-5. [DOI] [PubMed] [Google Scholar]
- 14.Vaccarino V, Abramson JL, Veledar E, Weintraub WS. Sex differences in hospital mortality after coronary artery bypass surgery: evidence for a higher mortality in younger women. Circulation. 2002;105:1176–81. doi: 10.1161/hc1002.105133. [DOI] [PubMed] [Google Scholar]
- 15.Misare BD, Krukenkamp IB, Levitsky S. Age-dependent sensitivity to unprotected cardiac ischemia: the senescent myocardium. J Thorac Cardiovasc Surg. 1992;103:60–5. [PubMed] [Google Scholar]
- 16.Calderone CA, Krukenkamp IB, Burns PG, Gaudette GR, Schulman J, Levitsky S. Blood cardioplegia in the senescent heart. J Thorac Cardiovasc Surg. 1995;109:269–74. doi: 10.1016/S0022-5223(95)70388-8. [DOI] [PubMed] [Google Scholar]
- 17.Humphrey LL, Chan BK, Sox HC. Postmenopausal hormone replacement therapy and the primary prevention of cardiovascular disease. Ann Intern Med. 2002;137:273–84. doi: 10.7326/0003-4819-137-4-200208200-00012. [DOI] [PubMed] [Google Scholar]
- 18.Tsai CH, Su FS, Chou TF, Lee TM. Differential effects of sarcolemmal and mitochondrial channels activated by 17β-estradiol on reperfusion arrhythmias and infarct size in canine hearts. J Pharmacol Exp Ther. 2002;301:234–40. doi: 10.1124/jpet.301.1.234. [DOI] [PubMed] [Google Scholar]
- 19.Booth EA, Marchesi M, Kilbournee J, Lucchesi BR. 17β-Estradiol as a receptor-mediated cardioprotective agent. J Pharmacol Exp Ther. 2003;307:395–401. doi: 10.1124/jpet.103.054205. [DOI] [PubMed] [Google Scholar]
- 20.Hale SL, Birnbaum Y, Kloner RA. Beta-estradiol, but not alpha-estradiol, reduced myocardial necrosis in rabbits after ischemia and reperfusion. Am Heart J. 1996;132:258–62. doi: 10.1016/s0002-8703(96)90419-6. [DOI] [PubMed] [Google Scholar]
- 21.Lee TM, Su SF, Tsai CC, Lee YT, Tsai CH. Cardioprotective effects of 17-beta-estradiol produced by activation of mitochondrial ATP-sensitive K(ATP) channels in canine hearts. J Mol Cell Cardiol. 2000;32:1147–58. doi: 10.1006/jmcc.2000.1167. [DOI] [PubMed] [Google Scholar]
- 22.Costa AD, Quinlan CL, Andrukhiv A, West IC, Jaburek M, Garlid KD. The direct physiological effects of mitoK(ATP) opening on heart mitochondria. Am J Physiol Heart Circ Physiol. 2006;290:H406–15. doi: 10.1152/ajpheart.00794.2005. [DOI] [PubMed] [Google Scholar]
- 23.Kowaltowski AJ, Seetharaman S, Paucek P, Garlid KD. Bioenergetic consequences of opening the ATP-sensitive K(+) channel of heart mitochondria. Am J Physiol Heart Circ Physiol. 2001;280:H649–57. doi: 10.1152/ajpheart.2001.280.2.H649. [DOI] [PubMed] [Google Scholar]
- 24.Jiang MT, Ljubkovic M, Nakae Y, et al. Characterization of human cardiac mitochondrial ATP-sensitive potassium channel and its regulation by phorbol ester in vitro. Am J Physiol Heart Circ Physiol. 2006;290:H1770–6. doi: 10.1152/ajpheart.01084.2005. [DOI] [PubMed] [Google Scholar]
- 25.Tsukamoto O, Asanuma H, Kim J, et al. A role of opening of mitochondrial ATP-sensitive potassium channels in the infarct size-limiting effect of ischemic preconditioning via activation of protein kinase C in the canine heart. Biochem Biophys Res Commun. 2005;338:1460–6. doi: 10.1016/j.bbrc.2005.10.109. [DOI] [PubMed] [Google Scholar]
- 26.McCully JD, Rousou AJ, Parker RA, Levitsky S. Age and gender differences in mitochondrial oxygen consumption and free matrix calcium during ischemia/reperfusion and with cardioplegia and diazoxide. submitted for review. [DOI] [PMC free article] [PubMed] [Google Scholar]


