Abstract
Two lick suppression studies were conducted with water-deprived rats to investigate the influence of spatial similarity in cue interaction. Experiment 1 assessed the influence of similarity of the spatial origin of competing cues in a blocking procedure. Greater blocking was observed in the condition in which the auditory blocking cue and the auditory blocked cue originated at the same spatial location. Recent investigations have demonstrated that manipulations that impact competition between cues trained together have similar effects on interference between cues trained apart. Therefore, Experiment 2 investigated the influence of similarity of the spatial origin in proactive interference of Pavlovian conditioning by separately pairing two auditory cues with a common outcome, originating at the same spatial location or different spatial locations. Greater proactive interference was observed in the condition in which the interfering cue and target cue originated at the same spatial location. The results are considered in light of the possibility that a similar mechanism may underlie interference between cues trained apart and cue competition between cues trained together.
Cue competition refers to situations in which the presence of a nontarget stimulus during reinforced training of a target cue attenuates conditioned responding to the target cue at the time of test. In other words, when two cues are compounded and paired with an outcome, an inverse relationship is observed between the behavioral control exerted by each of the cues when tested alone. Cue interference here refers to situations in which a target cue is paired with a reinforcer in Phase 1 (or 2) and a nontarget cue is paired with a reinforcer in Phase 2 (or 1), such that the nontarget cue trials impair the behavioral control of the target cue that would otherwise result. Alternatively stated, cue competition refers to a deleterious interaction between cues trained together, whereas cue interference refers to a deleterious interaction between cues trained apart.
The contemporary associative analysis of cue interaction has focused almost exclusively on the examination of cues that are presented in compound at some point during training (e.g., blocking, overshadowing, and the relative stimulus validity effect). In a blocking preparation (Kamin, 1968; Lashley, 1942), the blocking stimulus (A) is paired with an unconditioned stimulus (US) in an initial phase of treatment (i.e., A-US trials in Phase 1). The blocking cue (A) is then paired with the blocked cue (X) and the US in a subsequent phase of treatment (i.e., AX-US trials in Phase 2). The compound training trials facilitate Cue A competing with Cue X; thus, at the time of test weaker responding to Cue X is observed relative to a control condition that had not received prior training with A. The ability of a theory to account for such interaction between cues presented together is considered a critical benchmark for contemporary models of learning. For instance, the Rescorla-Wagner (1972) model assumes that any given US can support only a fixed amount of associative strength on any single trial. In the case of blocking, the Rescorla-Wagner model assumes that the blocking cue (A) accrues most, if not all, of the associative strength the US can support during Phase 1 training, thereby attenuating additional learning to the blocked stimulus (X) during Phase 2. Other models, such as those of Mackintosh (1975), Miller and Matzel (1988), Pearce-Hall (1980), and Wagner (1981) can all account for such interaction, albeit with different mechanisms. Importantly, historical and recent evidence of analogous interactions between cues trained apart (i.e., in the absence of compound training of the target and interfering cues; e.g., Matute & Pineño, 1998; Underwood & Estrand, 1968) exists in what is referred to as the interference literature. Because none of the contemporary associative models of learning were designed to account for situations in which the retrieval of a target association is impaired exclusively by independent training of another association with the same cue or same outcome, it might prove valuable to our understanding stimulus interaction if we identified variables as having similar or opposing effects on interaction between cues presented together (defined here as competition) and between cues presented apart (defined here as interference).
Interaction between cues trained apart is usually observed in the retroactive interference and proactive interference paradigms in which the target and interfering training occurs phasically (for reviews, see Bouton, 1993; Underwood, 1957). In proactive interference the associations acquired during Phase 1 interfere with the retrieval (or acquisition) of the target associations trained during Phase 2, and in retroactive interference the associations acquired during Phase 2 interfere with the retrieval of the target associations trained during Phase 1. The concepts of proactive interference and retroactive interference developed primarily within the early studies of verbal behavior, especially those using paired-associate stimuli (for reviews, see Slamecka & Ceraso, 1960; Swenson, 1941). In a typical study of retroactive cue interference in verbal paired-associate learning, human participants were first presented with a list of paired-associates (e.g., words, nonsense syllables, or trigrams) of the form A-B (e.g., cat-tree), then a second list of the form C-B (e.g., horse-tree). At test, participants were presented with the common associate (B) and asked to recall all stimuli that were paired with it. Although participants tended to learn the pairs of items over several trials, the probability that they could recall the target stimulus (A, in this example, cat) decreased if they had also been exposed to the C-B pairings (e.g., Johnston, 1968). That is, presumably Stimulus C (horse in this example) retroactively interfered with Stimulus A and thus, prevented the recall of Stimulus A compared to a condition in which the C-B list was not presented and no interference occurred to Stimulus A.
In 1998, Matute and Pineño reported a Pavlovian analogue to retroactive interference in humans, thereby encouraging the view that interference effects observed in verbal learning tasks also apply to Pavlovian learning. More recently researchers have used Pavlovian preparations to investigate retroactive interference and proactive interference in rats using a procedure akin to that used in the study of human verbal behavior (e.g., Amundson, Escobar, & Miller, 2003; Escobar, Matute, & Miller, 2001). In the investigation of proactive interference in rats, Amundson et al. used an A-O, X-O paradigm (in which X and A were stimulus events and O was an outcome, in this case a footshock) to demonstrate that rats exhibited less conditioned responding to X at test if the X-O pairings were preceded by A-O pairings than if they were preceded by unpaired presentations of A and O. The interaction seen between cues trained apart with a common outcome is in many respects highly reminiscent of the interaction found between cues trained together (e.g., Miller & Escobar, 2002). For example, both deficits are often attenuated when the competing association is extinguished (e.g., with cues trained together, Blaisdell, Gunther, & Miller, 1999; with cues trained apart, Amundson et al., 2003). These similarities raise the question, do other manipulations that affect interaction between cues trained together have a similar effect on interaction between cues trained apart?
Contiguity is recognized as a central factor in the establishment of associations. The establishment of an association is inversely related to the temporal and spatial separation of the two associates. Most studies of the role of stimulus similarity have focused on either qualitative features or temporal relationships such as those mentioned above. However, spatial contiguity between two stimuli (a form of similarity) has been found to influence the formation of associations between them. For example, Rescorla and Cunningham (1979) demonstrated that second-order conditioning was facilitated when the first-order CS and the second-order CS shared the same spatial location compared to when each originated at a different location. The facilitation of conditioning by spatial contiguity (or similarity) of the associates appears to be a robust phenomenon that generalizes across conditioning paradigms (e.g., for a taste aversion procedure, see Ellins & Von Kluge, 1987; for autoshaping and conditioned suppression, see Rescorla & Cunningham, 1979; for a review, see Chamizo, 2003). However, spatial contiguity of cues has not been examined for its effects on cue interaction.
One might expect that similar spatial location would also facilitate cue interaction between cues trained apart with a common outcome (i.e., cue interference) and between cues trained together with a common outcome (i.e., cue competition). That is, greater interaction might be expected when two associations are formed in the same spatial location, than when the two associations are formed in different locations. The current experiments address cue interaction between cues trained together and between cues trained apart by examining the role of spatial similarity in both blocking and proactive interference, respectively. We expected to observe greater blocking and greater proactive interference when the interacting cue originates from the same spatial location as the target cue, compared to conditions when the interacting cue originates from a different location than the target cue. In other words, the sharing of an attribute such as spatial location might make the target and nontarget cues more similar and consequently encourage conjoint processing of the two associations when either cue is presented alone. Therefore, cue interaction may be increased compared to a condition in which the cues are less similar.
Experiment 1
Experiment 1 examined the influence of spatial similarity on competition between cues trained together with a common outcome, specifically blocking. We expected that, when the competing cue and the target cue originated in the same spatial location, the competition would be stronger than when the competing cue and the target cue originated in different spatial locations. The results of an experiment similar to the current one suggested that subjects were not sufficiently discriminating the spatial location of the speakers. One possible reason for the inability of the subjects in that experiment to discriminate the spatial origins of the cues was the novelty of the cues. In the present experiment we gave prettraining exposure to the cues in order to reduce novelty. Moreover, to further direct attention to the spatial origins of the cues, in Phase 1of the blocking treatment we administered spatial discrimination training.
Method
Subjects
The subjects were 24 male (207 – 361 g) and 24 female (178 – 302 g) naïve Sprague-Dawley rats, bred in our colony. Subjects were individually housed in wire-mesh hanging cages in a vivarium maintained on 16-hr light/8 hr-dark cycle. Experimental manipulations occurred approximately midway through the light portion of the cycle. A progressive water deprivation schedule was imposed over the week prior to the beginning of the experiment until water availability was limited to 30 min per day. All subjects were handled for 30 s three times per week from weaning until the initiation of the study.
Apparatus
Twelve identical chambers, each measuring 23.5 x 8.5 x 12.5 cm (l x w x h), were used. By intent, these small chambers precluded the subjects walking toward or away from speakers, which might have changed the relative intensities of the speakers. The walls of each chamber were made of clear Plexiglas, and the floor was constructed of 0.5 cm diameter rods, spaced 1.5 cm center-to-center, and connected by NE-2 neon bulbs that allowed a 1-mA constant-current footshock to be delivered by means of a high voltage AC circuit in series with a 1.0-MΩ resistor. Each chamber was housed in an environmental isolation chest. Each environmental isolation chest had black vertical lines (1.0 cm black strip separated by 0.7 cm clear space) running along one long side and half of the front and the back end walls of the chest so as to create a strong visual asymmetry to the chamber which presumably made the spatial location of the stimuli easier to discriminate and less a function of each rat’s immediate location or posture. Each environmental isolation chest was dimly illuminated by a houselight (#1820 incandescent bulb) mounted on the ceiling of the experimental chamber. Each chamber was equipped with a water-filled lick tube (opening = 0.3 cm in diameter) that extended about 1 cm from the rear of a 4.5 cm deep cylindrical niche, 4.5 cm in diameter, that was left-right centered on one short wall, with its axis perpendicular to the wall and positioned 4 cm above the grid floor. An infrared photobeam was projected horizontally across the niche, 1 cm in front of the lick tube. In order to drink from the tube, subjects inserted their heads into the niche, thereby breaking the infrared photobeam. Thus, the amount of time the photobeam was disrupted was monitored and served as our dependent measure. There were two 45-Ω speakers, one mounted on the interior left side of the environmental chest and the other on the right side. The two locations were identified as Location 1 and Location 2, counterbalanced within groups. The speakers were used to deliver a white noise stimulus, a low frequency tone stimulus (400 Hz), and a high frequency tone stimulus (2000 Hz), each 8 dB above background. Ventilation fans in each chest provided a constant 72-dB background noise. All auditory cues were measured on the C-scale. The two tones served as Cues A and B (counterbalanced) and the white noise served as cue X. During training, all CSs were 10 s in duration.
Procedure
Subjects were randomly assigned to one of four groups: Acquisition-Control (Acq-Con), Block-Different-A1 (Blk-DiffA1), Block-Different-A2 (Blk-DiffA2), Block-Same (Blk-Same), counterbalanced for sex (ns = 12). The design is depicted in Table 1. The Acq-Con group was to control for possible overshadowing; no blocking was expected. The Blk-Same group was expected to exhibit maximal blocking because the blocking and blocked cues originated from the same spatial location. And the two Blk-Diff groups were expected to display a reduction in blocking due to the blocking and blocked cues emanating from different spatial locations. These last two groups differed only in where A originated in testing relative to where it originated in training to see if relocating A at test produced any generalization decrement.
Table 1.
Design of Experiment 1
| Group | Preexposure | Phase 1 | Phase 2 | Test X | Test A |
|---|---|---|---|---|---|
| Acq-Con | 2 A-, 2 X- | 12 B1 → US/12 B2 - | 4 A1 X1 → US | X1 | A1 or A2 |
| Blk-DiffA1 | 2 A-, 2 X- | 12 A2 → US/12 A1 - | 4 A2 X1 → US | X1 | A1 |
| Blk-DiffA2 | 2 A-, 2 X- | 12 A2 → US/12 A1 - | 4 A2 X1 → US | X1 | A2 |
| Blk-Same | 2 A-, 2 X- | 12 A1 → US/12 A2 - | 4 A1 X1 → US | X1 | A1 |
Note: The superscripts indicate the location of the CS (1 = Location 1 and 2 = Location 2). During preexposure each stimulus was presented from both Location 1 and Location 2. The numbers preceding the letters indicate the total number of presentations of the stimuli in that phase. Slashes separate unpaired presentations of CSs and USs. Stimuli A and B were a low frequency tone and a high frequency tone, counterbalanced. Stimulus X was white noise. → indicates “followed by.”
Acclimation and preexposure
On Days 1–3, all subjects were acclimated daily to the experimental context for 60 min. Subjects had free access to the water-filled lick tubes and no nominal stimuli were presented on Days 1 and 2. On Day 3, the water-filled lick tubes were removed and Cue A and Cue X were each presented twice without reinforcement. Cue X was presented at 12 and 57 min into the session, whereas Cue A was presented at 26 and 44 min into the session. Each cue was presented once from each of the two spatially separated speakers.
Phase 1. Competing association training
On Days 4–6 during the 120-min sessions, subjects in Groups Blk-Same, Blk-DiffA1 and Blk-DiffA2 received four daily A-US pairings and four daily A-only presentations. Subjects in Group Acq-Con received four daily B-US pairings and four daily B-only presentations. For the purpose of discrimination training, Cue A or B was reinforced when it emanated from one location (Location 1 for Groups Blk-Same and Acq-Con, and Location 2 for Groups Blk-DiffA1 and Blk-DiffA2) and not reinforced when it emanated from the other location with the spatial locations (1 and 2) left/right counterbalanced within groups. The US onset coincided with CS A or B termination. The footshock US duration was 0.5 s. Two different schedules were used. In Schedule 1 the reinforced Cue A or B presentation on Days 4 and 6 occurred at 10, 30, 80, and 100 min into the 120-min session whereas the nonreinforced presentations of Cue A or B occurred at 15, 45, 60, and 90 min into the 120-min session. In Schedule 2 the reinforced stimulus presentations on Day 5 occurred at 19, 54, 89, and 109 min into the 120-min session whereas the nonreinforced stimulus presentations occurred at 3, 28, 43, and 78 min into the session.
Phase 2. Target training
On Day 7 during the 60-min session, all subjects received four AX-US pairings. Cue X was generated from Location 1 for all groups. Cue A was generated from Location 1 for Groups Blk-Same and Acq-Con and Location 2 for Groups Blk-DiffA1 and Blk-DiffA2. Onset of the compound Cue AX occurred at 10, 25, 40, and 55 min into the 60-min session, with the footshock US being presented immediately after termination of Cue AX.
Reacclimation
On Days 8 and 9, all subjects were exposed to the experimental context for 60 min with the lick tubes again available. Stimuli A and X were not presented. This treatment served to reestablish a uniformly high baseline rate of drinking among all groups.
Testing
On Days 10, all subjects were tested for conditioned lick suppression to Cues X and A, respectively. We tested all subjects on cue X on Day 10 because it was the cue of primary interest and consequently we wanted to avoid any possible effect of our first testing cue A upon responding to cue X. The test stimulus was presented from Location 1 immediately upon completion of the first five cumulative seconds of licking. Consequently, all subjects were licking at the time of cue onset. The delay to this time constituted the pre-CS scores. On Day 10 in a 16-min session, all subjects were exposed to Cue X for 15 min and time to complete five cumulative seconds of licking in the presence of the stimulus was recorded (CS score). On Day 11, identical testing occurred with cue A, with cue A being generated from the same location from which it originated in Phase 1, except for Group Blk-DiffA1. Cue A for Group Blk-DiffA1 originated from the nonreinforced location. Following the established practice in our laboratory, the data from all animals that took longer than 60-s to complete their first five cumulative seconds of drinking in either test session (i.e., before cue onset) were eliminated from the analyses because such long latencies would reflect unusual fear of the experimental context. Based on this criterion the data from one animal in Group Blk-Same and one from Group Blk-DiffA1 were eliminated from the statistical analyses. Additionally, the data from one animal in Group Blk-DiffA1 had to be eliminated due to equipment problems. In order to better approximate the normal within-group distributions of scores assumed by parametric statistics, a log (base 10) transformation was performed on each measured time in Experiments 1 and 2.
Results and Discussion
Experiment 1 revealed that blocking was greater when the blocking and blocked cues emanated from the same location. The following analysis supported this conclusion. Because the only procedural difference between Groups Blk-DiffA1 and Blk-DiffA2 was the location in which Cue A was tested and these two groups did not differ significantly in suppression to X (p = .89), these groups were pooled during the assessment of conditioned fear to Cue X resulting in a single Blk-Diff group. A one-way analysis of variance (ANOVA) on the Day 10 baseline scores (i.e., time to complete 5 cumulative seconds of drinking prior to CS onset) of Groups Blk-Diff, Blk-Same, and Acq-Con recorded during testing revealed differences in initiation of drinking, F(2, 42) = 5.04, MSE = 0.21, p < .02. Therefore, a one-way analysis of covariance (ANCOVA) with the baseline (i.e., pre-CS) scores as the covariate was used to assess conditioned fear during the test presentation of Cue X. Notably, the ANCOVA only reduces any possible impact of the differences in baseline scores and does not eliminate them. This analysis revealed differences among groups, F(2, 41) = 16.52, MSE = 0.11, p < .005 (see Figure 1). Importantly, a visual comparison of the pre-CS and CS scores in Figure 1 reveals that the CS scores do not mirror the pre-CS scores. This suggests that the differences between the different and same conditions in responding to CS X are not related to baseline differences. Pairwise comparisons were conducted to illuminate the basis of the differences among the groups. These comparisons revealed that blocking occurred in both the same, F(1, 41) = 32.83, MSE = 0.11, p < .001, and different, F(1, 41) = 12.31, MSE = 0.11, p < .001, conditions, but Group Blk-Same suppressed less to Cue X than did Group Blk-Diff, F(1, 41) = 6.90, MSE = 0.11, p < .02, thereby indicating that greater blocking occurred when the blocking cue A originated from the same spatial location as the blocked target cue X. That is, the results support the view that blocking is facilitated when the blocking cue and the blocked target cue originated from the same spatial location.
Figure 1.
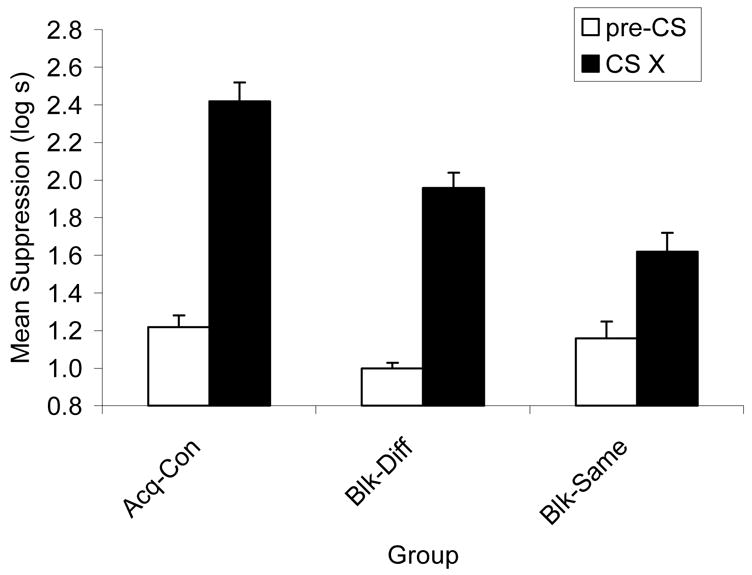
Experiment 1: CS X means are ANCOVA adjusted with pre-CS as the covariant. The Blk groups were those in which blocking was expected due to prior training of the competing cue in Phase 1. Higher bars indicate stronger suppression and lower bars indicate weaker suppression. Group Acq-Con served as a basic acquisition control. Enhanced blocking is evident in the lower responding to the target stimulus X in the same condition (Group Blk-Same) than in the different condition (Groups Blk-DiffA1 and Blk-DiffA2 pooled) and the control group.
Although there were no significant differences in baseline drinking on Day 11, ps > .05, in order to maintain consistency with the analysis of responding to Cue X, a one-way ANCOVA with the baseline (i.e., pre-CS) scores as the covariate was used to assess conditioned fear during the test presentation of Cue A. Of interest was whether the subjects learned to discriminate between the reinforced and nonreinforced locations. The ANCOVA on the Day 11 data revealed differences in suppression to CS A among groups, F(3, 40) = 3.97, MSE = 0.13, p < .02 (see Figure 2). A pairwise comparison of the difference between Groups Blk-DiffA1 and Blk-DiffA2, revealed no significant difference between these groups, p > .05, but visual inspection of the means indicates a nonsignificant tendency for subjects to discriminate between the reinforced location and the nonreinforced location suggesting that spatial location of the cues was learned. The fact that testing on Cue A followed testing of Cue X perhaps made it difficult to detect a difference between test locations for Cue A.
Figure 2.
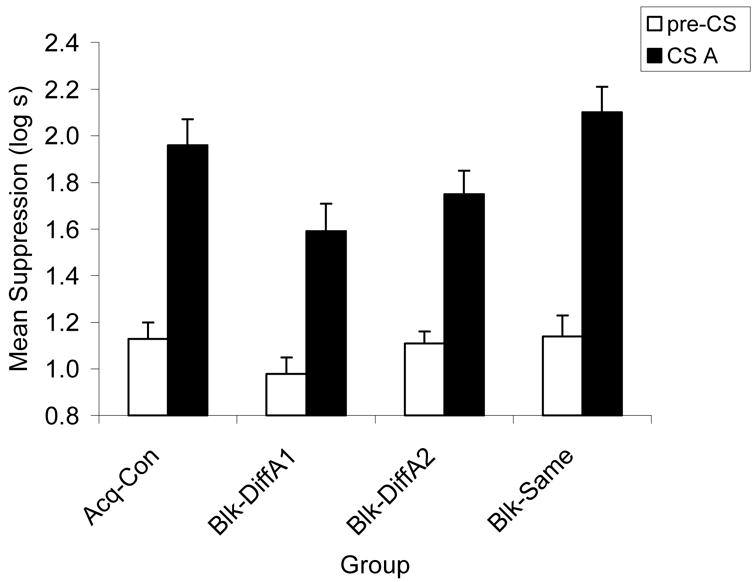
Experiment 1: CS A means are ANCOVA adjusted with pre-CS as the covariant. Groups Blk-DiffA2, Blk-Same, and Acq-Con were those in which strong responding to cue A was expected due to prior training of the competing cue in Phase 1 (Groups Blk-DiffA2 and Blk-Same) or Phase 2 (Group Acq-Con). Higher bars indicate stronger suppression and lower bars indicate weaker suppression. Weaker responding to cue A was expected in Group Blk-DiffA1 because at test cue A emanated from a location different from that when it had been reinforced. Discrimination of the reinforced and nonreinforced locations is (nonsignificantly) suggested in the lower responding to cue A in Group Blk-DiffA1 than in Group Blk-DiffA2.
Experiment 2
Experiment 1 successfully demonstrated the facilitative effects of spatial similarity on cue interaction between cues that are trained in compound. That is, when two cues originate from the same spatial location during blocking treatment, the interaction between them observed at the time of test is greater. The effects seen in Experiment 1 parallel similar effects observed when the role of temporal similarity in interaction between cues trained together was assessed (e.g., Barnet et al., 1993). That is, competition was enhanced when the blocking stimulus and blocked stimulus shared the same temporal relationship with the US. Importantly, the role of temporal similarity in interaction between cues trained apart (e.g., Escobar & Miller, 2003) seems to parallel the role it has in competition between cues trained together (i.e., similar temporal relationships of the interacting cues result in enhanced interference). Because of the parallel between the observed effects in prior demonstrations of the role of temporal similarity in cue competition and cue interference, one might expect the role of spatial similarity in cue competition and cue interference to also be parallel. Thus, the objective of Experiment 2 was to assess the influence of spatial similarity between the interfering association and the target association on proactive interference (i.e., cues trained apart) in first-order Pavlovian conditioning to see if the results of Experiment 1 could be obtained in an interference paradigm. That is, would greater interference be seen when the interfering cue trained in the first phase and the target cue trained in the second phase originate from the same location? Amundson et al. (2003) obtained proactive interference in first-order Pavlovian conditioning and investigated how several manipulations commonly used to control interaction between cues trained together also influenced interaction between cues trained apart, but they did not examine the effects of spatial similarity. The present experiment was designed using many of the parameters that they found yielded proactive interference.
Method
Subjects and Apparatus
The subjects were 24 male (190 – 260 g) and 24 female (167 – 192 g) naïve Sprague-Dawley rats, bred in our colony. Subjects were maintained and housed as described in Experiment 1. The apparatus was identical to that used in Experiment 1 except for the two tones that served as the target (X) and interfering (A) cues, counterbalanced within groups.
Procedure
Subjects were randomly assigned to one of four groups: Acquisition-Control (Acq-Con), Proactive-Interference-Different-A1 (PI-DiffA1), Proactive-Interference-Different-A2 (PI-DiffA2), and Proactive-Interference-Same (PI-Same), counterbalanced for sex (ns = 12). The design is depicted in Table 2. Acq-Con refers to the acquisition control group (i.e., no interference treatment) for which no interfering pairing was presented, PI-Diff refers to the groups for which the interfering and target cues were presented at different locations, and PI-Same refers to the group for which the interfering and target cues were presented at the same location. Maximal interference was anticipated in Group PI-Same due to the common physical location of the interfering and target cues. Weaker interference was expected in the two PI-Diff groups due to the dissimilar physical location of the two cues. The only difference between the PI-DiffA1 and PI-DiffA2 groups was the location from which Cue A emanated on its test day.
Table 2.
Design of Experiment 2
| Group | Preexposure | Phase 1 | Phase 2 | Test X | Test A |
|---|---|---|---|---|---|
| Acq-Con | 2 A-, 2 X- | 12 A2/12 US/12 A1 - | 2 X1 → US | X1 | A1 or A2 |
| PI-DiffA1 | 2 A-, 2 X- | 12 A2 → US/12 A1 - | 2 X1 → US | X1 | A1 |
| PI-DiffA2 | 2 A-, 2 X- | 12 A2 → US/12 A1 - | 2 X1 → US | X1 | A2 |
| PI-Same | 2 A-, 2 X- | 12 A1 → US/12 A2 - | 2 X1 → US | X1 | A1 |
Note: The superscripts indicate the location of the CS (1 = Location 1 and 2 = Location 2). During preexposure each stimulus was presented from both Location 1 and Location 2. The numbers preceding the letters indicate the total number of presentations of the stimuli in that phase. Slashes separate unpaired presentations of CSs and USs. Stimuli A and X were a low frequency tone and white noise, respectively. → indicates “followed by.”
Acclimation and preexposure
Days 1–3 were identical to those in Experiment 1.
Phase 1. Interfering association training
On Days 4–6, subjects in Groups PI-Same, PI-DiffA1 and PI-DiffA2 received four daily A-US pairings and four daily A-only presentations in 120-min sessions. For the purpose of discrimination training, Cue A from one location (Location 1 for Group PI-Same and Location 2 for Groups PI-DiffA1 and PI-DiffA2) was reinforced and from the other location was not reinforced, with the spatial locations (1 and 2) left/right counterbalanced within groups. The US onset coincided with Cue A termination. Two different schedules were used on alternate days. In Schedule 1 the reinforced stimulus presentations on Days 4 and 6 occurred at 10, 30, 80, 105 min into session, whereas the nonreinforced presentations of Cue A occurred at 15, 45, 60, and 90 min into the session. In Schedule 2 the reinforced stimulus presentations on Day 5 occurred at 4, 54, 89, and 109 min into the session, whereas the nonreinforced Cue A presentations occurred at 8, 23, 43, and 73 min into the session. Group Acq-Con received four explicitly unpaired presentations of Cue A and the US per day. One half of Group Acq-Con received four presentations of A from Location 1 and the other half of this group received four presentation of A from Location 2. For all subjects in this group stimulus presentations occurred at 3, 40, 60, and 95 min into the session for Schedule 1 (Days 4 and 6) and at 10, 36, 69, and 116 min into the session for Schedule 2 (Day 5). The footshock US presentations occurred simultaneously in all groups and lasted 0.5 s. The duration of the sessions in this and subsequent phases was 120 min, based on a prior demonstration of proactive interference in first-order Pavlovian conditioning in our laboratory (Amundson et al., 2003). This decreased the likelihood of obtaining a US-preexposure effect in Group Acq-Con (i.e., it presumably decreased the strength of association between the US and the context).
Phase 2. Target training
On Day 7, all subjects received two X-US pairings. Cue X was generated from Location 1. Onset of Cue X occurred at 30 and 90 min into the 120-min session, with the footshock US being presented immediately after termination of Cue X and lasting for 0.5 s.
Reacclimation
On Days 8 and 9, all subjects were exposed to the experimental context for 60 min with lick tubes available. Stimuli A and X were not presented. This treatment served to reestablish a uniformly high baseline rate of drinking among all groups.
Testing
On Days 10 and 11, all subjects in all groups were tested for conditioned lick suppression to Cues X and A, respectively. We tested all subjects on Cue X on Day 10 because it was the cue of primary interest. The test stimulus was presented from Location 1 immediately upon completion of the first five cumulative seconds of licking. On Day 10 in a 16-min session, all subjects were exposed to Cue X for 15 min and time to complete five cumulative seconds of licking in the presence of the cue was recorded. On Day 11, identical testing occurred with Cue A, with Cue A being generated from the same location from which it originated in Phase 1, except for Group PI-DiffA2 for which Stimulus A originated from the nonreinforced location. Based on the previously described pre-CS score criterion, the data from no animals were eliminated from the statistical analyses.
Results and Discussion
Experiment 2 demonstrated that proactive interference was greater when the interfering and target cues originated from the same location. The following statistics support this conclusion. Because the only difference between Groups PI-DiffA1 and PI-DiffA2 was the location from which cue A emanated on Day 11 and these two groups did not differ significantly in suppression to X (p = .42), these groups were pooled during the assessment of conditioned fear to cue X resulting in a single PI-Diff group. A one-way ANOVA on the Day 10 baseline scores (i.e., time to complete 5 cumulative seconds of drinking prior to CS onset) recorded during testing revealed no differences in initiation of drinking, F < 1.0. Therefore, a one-way ANOVA was used to assess conditioned fear during the test presentation of Cue X. This analysis revealed differences among groups, F(2, 45) = 7.16, MSE = 0.09, p < .005 (see Figure 3). Pairwise comparisons were conducted to better understand the basis of the differences among the groups. These comparisons revealed that Group PI-Same suppressed less to Cue X than did Group PI-Diff, F(1, 45) = 10.55, MSE = 0.09, p < .003, thereby suggesting that more interference occurred when, in an interference paradigm, the interfering Cue A shared the same spatial origin during training as Target Cue X. Additionally, a significant difference between Group PI-Same and Group Acq-Con, F(1, 45) = 11.98, MSE = 0.09, p < .005, and a nonsignificant difference between Group PI-Diff and Group Acq-Con, p > .77, support the conclusion that interference is facilitated when the interfering cue and the target cue share the same spatial location.
Figure 3.
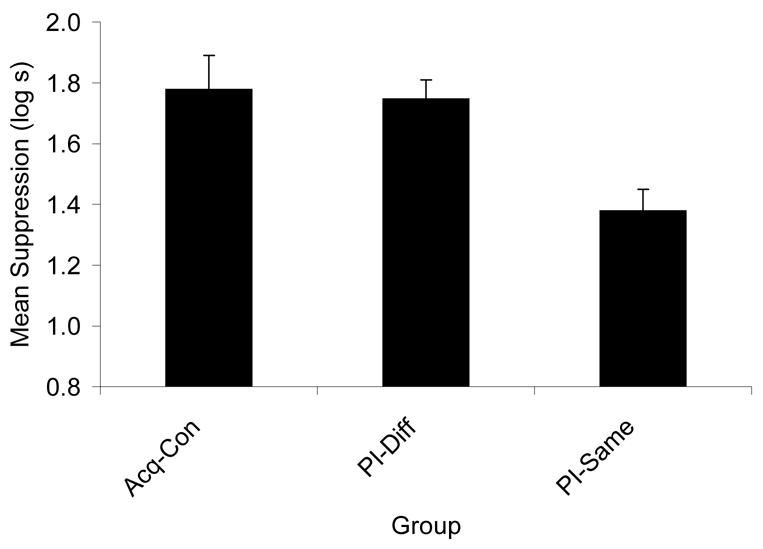
Means of Experiment 2 from the CS X test. The PI groups were those in which proactive interference was expected due to interference training of Phase 1. Group Acq-Con served as a basic acquisition control. Higher bars indicate stronger suppression and lower bars indicate weaker suppression. Enhanced interference is evident in the lower responding to the target stimulus X in same condition (Group PI-Same) than in the different condition (Groups PI-DiffA1 and PI-DiffA2 pooled) and the control group.
An ANOVA was used to assess conditioned fear during the test presentation of Cue A on Day 11. Of interest was whether the subjects learned to discriminate between Cue A coming from the reinforced and nonreinforced locations. This analysis revealed differences among groups, F(3, 44) = 3.62, MSE = 0.05, p < .02 (see Figure 4). Pairwise comparisons detected differences between Group PI-Same and Group Acq-Con, F(1, 44) = 7.75, MSE = 0.05, p < .01 and between Group PI-DiffA2 and Group Acq-Con, F(1, 44) = 6.63, MSE = 0.05, p < .02, indicated that an association was formed between Cue A and the footshock when these two events were paired in Phase 1 but not when these events were unpaired (Group Acq-Con). Additionally, a pairwise comparison of the difference between Groups PI-DiffA1 and PI-DiffA2, revealed no significant difference between these groups, p > .05, but visual inspection of the means indicates a nonsignificant tendency for subjects to discriminate between the reinforced location and the nonreinforced location suggesting that spatial location of the cues was learned. Again, the fact that testing on Cue A followed testing of Cue X perhaps made it difficult to detect a difference between test locations for Cue A..
Figure 4.
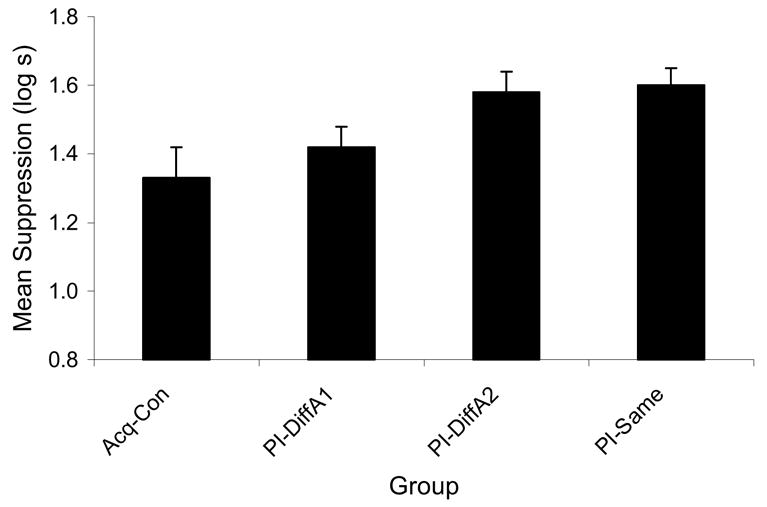
Means of Experiment 2 from the CS A test. The PI-DiffA2 and PI-Same groups were those in which strong responding to cue A was expected due to prior training of the competing cue in Phase 1. Higher bars indicate stronger suppression and lower bars indicate weaker suppression. Weaker responding to cue A was expected in Group PI-DiffA1 because cue A emanated from a location different from that when it had been reinforced. Discrimination of the reinforced and nonreinforced locations is evident in the lower responding to cue A in Group PI-DiffA1 than in Group PI-DiffA2.
General Discussion
The current results indicate that similarity in spatial origin of two cues with a common outcome encourages interaction between cues both in a condition in which cues are trained together (blocking; Experiment 1) and a condition in which cues are trained apart (proactive interference; Experiment 2). That is, greater competition and greater interference occurred between cues originating from the same spatial location compared to a condition in which the cues originated in different spatial locations.
Prior demonstrations of the role of similarity in cue interaction between cues trained together focused solely on the influence of the temporal similarity of the target association relative to the nontarget association (e.g., Barnet, Grahame, & Miller, 1993; Escobar & Miller, 2003). The results of Experiment 1 provide evidence for the importance of spatial similarity in cue interaction between cues trained together. Specifically, the results demonstrate that high spatial similarity, like high temporal similarity, facilitates the interaction of the competing cue and the target cue. When the nontarget cue and the target cue in Group Blk-Same originated from the same spatial location, we observed stronger attenuation of conditioned responding to the target cue (i.e., enhanced blocking) relative to responding when the nontarget cue and the target cue in Group Blk-Diff originated from different spatial locations. With an interference paradigm, Experiment 2 revealed effects parallel to Experiment 1. That is, attenuated conditioned responding to the target cue (i.e., proactive interference) was observed when the interacting cue and the target cue emanated from the same spatial location. The parallel effects observed when the temporal similarity of the interfering association was manipulated relative to the target association (e.g., Barnet et al., 1993, in a blocking procedure; Escobar & Miller, 2003 in a retroactive interference procedure) suggest that spatial and temporal similarity have comparable effects on stimulus interaction.
In contrast to the current findings, Glautier (2002) reported that similar spatial origin of two cues to a common outcome can attenuate competition between the competing and target cues. Glautier, using a contingency learning task with humans, assessed the effects of spatial contiguity on blocking. Participants were trained with a computerized card game to predict, based on the colors and symbols on the backs of the cards (i.e., the blocking and blocked cues), which cards resulted in the best payout (i.e., the outcome). The results showed that, when the blocking cue and blocked cue were on the same card, blocking did not occur. In contrast, if the cues were on different cards, then blocking occurred. Consequently, Glautier concluded that spatial separation of the cues facilitated blocking. However, there is another possible interpretation for his results. In Glautier’s same-card condition, the symbol on the color may have reduced the color to background status, thereby undermining its ability to block the symbol. Alternatively, there may have been generalization decrement between Phases 1 and 2 going from one color and symbol pair to a pair composed of the same color and a different symbol. Both of these factors could have reduced blocking when the symbol was superimposed on the color. Nonetheless, Glautier’s findings suggest that similar spatial relationships of a target cue and a competing cue, in some circumstances, can promote an increase in responding to a target cue rather than an attenuation of responding to a target cue in cue competition. That is, one might expect a facilitation of responding to the target cue when conditions favor generalization between the nontarget cue and the target cue.
A similar effect was observed by Martin and Levey (1991). They used human participants in an eyeblink preparation and observed weaker blocking when the blocking and blocked stimuli were next to one another than when the blocking and blocked stimuli were separated by one panel in their array of colored lights. However, this effect of spatial separation was observed only across experiments and the authors noted that high variability both in the between and within-subjects comparisons obscured their experimental effects. Thus, any conclusions one can make from this finding are tenuous and further emphasize the need for the current experiments.
A potential issue for the current findings is that in both experiments the discrimination procedure used (A from one location being reinforced, A from the second location not being reinforced) potentially made the nonreinforced spatial location inhibitory. In principle, this could have resulted in superconditioning of the target cue (X) when it emanated from the location that had become inhibitory. That is, rather than weaker suppression in the Same conditions of Experiments 1 and 2 reflecting greater blocking and proactive interference, respectively, the difference between the Same and Different conditions may have arisen from enhanced suppression due to superconditioning of X in the Diff condition. Although this is a possibility, it seems unlikely to account for the present results. In Experiment 1, the presentation of the excitatory A stimulus during Phase 2 trials should have counteracted any potential inhibitory properties of the nonreinforced location. That is, in Phase 2 of Experiment 1 both the potential inhibitory stimulus (the location) and the excitatory blocking cue A were present and thus, one might expect little superconditioning of X. Additionally, in both Experiments 1 and 2, at test the presumed inhibitory location and the target cue X were presented together. Consequently, one would expect reduced responding to the target cue X (i.e., a reversal of the superconditioning effect). However, stronger responding was observed when X emanated from the different spatial location (i.e., location in which A was not reinforced), which suggests that the location did not inhibit responding to X.
As mentioned in the introduction, most contemporary associative learning models (e.g., Gallistel & Gibbon, 2000; Mackintosh, 1975; McLaren & Mackintosh, 2000; Miller & Matzel, 1998; Pearce & Hall, 1980; Rescorla & Wagner, 1972) were designed to account for competition between cues trained together that predict a single outcome. The typical explanation used by these models to account for cue competition effects is that the relatively low responding to the target cue is a consequence of the target cue’s failing to acquire an association to the outcome (e.g., Rescorla & Wagner, 1972) or a failure to retrieve the target cue-outcome association at the moment of testing (e.g., Miller & Matzel, 1988). The combined results of Experiments 1 and 2 demonstrate a commonality between interaction between cues trained together and between cues trained apart. This commonality suggests that a similar mechanism may underlie these two types of cue interaction. However, the aforementioned models fail to account for interference between cues or outcomes that are trained apart.
The findings from situations in which a high degree of temporal similarity facilitates cue interaction in conjunction with the current findings requires one to accept the notion that spatial location of stimuli as well as temporal location of stimuli are ordinarily encoded during learning; that is, it invites a spatial coding hypothesis to parallel the temporal coding hypothesis (Miller & Barnet, 1993), or better, a unified spatiotemporal coding hypothesis. In order to tailor such an approach to explain the role of spatiotemporal attributes in stimulus representation, one might consider the tenets of the temporal coding hypothesis. The tenets of the temporal coding hypothesis state that: a) contiguity alone is necessary for the formation of an association, b) the temporal relationship between the associated events is automatically encoded as part of the association (i.e., temporal maps are formed that link events), c) temporal information plays a role in the nature, magnitude, and timing of the conditioned response, and d) temporal maps are superimposed when elements common to those maps are presented together even when the elements have been trained apart (i.e., temporal information from different training situations can be integrated). A spatial version of these tenets can easily be applied to the current findings. That is, spatial information is automatically encoded as part of the association, plays a role in the nature and magnitude of a conditioned response, and spatial maps can be superimposed when there are elements common to those maps (see Blaisdell & Cook, 2005) for data supportive of this view). The current findings indicate that spatial information is relevant to stimulus interaction regardless of the type of interaction (i.e., cues trained together, e.g., blocking in Experiment 1, or cues trained apart, e.g., proactive interference in Experiment 2).
Additionally, one might consider an attentional account of the current findings. For example, in both experiments spatial similarity perhaps facilitated cue interaction by focusing attention on the local context of the competing cue (Experiment 1) or the interfering cue (Experiment 2). However, the target cues (i.e., the blocked cue in Experiment 1 and the cue subject to proactive interference in Experiment 2) were both trained in Phase 2 after the blocking and interfering cues were trained in Phase 1. Hence, greater attention to the location from which the target cues emanated should have resulted in superior learning and hence superior stimulus control of behavior, not more impaired stimulus control relative to the target cues emanating from different locations than the blocking and interfering cues as was observed in the Same condition of both experiments. This raises the question, why was more responding to the target cue not observed in the Same condition? In order to answer this one can consider the early theoretical accounts of similarity in interference put forth by Skaggs (1925) and Robinson (1927). They proposed an inverted U-shaped function in which interference was greatest when an intermediate level of similarity existed between the target association and the interfering association (e.g., the apex of the U), weaker when the two associations were completely dissimilar (e.g., points on the arc to the left of the apex), and no interference (in fact facilitation) when the two associations were identical (e.g., points on the arc to the right of the apex). In the case of the current experiments one might assume that the Different condition represents points on the arc to the left of the apex and the Same condition represents a point near the apex. In order to effectively assess this, one would need to design an experiment in which similarity was examined at a large number of points along a single [spatial] dimension rather than simply two points as were examined in the present research while holding other attributes constant (e.g., type of auditory stimulus).
Further consideration of the current findings suggests that the representation of an association includes information about both where the CS originates and where the US originates. That is, relative to prior studies demonstrating that the CS signals to the subject the what (e.g., Kehoe, Poulos, & Gormezano, 1985), where (e.g., Christie, 1996), and when (e.g., Kirkpatrick & Church, 2003) of the US, the current findings suggest that the representation of a CS also includes the what, where, and when for the CS. In other words, perhaps the representation of the CS not only denotes what the CS is but also when (and potentially where) it occurs relative to other stimuli. However, this notion can only be supported by the current evidence in conjunction with evidence from prior studies and therefore, one would need to conduct an experiment in which both the location of the CS and US were manipulated in order to differentiate the roles of the two wheres.
The parallels demonstrated between spatial and temporal location in both types of stimulus interaction raise the question as to what the benefit is in encoding each as part of an association. Besides response timing and locating, it appears that each serves, like other attributes, to help the subject differentiate between related memories. High similarity of such attributes increases the likelihood that one memory will influence the interpretation of the other memory. However, if the spatial or temporal information is less similar, then the organism can make a clearer distinction between the interfering and target associations, and consequently they are less apt to interact through comparator or priming processes. If spatial (and temporal) similarity facilitates cue interaction, then it seems important that contemporary learning theories embrace the acquisition, storage, retrieval, and use of spatial (and temporal) information.
Footnotes
Support for this research was provided by NIMH Grant 33881. We thank Olga Lipatova, Gonzalo Urcelay, Kouji Urushihara, and Daniel Wheeler for their comments on a preliminary version of this manuscript. Requests for information concerning this research should be addressed to Ralph R. Miller, Department of Psychology, SUNY-Binghamton, NY 13902-6000, USA; e-mail: rmiller@binghamton.edu.
References
- Amundson JC, Escobar M, Miller RR. Proactive interference between cues trained with a common outcome in first-order Pavlovian conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 2003;29:311–322. doi: 10.1037/0097-7403.29.4.311. [DOI] [PubMed] [Google Scholar]
- Barnet RC, Grahame NJ, Miller RR. Temporal encoding as a determinant of blocking. Journal of Experimental Psychology: Animal Behavior Processes. 1993;19:327–341. doi: 10.1037//0097-7403.19.4.327. [DOI] [PubMed] [Google Scholar]
- Blaisdell AP, Gunther LM, Miller RR. Recovery from blocking achieved by extinguishing the blocking CS. Animal Learning & Behavior. 1999;27:63–76. [Google Scholar]
- Blaisdell AP, Cook RG. Integration of spatial maps in pigeons. Animal Cognition. 2005;1:7–16. doi: 10.1007/s10071-004-0223-1. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychological Bulletin. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Chamizo VD. Acquisition of knowledge about spatial location: Assessing the generality of the mechanism of learning. Quarterly Journal of Experimental Psychology. 2003;56B:102–113. doi: 10.1080/02724990244000205. [DOI] [PubMed] [Google Scholar]
- Christie J. Spatial contiguity facilitates Pavlovian conditioning. Psychonomic Bulletin & Review. 1996;3:357–359. doi: 10.3758/BF03210760. [DOI] [PubMed] [Google Scholar]
- Ellins SR, Von Kluge S. Preexposure and extinction effects of lithium chloride induced taste-potentiated aversions for spatially contiguous auditory food cues in rats. Behavioral Neuroscience. 1987;101:164–169. doi: 10.1037//0735-7044.101.2.164. [DOI] [PubMed] [Google Scholar]
- Escobar M, Matute H, Miller RR. Cues trained apart compete for behavioral control in rats: Convergence with the associative interference literature. Journal of Experimental Psychology: General. 2001;130:97–115. doi: 10.1037/0096-3445.130.1.97. [DOI] [PubMed] [Google Scholar]
- Escobar M, Miller RR. Timing in retroactive interference. Learning & Behavior. 2003;31:257–272. doi: 10.3758/bf03195987. [DOI] [PubMed] [Google Scholar]
- Gallistel CR, Gibbon J. Time, rate, and conditioning. Psychological Review. 2000;107:289–344. doi: 10.1037/0033-295x.107.2.289. [DOI] [PubMed] [Google Scholar]
- Glautier S. Spatial separation of target and competitor cue enhances blocking in human causality judgments. Quarterly Journal of Experimental Psychology. 2002;55B:121–135. doi: 10.1080/02724990143000207. [DOI] [PubMed] [Google Scholar]
- Kamin LJ. ‘Attention-like’ processes in classical conditioning. In: Jones MR, editor. Miami symposium on the prediction of behavior: Aversive stimulation. Miami, FL: University of Miami Press; 1968. pp. 9–33. [Google Scholar]
- Kehoe EJ, Poulos CX, Gormezano I. Appetitive differential conditioning of the rabbit's jaw movement response: Effects of cue similarity and US magnitude. Pavlovian Journal of Biological Science. 1985;20:29–38. [PubMed] [Google Scholar]
- Kirkpatrick K, Church RM. Tracking of the expected time to reinforcement in temporal conditioning procedures. Learning & Behavior. 2003;31:3–21. doi: 10.3758/bf03195967. [DOI] [PubMed] [Google Scholar]
- Johnston WA. Bidirectional interference in an A-B, C-B paradigm. Journal of Verbal Learning and Verbal Behavior. 1968;7:305–311. [Google Scholar]
- Lashley KS. An examination of the continuity theory as applied to discriminative learning. Journal of General Psychology. 1942;26:241–265. [Google Scholar]
- Mackintosh NJ. A theory of attention: Variations in the associability of stimuli with reinforcement. Psychological Review. 1975;82:276–298. [Google Scholar]
- Martin I, Levey AB. Blocking observed in human eyelid conditioning. Quarterly Journal of Experimental Psychology. 1991;43B:233–256. [PubMed] [Google Scholar]
- Matute H, Pineño O. Stimulus competition in the absence of compound conditioning. Animal Learning & Behavior. 1998;26:3–14. [Google Scholar]
- McGeoch JA, Nolen ME. Studies of Retroactive inhibition: Temporal point of interpolation and degree of retroactive inhibition. Journal of Comparative Psychology. 1933;15:407–417. [Google Scholar]
- McLaren IPL, Mackintosh NJ. An elemental model of associative learning: I. latent inhibition and perceptual learning. Animal Learning & Behavior. 2000;28:211–246. [Google Scholar]
- Miller RR, Matzel LD. The comparator hypothesis: A response rule for the expression of associations. In: Bower GH, editor. The psychology of learning and motivation. Vol. 22. San Diego, CA: Academic Press; 1988. pp. 51–92. [Google Scholar]
- Pearce JM, Hall G. A model for Pavlovian learning: Variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychological Review. 1980;87:532–552. [PubMed] [Google Scholar]
- Rescorla RA, Cunningham CL. Spatial contiguity facilitates Pavlovian second-order conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1979;5:152–161. doi: 10.1037//0097-7403.5.2.152. [DOI] [PubMed] [Google Scholar]
- Robinson ES. The similarity factor in retroaction. American Journal of Psychology. 1927;39:297–312. [Google Scholar]
- Skaggs EB. Further studies in retroactive inhibition. In: Frantz SI, Warren HC, Watson JB, Bently M, Ferneberger SW, editors. Psychological Monographs. Vol. 34. Princeton, NJ: Psychological Review Company; 1925. [Google Scholar]
- Slamecka NJ, Ceraso J. Retroactive and proactive inhibition of verbal learning. Psychological Bulletin. 1960;57:449–475. [Google Scholar]
- Swenson EJ. University of Minnesota Studies in Education. Vol. 1. Minneapolis: University of Minnesota Press; 1941. Retroactive inhibition: A review of the literature; pp. 7–41. [Google Scholar]
- Underwood BJ. Interference and forgetting. Psychological Review. 1957;64:49–60. doi: 10.1037/h0044616. [DOI] [PubMed] [Google Scholar]
- Underwood BJ, Estrand BR. Linguistic associations and retention. Journal of Verbal Learning & Verbal Behavior. 1968;1:162–171. [Google Scholar]


