Abstract
In Pavlovian fear conditioning, context-mediated decrements in conditioned responding (e.g., the US-preexposure effect) can counteract competition between cues trained together (e.g., overshadowing). Two experiments were conducted using rats in a conditioned lick suppression preparation to determine whether context-mediated competition also counteracts competition between cues trained apart (retroactive interference). In Experiment 1, a combination of degraded contingency and retroactive interference treatments produced less of a decrement in conditioned responding than either of those treatments alone. Experiment 2 showed that retroactive interference treatment attenuates the normally deleterious effect of trial massing. The results suggest that there are empirical similarities shared by interference between cues trained apart and competition between cues trained together.
The field of Pavlovian conditioning has largely been concerned with the factors that impact the acquisition or extinction of associations that form between conditioned stimuli (CSs) and unconditioned stimuli (USs). These factors include variables such as CS salience, US intensity, contingency, contiguity, trial spacing, and CS duration, among others. In recent decades, many studies of Pavlovian conditioning have investigated the ways that stimuli interact when they are trained together (i.e., in compound) with a common US. Compound training often results in reduced expression of the CS-US association by one or both of the CSs, relative to a condition in which the CSs are trained alone (i.e., elementally). Overshadowing (Pavlov, 1927, pp. 142–143 and 269–270) is a classic example of this sort of cue competition in which two CSs are trained together and conditioned responding to one of the CSs (typically the less salient CS) is reduced relative to a situation in which that same CS is trained elementally.
Research suggests that some of the factors that affect conditioned responding to an elementally trained CS have a different impact on a CS that is trained in compound with another CS. For example, when a CS is preexposed without reinforcement prior to CS-US training trials, the expression of the CS-US association will be retarded in the initial trials (i.e., latent inhibition; e.g., Lubow & Moore, 1959). Latent inhibition is a ubiquitous effect that has been observed across many tasks and species (for reviews, see Lubow, 1989; Lubow & Gerwitz, 1995), and at least operationally CS preexposure may be considered as a reliable influence of CS-US contingency on Pavlovian conditioning. However, when a CS is trained in compound with another CS, CS preexposure does not always lead to retarded conditioned responding. In certain compound conditioning situations, CS preexposure actually attenuates the overshadowing effect that otherwise occurs as a result of compound training (Blaisdell, Bristol, Gunther, & Miller, 1998; Loy & Hall, 2002; but see Nakajima, Ka, & Imada, 1999; Nakajima & Nagaishi, 2005). In a conditioned suppression preparation with rat subjects, Blaisdell et al. observed that preexposure to a CS (X) before reinforcing it in compound with a more salient CS (A) results in conditioned responding to X that is stronger than in a situation in which X is not preexposed prior to compound training. Furthermore, the conditioned responding to X after CS preexposure and compound conditioning was stronger relative to a situation in which X was preexposed and then reinforced elementally. In summary, the otherwise response-degrading effects of overshadowing training (AX-US) and latent inhibition training (X- followed by X-US) seem to counteract each other when the two treatments are combined (X- followed by AX-US). The counteraction observed by Blaisdell et al. was robust to the point that responding to X after combined latent inhibition and overshadowing treatments appeared to be little different than responding to X after it had been reinforced elementally with no nonreinforced preexposure to X.
The results of Blaisdell et al. (1998) suggest that at least one of the factors that reliably impacts conditioned responding to an elementally trained CS can have a different effect on responding to a CS that is trained in compound with another CS. Their pioneering result has been developed further by other studies that investigated factors that usually negatively impact conditioned responding other than CS preexposure. It has been found that the effect of overshadowing treatment is attenuated by normally response-degrading treatments such as unsignalled US presentations prior to conditioning (i.e., US preexposure; Urushihara & Miller, in press), interspersed unsignalled US presentations (i.e., degraded contingency treatment; Urcelay & Miller, 2006), interspersed unreinforced CS presentations (i.e., partial reinforcement; Urushihara & Miller, 2006), long CS durations (Urushihara, Stout, & Miller, 2004; Westbrook, Homewood, Horn, & Clarke, 1983), and temporally massed training trials (Stout, Chang, & Miller, 2003). Taken together, these results provide strong evidence of a dissociation in processing between CSs that are trained in compound and CSs that are trained elementally.
Miller and his colleagues have suggested that results such as those presented above indicate that the factors that impact conditioning can be different for elementally trained stimuli than for stimuli trained in compound (e.g., Urushihara et al., 2004). This broad statement is somewhat misrepresentative of Miller et al.’s explicit account of the data (Denniston, Savastano, & Miller, 2001). More specifically, they claim that two response-degrading treatments may counteract each other because the stimuli that compete with a common target are themselves subject to competition. In all of the situations described above, there are two potential cues that may compete with the target stimulus (X). The more salient overshadowing stimulus is the obvious competing cue. Additionally, the context can also be viewed as a cue that competes with X in situations in which the context has an exceptionally strong association with the US (e.g., degraded contingency and US preexposure), the CS (e.g., CS preexposure and partial reinforcement), or both the CS and the US (e.g., situations with massed training trials). Miller et al. posit that, in certain situations, two competing cues (A and the context) can essentially compete with and counteract each other, resulting in little combined competition for X (a more detailed description of this account is included in the General Discussion). Notably, this explanation indicates that the most important factor in the aforementioned counteraction effects is the presence of two effective competing cues that can compete with one another.
The present experiments were conducted to investigate whether competing cues would counteract each other in situations in which one of the competing cues is not presented in compound with the target cue. In order to address this question, degraded contingency (Experiment 1) and trial massing (Experiment 2) treatments were combined with a retroactive interference (RI) paradigm (e.g., Escobar, Matute, & Miller, 2001). In this paradigm, RI is obtained when a nontarget cue (A) is paired with an outcome (O) after a target cue (X) is trained with the same outcome (X-O followed by A-O). Retroactive interference is evidenced by weaker expression of the X-O association relative to a control condition that receives no A-O pairings after X-O training. A-O training apparently retroactively interferes with the expression of the X-O association. Because X and A are never presented simultaneously, most models of Pavlovian conditioning do not anticipate RI.
Escobar et al. (2001) only observed RI if the outcome used during training was not an actual US. In a first order-conditioning preparation, no RI was observed presumably because a first-order CS tends to be resistant to reductions in conditioned responding potentially induced through indirect means, such as posttraining reinforcement of a competing CS (e.g., Miller & Matute, 1996). To avoid the use of a US during training, demonstrations of RI in Pavlovian conditioning have used a sensory preconditioning preparation. In this preparation, A, X, and O are all behaviorally neutral stimuli during training. After X-O and A-O training, O is reinforced with a US. Responding to the X stimulus is then assessed, with the assumption that any responding to X is dependent on the X-O and O-US associations. Because the impact of O-US association is presumed to be equal between the RI treatment and the control treatment (Escobar et al., 2001), any differences in responding to X may be attributed to the expression of the X-O association.
Experiment 1
Experiment 1 investigated whether degraded contingency treatment would interact with competition between cues trained apart (RI) in the same way that it interacts with competition between cues trained together (e.g., overshadowing; Urcelay & Miller, 2006). A cue-outcome contingency can be degraded in two ways. Either the cue can be presented without the outcome (i.e., partial reinforcement), or the outcome can be presented without the cue. In the Pavlovian conditioning literature, the degraded contingency effect specifically refers to situations in which interspersed unsignalled US presentations among CS-US training trials result in a reduction in responding to the CS (e.g., Rescorla, 1968). The degraded contingency effect is observed in both first-order conditioning and sensory preconditioning (e.g., Gunther & Miller, 2000), and it is attenuated when the target stimulus is trained in compound with an overshadowing stimulus (Urcelay & Miller, 2006).
Urcelay and Miller (2006) demonstrated that a target cue exposed to both overshadowing and degraded contingency treatments produced responding greater than a target cue that is exposed to either of those treatments alone. The following study incorporates Urcelay and Miller’s degraded contingency manipulation with Escobar et al.’s (2001) RI paradigm. In this study, RI (RI) was produced by training a cue (A) with an outcome (O) after the same outcome had been paired with another cue (X). The RI control (Ctrl) received X-O pairings in Phase 1, but only exposure to the A stimulus in Phase 2 (see Table 1). This control for interference was selected because it did not involve the presentation of any unsignaled exposures of O. In addition to the RI treatment, half of the subjects experienced degraded contingency (DC) treatment, that is, unsignalled O presentations in Phases 1 and 2. The other half received no degraded contingency treatment (NoDC). After Phases 1 and 2, all subjects experienced pairings of O with a footshock US in a third phase of training. Afterward, X was tested alone to assess the expression of the X-O association.
Table 1.
The design of Experiment 1
| Group | Phase 1 in Context A | Phase 2 in Context A | Phase 3 in Context B | Test X in Context B |
|---|---|---|---|---|
| Ctrl-NoDC | 8 X→O | 8 A | 8 O→US | CR |
| Ctrl-DC | 8 X→O / 12 O | 8 A / 12 O | 8 O→US | Cr |
| RI-NoDC | 8 X→O | 8 A→O | 8 O→US | Cr |
| RI-DC | 8 X→O / 12 O | 8 A→O / 12 O | 8 O→US | ? |
Note: Ctrl = Control, RI = Retroactive Interference, NoDC = no degraded contingency, DC = degraded contingency. X = complex tone, A = click train, O = white noise, US = footshock. The strength of the expected conditioned response is denoted by the size of the CR. The question mark denotes an uncertain outcome.
Because the degraded contingency manipulation intentionally varied the exposure of the outcome in the training context, we used a different context for Phase 3 and testing to avoid any differences in the excitatory value of the context during testing. If the extra O presentations in the RI and DC conditions caused an enhancement of the excitatory value of the test context, responding between groups could be differentially affected by summation of responding to the target cue and responding to the test context. Additionally, switching the context between Phases 2 and 3 should have had the additional benefit of reducing the potential effect of latent inhibition on the outcome that can be caused by nonreinforced exposure of a stimulus before reinforcement, which could also cause differences in responding between groups in this design (e.g., Lovibond, Preston, & Mackintosh, 1984).
There were two well-founded expectations for Experiment 1. Retroactive interference was expected, denoted by less conditioned suppression in group RI-NoDC compared to group Ctrl-NoDC. Also, a degraded contingency effect was expected, with less conditioned suppression in group Ctrl-NoDC compared to group Ctrl-DC. Of critical interest was whether the two combined treatments (RI-DC) would produce more or less conditioned suppression than either treatment alone (RI-NoDC or RI-DC). Intuitively, summation of response-degrading treatments might be expected. However, considering the research discussed above, it was possible that degraded contingency treatment would counteract RI, and vice-versa.
Method
Subjects
Subjects were 24 male (279 – 341 g) and 24 female (195 – 250 g), experimentally naive, Sprague-Dawley descended rats obtained from our own breeding colony. Subjects were randomly assigned to one of four groups (ns = 12), counterbalanced within groups for sex. The animals were individually housed in standard hanging stainless-steel wire-mesh cages in a vivarium maintained on a 16/8-hr light/dark cycle. Experimental manipulations occurred near the middle portion of the light phase. The animals received free access to Purina Lab Chow, whereas water availability was limited to 20 min per day following a progressive deprivation schedule initiated one week prior to the start of the study. From the time of weaning until the start of the study, all animals were handled for 30 s, three times per week.
Apparatus
Two types of enclosures, R and V, were used as Contexts A and B, counterbalanced. Acclimation, Phase 3, reacclimation, and testing took place in Context B, whereas Phases 1 and 2 took place in Context A. Enclosure R was a clear, Plexiglas chamber in the shape of a rectangular box 22.75 x 8.25 x 13.0 cm (l x w x h) with a floor constructed of 0.48 cm diameter rods 1.5 cm apart center-to-center, connected by NE-2 neon bulbs, which allowed constant-current footshock to be delivered by means of a high voltage AC circuit in series with a 1.0-MΩ resistor. Each of six copies of Enclosure R had its own environmental isolation chest. Enclosure R was dimly illuminated by a 2-W (nominal at 120 VAC) incandescent bulb driven at 80 VAC mounted on an inside wall of the environmental isolation chest approximately 30 cm from the animal enclosure. Background noise (primarily from a ventilation fan) was 74 dB (C scale, SPL).
Enclosure V was a 25.5-cm long box in the shape of a truncated-V (28 cm high, 21 cm wide at the top, 5.25 cm wide at the bottom). Each of six copies of Enclosure V had its own environmental isolation chest. The floor and sides were constructed of sheet metal. The ceiling was clear Plexiglas. The floor consisted of two parallel metal plates each 2-cm wide with a 1.25-cm gap between them. Enclosure V was dimly illuminated by a 7-W (nominal at 120 VAC) bulb driven at 80 VAC mounted on an inside wall of the environmental isolation chest approximately 30 cm from the animal enclosure with the light entering the animal enclosure primarily reflected from the roof of the environmental chest. Due to differences in opaqueness of the enclosures, this level of illumination roughly matched that of Enclosure R. Background noise (primarily from a ventilation fan) was 74 dB(C).
Each chamber (R and V) was also equipped with three 45-Ω speakers mounted on three different interior walls of each environmental chest, which could deliver a complex tone (800 and 1000 Hz) of 8 dB (C) above background, a 6 per s click of 8 dB (C), and a white noise of 8 dB (C) above the ambient background sound. In this experiment, the tone served as cue X, the clicks served as cue A, and the white noise served as the outcome. A and X were 30 s, whereas O was 10 s in duration. The US was a 0.5-s 1.0-mA footshock. Each chamber was dimly illuminated by a #1820 houselight. Chamber assignments between the four groups were counterbalanced.
Procedure
Acclimation
On Day 1, all the groups were acclimated to Context B (the test context) for 60 min to establish baseline drinking. During this session, the animals had free access to the water-filled tubes and no discrete stimuli used for training were presented. After acclimation, all of the water-filled lick tubes were removed.
Phases 1 and 2
As previously stated, Phases 1 and 2 took place in Context A. Phase 1 training occurred on Day 2 in a 77-min session. Subjects in all groups received 8 X→O pairings in which the onset of the 10-s O occurred as the 30-s X terminated. The mean intertrial interval (ITI) between stimulus X onsets was 578 ± 280 s. Subjects in the DC condition received an additional 12 exposures of O interspersed with this training. The mean ITI in this latter condition was 200±120 s between X and unsignalled O onsets. Phase 2 occurred on Day 3, and consisted of the same training as Phase 1, except the X stimulus was replaced by the A stimulus. Also, the A presentations were not followed by the outcome in the Ctrl condition.
Phase 3
On Days 4 and 5, all subjects received 4 O→US pairings per day in Context B. The 10-s O stimulus coterminated with the 0.5-s footshock. Trials occurred 10, 23, 37, and 48 min into the 60-min session.
Reacclimation
On Days 6 and 7, reacclimation took place in Context B for 60 minutes. During these sessions, the animals had free access to the water-filled tubes and no discrete stimuli used during training were presented. On Day 6, an extra 30-min reacclimation session was given to all of the subjects. The purpose of these reacclimation sessions was to stabilize baseline drinking that is usually disrupted by footshock.
Test of X
On Day 8, testing with CS X took place in Context B. After placement of the subjects in the testing context, the time required to complete the first five cumulative seconds of licking was recorded (preCS score) for each subject. Immediately following five cumulative seconds of licking, CS X was presented for 15 min, and the time it took the subject to complete five more cumulative seconds of licking (now in the presence of the CS) was recorded as our critical dependent variable (CS score). Thus, all subjects were drinking at the time of CS onset. As is the convention in our laboratory, any subjects that took more than 60 s to complete the first five cumulative seconds of licking were scheduled to be excluded from all the subsequent analyses because such high preCS scores would indicate an unusually strong fear to the testing context. This resulted in the elimination of data from one subject from group RI-DC. All the drinking latencies (both preCS and during the CS presentation) were converted into log (base 10) times to better approximate the within-groups normality assumption of parametric tests. An α level of .05 was selected as our criterion for significance.
Results and Discussion
The data are displayed in Figure 1. The Ctrl-NoDC group showed strong suppression to the target stimulus, which suggests that sensory preconditioning was observed (although there was no unpaired control in this experiment). Suppression was noticeably lower if X-O pairings were followed by A-O pairings (group RI-NoDC), denoting RI. Also, interspersed unsignalled outcome presentations among X-O pairings (group Ctrl-DC) caused low suppression, denoting a degraded contingency effect. However, subjects that experienced both A-O pairings and unsignalled outcome presentations showed levels of suppression that approached that of the Ctrl-NoDC group. Thus, it appears that although RI and degraded contingency lowered conditioned suppression to the target when they were administered alone, they were not as effective when they were combined. Alternatively stated, the two treatments seemed to counteract each other. The following statistical analyses support these observations.
Figure 1.
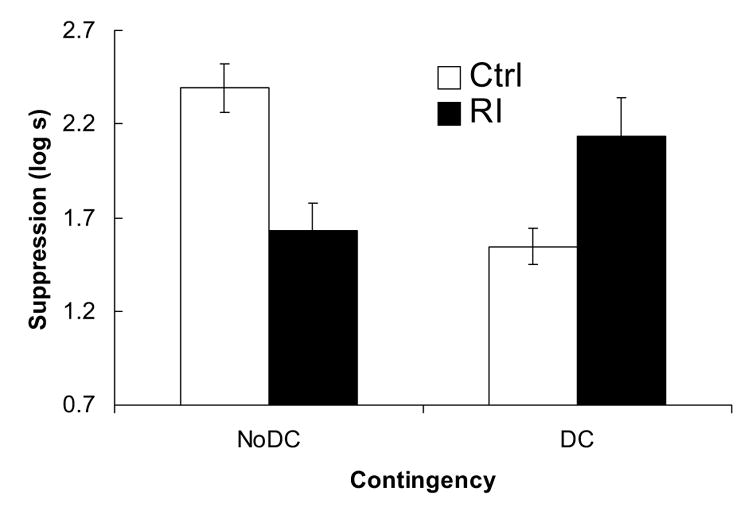
Experiment 1. The bars depict the mean suppression scores for the four groups. Larger bars denote longer latencies to resume drinking in the presence of the CS. Thus, smaller bars suggest less fear to the target stimulus. The error bars represent the standard error for each group.
Prior to analyzing the CS scores, the preCS scores were analyzed to determine whether the groups appreciably differed in fear of the test context. A 2 (Treatment: RI vs. Ctrl) x 2 (Contingency: DC vs. NoDC) analysis of variance (ANOVA) did not detect any significant effects or interaction, all ps > .19. Thus, we assumed that any group differences in suppression to the CS were not driven by differences in generalized fear of the context. A similar ANOVA was used to analyze the CS scores, which detected a significant interaction between treatment and contingency, F(1, 43) = 19.46, but no other significant effects, ps > .27. Planned comparisons were conducted to determine the source of the interaction. Group Ctrl-NoDC showed more fear than group Ctrl-DC, F(1, 43) = 15.56. Thus, a degraded contingency effect was observed when A-O pairings did not follow X-O training. Also, group Ctrl-NoDC expressed more fear than group RI-NoDC, F(1, 43) = 12.63, which indicates a RI effect when no unsignalled outcome presentations were interspersed with training. However, suppression in group RI-DC was greater than in group Ctrl-DC, F(1, 43) = 7.27, and group RI-NoDC, F(1, 43) = 5.35. Thus, when the two manipulations were combined, both of the effects were attenuated.
It is possible to explain the degraded contingency and RI effects observed here as a result of latent inhibition. Unsignaled outcome presentations could have caused latent inhibition of O, resulting in weaker sensory preconditioning in the Ctrl-DC and RI-NoDC groups relative to the Ctrl-NoDC group. However, this explanation does not account for the high level of fear observed in the RI-DC group. The subjects in this group received the largest number of O presentations prior to first-order conditioning, and still exhibited more fear than those in the Ctrl-DC and RI-NoDC groups. Thus, the lack of fear in the Ctrl-DC and RI-NoDC groups is not likely due to simple latent inhibition. Further support for this conclusion comes from Escobar et al.’s (2001) Experiment 2, in which retroactive interference was observed relative to three different controls. In that experiment, control subjects received A-alone, O-alone, or neither treatment in Phase 2. None of these control conditions differed from each other, suggesting that latent inhibition of O should not be a factor in the retroactive interference effect observed in the present studies given the high similarities in parameters and procedures between that study and the present one. We omitted the O-alone control here because we knew from Escobar et al.’s study that it would not differ from the A-alone control that we included, and because we wanted a clearer distinction between our degraded contingency manipulation and our retroactive interference manipulation.
The apparent counteraction between the RI and DC treatments could have occurred because the extensive presentations of O in the RI-DC group produced an increase in generalized fear. That is, the large number of O presentations might have caused a great expectation of O regardless of the test stimulus. In the present series this possibility is somewhat dubious because the animals failed to show any differences in their fear to the test context in the preCS scores. In addition, Urushihara, Wheeler, and Miller’s (2004) studies of outcome postexposure administered a similarly large number of total O presentations (36 compared to our 40) and observed a profound reduction in responding to the target cue. Finally, it is unclear why an increase in generalization between stimuli would occur in group RI-DC without any evidence of increases in responding in groups RI-NoDC and Ctrl-DC. In particular, one might expect to see generalization between X and A because they are both 30-s auditory stimuli that shared a common relationship with O. However, the addition of A-O pairings appeared to reduce responding to X in group RI-NoDC. Furthermore, other similar investigations of retroactive interference have explicitly shown an inverse relationship between responding to X and responding to A (Escobar et al., 2001).
We contend that the results indicate that RI interacts with degraded contingency in a way that is similar to the interaction between overshadowing and degraded contingency (Urcelay & Miller, 2006). In this situation, RI may have been reduced because the degraded contingency treatment decreased expression of the A-O situation as well as the X-O association. This explanation accounts for the attenuation of RI in the RI-DC group, but it does not account for the fact that degraded contingency treatment was also less effective. Even if it is assumed that unsignalled outcome presentations reduced A’s potential to interfere with X, the unsignalled outcome presentations should still have reduced responding to X. Instead, the results indicate that the two response-degrading treatments interacted and mutually reduced the effectiveness of each other.
Experiment 2
Experiment 1 revealed a correspondence between competition between cues trained together (e.g., overshadowing; Urcelay & Miller, 2006) and cues trained apart (e.g., RI; the present research). Experiment 2 was designed to further investigate whether interacting cues would counteract each other in situations in which one of the competing cues is not presented in compound with the target cue. In Experiment 2, RI treatment was combined with trial massing treatment, which itself is known to attenuate conditioned responding.
Stout et al. (2003) observed an interaction between overshadowing and the trial massing effect. Typically, an elementally trained cue elicits more conditioned responding when training trials are temporally spaced relative to when the trials are temporally massed (for reviews, see Barela, 1999; Gibbon & Balsam, 1981). Stout et al. found that this effect is reversed when a target stimulus is trained in compound with an overshadowing stimulus. Experiment 2 of the present series investigated the potential interaction between RI and trial spacing. As in Experiment 1, half of the subjects were given RI treatment (condition RI), and the other half were given a control treatment (Ctrl). Unlike Experiment 1, the session durations and ITIs were manipulated so that trials in Phases 1 and 2 were either massed or spaced (see Table 2). All CS durations were identical to those used in Experiment 1. The trial spacing in the Spaced condition in Phases 1 and 2 was the same as the trial spacing that was used in Experiment 1, with an average of 577.5 s between trial onsets (this includes time spent in the context at the beginning and the end of the session). The average ITI in the Massed condition was much shorter (90 s). The mean ITI for Phase 3 was the geometric mean of the average Massed and Spaced ITIs (228s). Also, because of the high responding observed in Experiment 1, the shock level was lowered from 1.0 mA to 0.8 mA.
Table 2.
The design of Experiment 2
| Group | Phase 1 in Context A | Phase 2 in Context A | Phase 3 in Context B | Test X in Context B |
|---|---|---|---|---|
| Ctrl-Spaced | 8 X→O (577.5) | 8 A (577.5) | 8 O→US (228) | CR |
| Ctrl-Massed | 8 X→O (90) | 8 A (90) | 8 O→US (228) | Cr |
| RI-Spaced | 8 X→O (577.5) | 8 A→O (577.5) | 8 O→US (228) | Cr |
| RI-Massed | 8 X→O (90) | 8 A→O (90) | 8 O→US (228) | ? |
Note: Ctrl = Control, RI = Retroactive Interference, Spaced = relatively spaced ITIs (577.5 s), Massed = relatively massed ITIs (90 s), X = complex tone, A = clicks, O = white noise, US = footshock. The strength of the expected conditioned response is denoted by the size of the CR. The question mark denotes an uncertain outcome.
In light of the results of Experiment 1, the expected result of Experiment 2 was a bit clearer. In Experiment 1, degraded contingency treatment interacted with RI treatment. The combination of the two produced greater conditioned suppression to the target cue than that produced when either treatment was administered alone. A similar result in Experiment 2 would be revealed by a significant interaction between RI and trial spacing. Alternatively, Experiment 2 could reveal summation of the two treatments, which would be reflected by lower conditioned suppression in group RI-Massed relative to both groups RI-Spaced and Ctrl-Massed. Although the latter possibility is more intuitive, the former might be considered to be more likely based on the results of Experiment 1.
Method
Subjects
Subjects were 24 male (226–295 g) and 24 female (180–218 g), experimentally naive, Sprague-Dawley descended, water-deprived rats obtained from our own breeding colony. Subjects were randomly assigned to one of four groups (ns = 12), counterbalanced within groups for sex. The animals were housed, handled, and water deprived as in Experiment 1.
Apparatus
The apparatus was the same as that used in Experiment 1. The stimuli were the same except the footshock, which was 0.8 mA.
Procedure
Acclimation
On Day 1, all the groups were acclimated to Context B (the test context) for 60 min to establish baseline drinking. During this session, the animals had free access to the water-filled tubes and no nominal stimuli were presented. After acclimation, all of the water-filled lick tubes were removed.
Phases 1 and 2
As in Experiment 1, Phases 1 and 2 took place in Context A (the training context). In Phase 1, subjects in all groups received 8 X→O pairings in which the onset of the 10-s O occurred as the 30-s X terminated. For subjects in the Spaced condition, these pairings occurred 5, 19, 24, 36, 43, 54.5, 59.5, and 69.5 min into the 77-min session. For subjects in the Massed condition, the pairing occurred 47, 144, 224, 337, 402, 490, 556, and 650 s into a 12-min session. Phase 2 occurred on Day 3, and consisted of the same training as Phase 1, except the X stimulus was replaced by the A stimulus, and the A presentations were not followed by the outcome in the Ctrl condition. Thus, the ITIs for conditions Massed and Spaced was the same as in Phase 1.
Phase 3
On Days 4 and 5, all subjects received four O→US pairings per day in Context B. The 10-s O stimulus coterminated with the 0.5-s footshock. The average time between footshocks was 228 s, the geometric mean of 90 and 577.5 s. Trials occurred 3, 7, 10, and 14 min into each 15.2-min session.
Reacclimation and Testing
On Days 6–8, reacclimation, testing, and data transformation were conducted as they were in Experiment 1. All subjects completed their first five cumulative seconds of licking within 60 s; consequently no subjects were eliminated based on that criterion.
Results and Discussion
The results of Experiment 2 are displayed in Figure 2. Subjects in the Ctrl-Spaced group expressed the most fear of the CS. As in Experiment 1, RI was observed when A-O pairings followed X-O pairings in long experimental sessions (group RI-Spaced). Also, subjects that experienced temporally massed X-O pairings with no A-O exposure (group Ctrl-Massed) showed the weakest level of suppression. When the two treatments were combined (group RI-Massed), conditioned suppression was greater than that observed in group Ctrl-Massed, but not group RI-Spaced. These observations are supported by the following statistical analyses.
Figure 2.
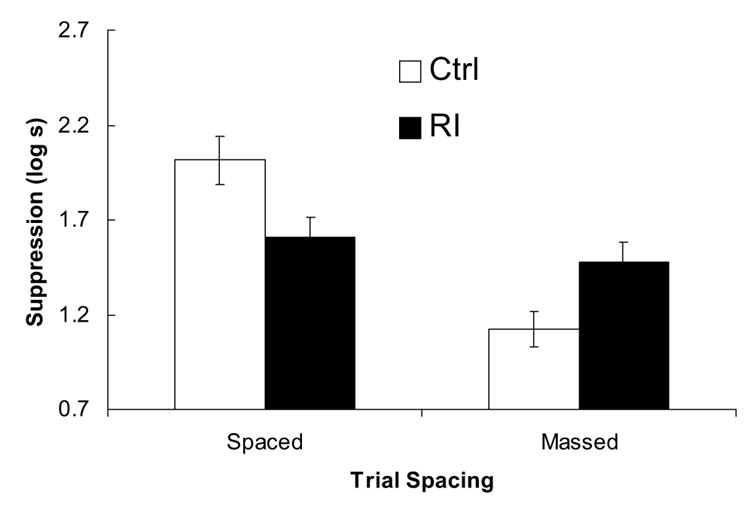
Experiment 2. The bars depict the mean suppression scores for the four groups. Larger bars denote longer latencies to resume drinking in the presence of the CS. Thus, smaller bars suggest less fear to the target stimulus. The error bars represent the standard error for each group.
Before analyzing any of the CS scores, the preCS scores were analyzed with a 2 (Treatment: RI vs. Ctrl) x 2 (Trial Spacing: Spaced vs. Massed). This analysis revealed no significant main effects or interaction, ps > .29. A similar analysis of the CS scores showed a main effect of trial spacing, F(1, 44) = 21.93, and importantly an interaction between treatment and trial spacing, F(1, 44) = 12.13, but no main effect of treatment, p > .80. Planned comparisons were conducted to determine the source of the interaction. A comparison of group RI-Spaced and Ctrl-Spaced showed that RI was apparent when the trials were sufficiently spaced, F(1, 44) = 6.99. Also, group Ctrl-Spaced responded more than group Ctrl-Massed, indicating that massed training reduced conditioned suppression to X when X-O training was not followed by A-O training, F(1, 44) = 33.35. In the group for which the two response-degrading treatments were combined (RI-Massed), suppression was greater than that observed in group Ctrl-Massed, F(1, 44) = 5.21. However, conditioned fear in groups RI-Massed and RI-Spaced did not differ significantly, F(1, 44) = 0.72, p > .40. Thus, there was an interaction between RI and trial massing treatments, but the conditioned fear produced by combining the two treatments was not significantly stronger than fear observed in the RI-Spaced group.
There are at least two primitive interpretations of the results of Experiment 2. One interpretation of the results is that RI attenuated the response-degrading effects of trial massing, but not vice-versa. The level of suppression observed in group RI-Massed was greater than in group Ctrl-Massed (which indicates an attenuation of the trial massing effect), but not greater than in group RI-Spaced. This account is supported by the analyses employed here, but it is not the only way to interpret the results of Experiment 2. Another potential explanation is that the RI treatment only partially attenuated the effect of trial massing. The RI effect observed in Experiment 2 was less robust than the trial massing effect as suggested by a main effect of trial spacing but not of interference treatment. Therefore, the symmetrical counteraction observed in Experiment 1 might be absent here because the independent effects were not of equal strength. The trial massing manipulation might have been sufficient to attenuate RI, but the RI effect might not have been sufficiently strong to completely reduce the trial massing effect. Although the two treatments did not completely counteract each other as in Experiment 1, there was clearly an interaction, and no suggestion of summation.
General Discussion
The results presented here show some correspondence between situations in which there is competition between cues trained together (e.g., overshadowing) and situations in which there is competition between cues trained apart (e.g., RI). In Experiment 1, RI attenuated the degraded contingency effect, and vice-versa. This result mirrors the results of Urcelay and Miller (2006), who found that degraded contingency treatment and overshadowing treatment reciprocally counteract one another. In Experiment 2, RI treatment did appear to attenuate the effect of trial massing, but there was not a symmetrical counteraction. Subjects that experienced the combined treatments exhibited more conditioned suppression than those that received only massed trials, and approximately equivalent suppression as subjects that received only RI treatment. Thus, the present results do not show complete correspondence with the findings of Stout et al. (2003), who showed that overshadowing treatment and trial massing counteract each other. Even so, there was clearly an interaction between the two variables, and no indication that the two effects summated.
At the present time, no single theory of learning can account for the results presented here. Most contemporary theories account for competition between cues trained together, and do not predict that RI would occur between cues trained apart (e.g., Gallistel & Gibbon, 2000; Mackintosh, 1975; Miller & Matzel, 1988; Pearce & Hall, 1980; Rescorla & Wagner, 1972; Wagner, 1981). Even theories that can account for backward blocking (i.e., AX-O followed by A–O training) rely on the formation of a within-compound association, and would not predict the RI effect observed in Experiments 1 and 2 because X and A were never trained together (e.g., Dickinson & Burke, 1996; Miller & Matzel, 1988; Van Hamme & Wasserman, 1994). Miller and Escobar (2002) suggested a framework that could account for RI by combining a theory that accounts for interference between cues trained apart with Miller and Matzel’s comparator account of competition between cues trained together. Although their theory does not completely account for the results observed here, it does account for much of the data.
To explain competition between cues trained together, Miller and Escobar (2002) appealed to Miller and Matzel’s (1988) comparator hypothesis (see also Denniston, Savastano, & Miller, 2001). According to this theory, competition between cues trained together occurs when a target cue is tested, and is mediated by the current associative status of any cue that has a direct association with the target cue and the outcome (called a comparator stimulus). Responding to the target cue is directly related to the strength of its association with the outcome (called Link 1 in the framework of the comparator hypothesis). At the same time, responding to the target is reduced by the strength of the within-compound association between the target cue and the comparator stimulus (Link 2) and the strength of the association between the comparator stimulus and the outcome (Link 3). Specifically, the product of Links 2 and 3 reduces the expression of Link 1.
The comparator mechanism can account for some of the results observed in Experiments 1 and 2 if it is assumed that the context of training can act as a comparator stimulus. For example, in Experiment 1, subjects in the control condition received X-O pairings with no RI treatment. According to the comparator hypothesis, expression of the X-O association at test will be reduced if the context has an exceptionally strong association with X and/or O. In group Ctrl-DC, the X-O pairings were accompanied by interspersed unsignalled O presentations, which should have augmented the strength of the context-O association (Link 3 according to the comparator hypothesis). Thus, the comparator hypothesis predicts an effect of degraded contingency treatment in Experiment 1 based on a strong association between O and the training context.1 This account of degraded contingency has been supported by similar studies from our laboratory that indicate that subjects can recover from the effect of degraded contingency if the training context is extinguished after target-stimulus training (e.g., Urcelay & Miller, 2006; Witnauer & Miller, 2006). Thus, degraded contingency appears to be dependent on associative status of the context at test. In a similar way, the trial-massing effect observed in Experiment 2 can be explained by assuming that the training context can act as a comparator stimulus. In this case, both the context-X (Link 2) and the context-O (Link 3) associations are strong in the Ctrl-Massed group because there is little extinction of the context between trials in the massed condition relative to the spaced condition. As with degraded contingency, posttraining context extinction after massed training trials alleviates the trial-massing effect (Stout et al., 2003). Although comparator theory does effectively explain degraded contingency and trial massing effects, it fails to account for RI.
In order to explain competition between cues trained apart, such as the RI effect observed in the present studies, Miller and Escobar (2002) posited that interference can occur between two cues that are not directly associated to each other as a result of a priming mechanism that operates independently from the comparator mechanism. For interference to be strong, the interfering cue must share similar attributes with the target cue, including their associations with a common outcome. (However, if the two associations are not discriminable, enhancement will be observed because the nontarget trials will be essentially more target training trials.) Also, the interfering cue must be primed when the target stimulus is tested. Priming can be achieved by presenting an associate of the interfering stimulus or through recency, which leads to stronger expression of more recent learning. Applied to the RI effect observed here, the A-O association is primed because it has been recently trained compared to the target association. Furthermore, A and X share a similar association with the outcome, which also facilitates interference. Therefore, when X is tested, the expression of the X-O association is disrupted by the primed A-O association (see Anderson, Bjork, & Bjork, 2000 for an example of this sort of impaired retrieval in the human learning literature).
Miller and Escobar’s (2002) dual-process model can account for the individual response-degrading effects observed in Experiments 1 and 2 (i.e., degraded contingency, trial massing, and RI). However, a straightforward application of Miller and Escobar’s model does not explain the counteraction observed in Experiment 1 when two response-degrading treatments were combined. Specifically, the model would fail to predict that the presence of unsignaled outcomes in Phase 2 would reduce of RI. According to the model, the degraded contingency effect relies on the comparator process, and the RI effect relies on the priming process. Because the two processes operate independently in the model, there is no reason to expect that a comparator process involving the context and A would modulate the priming process involving A and X. In an attempt to accommodate the present results, one could assume that the priming process and comparator process are not independent, but such an assumption would violate one of the primitives of the model. Furthermore, making such a fundamental alteration of the model may have an unforeseen consequence for established predictions.
More important than the specific theoretical implications, the two experiments presented here converge with other studies that find counteractions between select treatments that normally reduce responding to a CS. Generally, most theories would predict that two response-degrading treatments would summate when combined, and result in even weaker responding. The results presented here show two situations that contribute to the aforementioned body of results in which this is not the case. It is important to note, however, that response-degrading treatments do not always counteract each other. For example, Nakajima et al. (1999) observed summation of latent inhibition and overshadowing treatments in a conditioned taste aversion preparation, failing to replicate the counteraction observed by Blaisdell et al. (1998) and replicated by Ishii (1999). Analogous to Nakajima et al., an unpublished experiment from our laboratory strongly suggests that latent inhibition and RI treatments summate. In that experiment, preexposure of the target cue prior to RI treatment resulted in very weak responding to the target cue. Although the data suffer from a statistical ambiguity that prevents us from determining whether summation occurred, there was certainly no counteraction effect. Indeed, even in the results of the present Experiment 2, the interaction between trial massing and RI did not produce a symmetrical counteraction of the two effects. It is clear that the variables that determine how different competing stimuli interact call for further study (e.g., Urushihara & Miller, 2006).
Although Miller and Escobar (2002) proposed two separate mechanisms to explain cue competition effects that occur when cues are trained together and cue interference effects that occur when cues are trained apart, the results of the experiments presented here and elsewhere show some intriguing empirical similarities between the two types of cue interaction. Broadly speaking, the results of Experiments 1 and 2 indicate that the counterintuitive interactions between response-degrading effects previously observed by Miller and colleagues (among others, e.g., Ishii, 1999; Loy & Hall, 2002; Maier, Jackson, & Tomie, 1987; Westbrook et al., 1983) are not unique to situations in which compound training occurs. Instead, these counteraction effects appear to arise more generally in situations in which cues interact with each other independent of whether they have been trained together or apart. Future studies should focus on the parameters that determine when counteraction will occur.
Footnotes
The training context, not the test context, acts as an effective comparator stimulus for the target here because it is associated with the target stimulus and the outcome. The test context should have little impact on responding to the target, assuming that the potential for summation is minimized, as was the case here (e.g., Kasprow, Schachtman, & Miller, 1987).
NIMH Grant 33881 provided support for this research. We thank Jim Esposito for his technical assistance, and Jeffrey C. Amundson, Jonah Grossman, David Guez, Rachael Hessner, Olga Lipatova, Alyssa Orinstein, Olga Perelmuter, Gonzalo Urcelay, Kouji Urushihara, and Jim Witnauer for comments on an earlier version of the manuscript.
References
- Anderson MC, Bjork EL, Bjork RA. Retrieval-induced forgetting: Evidence for a recall-specific mechanism. Psychonomic Bulletin & Review. 2000;7:522–530. doi: 10.3758/bf03214366. [DOI] [PubMed] [Google Scholar]
- Barela PB. Theoretical mechanisms underlying the trial-spacing effect in Pavlovian fear conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1999;25:177–193. doi: 10.1037//0097-7403.25.2.177. [DOI] [PubMed] [Google Scholar]
- Blaisdell AP, Bristol AS, Gunther LM, Miller RR. Overshadowing and latent inhibition counteract each other: Support for the comparator hypothesis. Journal of Experimental Psychology: Animal Behavior Processes. 1998;24:335–351. doi: 10.1037//0097-7403.24.3.335. [DOI] [PubMed] [Google Scholar]
- Denniston JC, Savastano HI, Miller RR. The extended comparator hypothesis: Learning by contiguity, responding by relative strength. In: Mower R, Klein S, editors. Handbook of contemporary learning theories. Mahwah, NJ: Erlbaum; 2001. pp. 65–117. [Google Scholar]
- Dickinson A, Burke J. Within-compound associations mediate the retrospective revaluation of causality judgements. Quarterly Journal of Experimental Psychology. 1996;49B:60–80. doi: 10.1080/713932614. [DOI] [PubMed] [Google Scholar]
- Escobar M, Matute H, Miller RR. Cues trained apart compete for behavioral control in rats: Convergence with the associative interference literature. Journal of Experimental Psychology: General. 2001;130:97–115. doi: 10.1037/0096-3445.130.1.97. [DOI] [PubMed] [Google Scholar]
- Gallistel CR, Gibbon J. Time, rate, and conditioning. Psychological Review. 2000;107:289–344. doi: 10.1037/0033-295x.107.2.289. [DOI] [PubMed] [Google Scholar]
- Gibbon J, Balsam P. Spreading association in time. In: Locurto CM, Terrace HS, Gibbon J, editors. Autoshaping and conditioning theory. New York: Academic Press; 1981. pp. 219–253. [Google Scholar]
- Gunther LM, Miller RR. Prevention of the degraded-contingency effect by signalling training trials. Quarterly Journal of Experimental Psychology. 2000;53B:97–119. doi: 10.1080/713932719. [DOI] [PubMed] [Google Scholar]
- Ishii K. Attenuation of latent inhibition after compound conditioning. Japanese Psychological Research. 1999;41:102–111. [Google Scholar]
- Kasprow WJ, Schachtman TR, Miller RR. The comparator hypothesis of conditioned response generation: Manifest conditioned excitation and inhibition as a function of relative excitatory strengths of CS and conditioning context at the time of testing. Journal of Experimental Psychology: Animal Behavior Processes. 1987;13:395–406. [PubMed] [Google Scholar]
- Lovibond PF, Preston GC, Mackintosh NJ. Context specificity of conditioning, extinction, and latent inhibition. Journal of Experimental Psychology: Animal Behavior Processes. 1984;10:360–375. [Google Scholar]
- Loy I, Hall G. Taste aversion after ingestion of lithium chloride: An associative analysis. Quarterly Journal of Experimental Psychology. 2002;55B:365–380. doi: 10.1080/02724990244000070. [DOI] [PubMed] [Google Scholar]
- Lubow RE. Latent inhibition and conditioned attention theory. Cambridge University Press; New York, NY: US: 1989. [Google Scholar]
- Lubow RE, Gewirtz JC. Latent inhibition in humans: Data, theory, and implications for schizophrenia. Psychological Bulletin. 1995;117:87–103. doi: 10.1037/0033-2909.117.1.87. [DOI] [PubMed] [Google Scholar]
- Lubow RE, Moore AU. Latent inhibition: The effect of nonreinforced pre-exposure to the conditional stimulus. Journal of Comparative and Physiological Psychology. 1959;52:415–419. doi: 10.1037/h0046700. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. A theory of attention: Variations in the associability of stimuli with reinforcement. Psychological Review. 1975;82:276–298. [Google Scholar]
- Maier SF, Jackson RL, Tomie A. Potentiation, overshadowing, and prior exposure to inescapable shock. Journal of Experimental Psychology: Animal Behavior Processes. 1987;13:260–270. [Google Scholar]
- Miller RR, Escobar M. Associative interference between cues and between outcomes presented together and presented apart: An integration. Behavioural Processes. 2002;57:163–185. doi: 10.1016/s0376-6357(02)00012-8. [DOI] [PubMed] [Google Scholar]
- Miller RR, Matute H. Biological significance in forward and backward blocking: Resolution of a discrepancy between animal conditioning and human causal judgment. Journal of Experimental Psychology: General. 1996;125:370–386. doi: 10.1037//0096-3445.125.4.370. [DOI] [PubMed] [Google Scholar]
- Miller RR, Matzel LD. The comparator hypothesis: A response rule for the expression of associations. In: Bower GH, editor. The psychology of learning and motivation. Vol. 22. San Diego, CA: Academic Press; 1988. pp. 51–92. [Google Scholar]
- Nakajima S, Ka H, Imada H. Summation of overshadowing and latent inhibition in rats' conditioned taste aversion: Scapegoat technique works for familiar meals. Appetite. 1999;33:299–307. doi: 10.1006/appe.1999.0247. [DOI] [PubMed] [Google Scholar]
- Nakajima S, Nagaishi T. Summation of latent inhibition and overshadowing in a generalized bait shyness paradigm of rats. Behavioural Processes. 2005;69:369–377. doi: 10.1016/j.beproc.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflexes. New York: Dover; 1927. [Google Scholar]
- Pearce JM, Hall G. A model for pavlovian learning: Variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychological Review. 1980;87:532–552. [PubMed] [Google Scholar]
- Rescorla RA. Probability of shock in the presence and absence of CS in fear conditioning. Journal of Comparative and Physiological Psychology. 1968;66:1–5. doi: 10.1037/h0025984. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and non-reinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II: Current research and theory. New York: Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- Stout SC, Chang R, Miller RR. Trial spacing is a determinant of cue interaction. Journal of Experimental Psychology: Animal Behavior Processes. 2003;29:23–38. [PubMed] [Google Scholar]
- Urcelay GP, Miller RR. Counteraction between overshadowing and degraded contingency treatments: Support for the extended comparator hypothesis. Journal of Experimental Psychology: Animal Behavior Processes. 2006;32:21–32. doi: 10.1037/0097-7403.32.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushihara K, Miller RR. Overshadowing and the outcome alone exposure effect counteract each other. Journal of Experimental Psychology: Animal Behavior Processes. doi: 10.1037/0097-7403.32.3.253. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushihara K, Miller RR. Partial reinforcement and CS duration effects counteract overshadowing in select situations. 2006. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushihara K, Stout SC, Miller RR. The basic laws of conditioning differ for elemental cues and cues trained in compound. Psychological Science. 2004;15:268–271. doi: 10.1111/j.0956-7976.2004.00664.x. [DOI] [PubMed] [Google Scholar]
- Van Hamme LJ, Wasserman EA. Cue competition in causality judgments: The role of nonpresentation of compound stimulus elements. Learning and Motivation. 1994;25:127–151. [Google Scholar]
- Wagner AR. SOP: A model of automatic memory processing in animal behavior. In: Spear NE, Miller RR, editors. Information processing in animals: Memory mechanisms. Hillsdale, NJ: Erlbaum; 1981. pp. 5–47. [Google Scholar]
- Westbrook RF, Homewood J, Horn K, Clarke JC. Flavour-odour compound conditioning: Odour-potentiation and flavour-attenuation. Quarterly Journal of Experimental Psychology. 1983;35B:13–33. doi: 10.1080/14640748308400911. [DOI] [PubMed] [Google Scholar]
- Witnauer JE, Miller RR. Degraded contingency revisited: Posttraining extinction of a cover stimulus attenuates a target cue’s behavioral control. 2006. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]


