Abstract
The heightened concern about the intentional release of variola virus has led to the need to develop safer smallpox vaccines. While subunit vaccine strategies are safer than live virus vaccines, subunit vaccines have been hampered by the need for multiple boosts to confer optimal protection. Here we developed a protein-based subunit vaccine strategy that provides rapid protection in mouse models of orthopoxvirus infections after a prime and single boost. Mice vaccinated with vaccinia virus envelope proteins from the mature virus (MV) and extracellular virus (EV) adjuvanted with CpG-ODN and alum were protected from lethal intranasal challenge with vaccinia virus and the mouse-specific ectromelia virus. Organs from mice vaccinated with three proteins (A33, B5 and L1) and then sacrificed after challenge contained significantly lower titers of virus when compared to control groups of mice that were not vaccinated or that received sub-optimal formulations of the vaccine. Sera from groups of mice obtained prior to challenge had neutralizing activity against the MV and also inhibited comet formation indicating anti-EV activity. Long-term partial protection was also seen in mice challenged with vaccinia virus 6 months after initial vaccinations. Thus, this work represents a step toward the development of a practical subunit smallpox vaccine.
Keywords: smallpox, vaccinia virus, CpG
1. INTRODUCTION
Concern about the intentional release of variola virus by terrorists has stimulated efforts to develop safer smallpox vaccines [1,2]. While the current licensed live vaccinia virus-based vaccine is extremely effective [3] and results in remarkably long-lived immune responses (e.g., [4]), it has an unacceptable safety profile [5,6] for wide use when considered in today’s setting of global eradication of active smallpox disease. Approaches to develop safer smallpox vaccines have ranged from the study of more attenuated live vaccinia viruses (such as MVA [7–9] or LC16m8 [10,11]) to subunit vaccines that rely on specific viral targets delivered either as DNA plasmids [12–15] or purified proteins [16–19].
These subunit vaccine strategies mainly target key proteins found on the envelopes of the infectious virions [20,21]. Orthopoxviruses, like variola virus, monkeypox virus, ectromelia virus, and vaccinia virus generate two major infectious forms [22]. The preponderance of progeny virus made during an infection is called the mature virus (MV), which contains an outer membrane studded with viral proteins. This form is the main component of live viral preparations and is believed to be the form of virus that is naturally transmitted from host to host. Some key targets of neutralizing antibody found on the MV envelope are encoded by the L1R [23–25] and A27R [26–28] [] genes. During an infection, a small portion of the MV subsequently acquires a second viral envelope and exits the cell as an infectious form termed the extracellular virus (EV). EV is critical for spread of the virus from cell-to-cell and to distant sites within a host. Some key targets on the EV envelope that are important for protection are encoded by the A33R [29] and B5R [30,31] genes. Because the viral proteins present on MV and EV envelopes differ, there is rationale and evidence [12–14,17] to include proteins from both virion forms to confer maximal protection. Theoretically, antibody responses to MV proteins could neutralize some of the initial infecting inoculum, while antibodies to EV targets would then limit spread of the progeny virus within the infected host. This pre-existing humoral defense would then allow the host immune response to develop and eradicate the infection.
The MV protein target L1 and the EV protein targets A33 and B5 have been studied previously in a protein vaccination strategy [17]. Using 10 μg of each protein in a Ribi adjuvant system (MPL TDM) or the saponin adjuvant QS-21, it was found that three to four subcutaneous vaccinations given every 3 weeks were required for optimal protection. We sought to build on this information to determine if we could develop a strategy that would confer protection more rapidly and using lower amounts of proteins, ideally after only two vaccinations given over a two-week period. In the setting of a smallpox outbreak, this goal would allow safe vaccination of large populations of unexposed but susceptible hosts [32,33]. We selected the adjuvant CpG-ODN, a synthetic toll-like receptor (TLR)-9 agonist [34], which has shown promise as a potent adjuvant to other protein subunit vaccines in various animal models [35,36] as well as in humans [36,37]. We also investigated if this approach would require the addition of a fourth viral protein target. Finally, we examined whether this vaccination strategy would confer protection against challenge infection with the heterologous orthopoxvirus, ectromelia virus, in a susceptible mouse strain. Our results support the feasibility/effectiveness of this vaccination scheme, and encourage us to consider that a subunit vaccine approach be pursued as a way to provide protection against orthopoxvirus infections in a relatively short period of time.
2. MATERIALS AND METHODS
2.1. Proteins and vaccine formulations
Histidine-tagged ectodomains of the vaccinia virus B5 and L1 proteins were expressed in a baculovirus system and purified from infected insect cell supernatants as previously reported [38,39]. The A27L and A33R open reading frames (ORF) were PCR amplified from the cloned HindIII A fragment of the vaccinia virus (WR) genome. For A27, the forward primer used for PCR amplification was: 5'-gcgcggatccaatggacggaactcttttccccggagat-3' (adds BamHI (bold)) and the reverse primer was 5'-gcgcgaattcagtggtgatggtgatgatgctcatatggacgccgtccagtctgaa-3' (adds six histidine codons (underlined) to the 3’ end of A27L ORF as well as an EcoRI site (bold)). A similar strategy was used to produce a soluble, secreted form of A33. Thus, the predicted transmembrane domain of the full-length A33 protein (residues 41–57) was excluded and only residues 58–185 were expressed. The forward primer for PCR amplification of the truncated A33R ORF was: 5'-cgccggatccacgcctaaatcaatgcatg -3' (adds BamHI site (bold)) and the reverse PCR primer was 5'-cgccgaattcaatggtgatggtgatgatggttcattgttttaacacaa -3' (adds 6 histidine codons (underlined) to the 3’ end of A33R ORF as well as an EcoRI site (bold)). The PCR products were digested with BamHI and EcoRI and ligated into the baculovirus transfer plasmid, pVT-Bac [40] that was digested with BamHI and EcoRI. Thus, the final recombinant plasmids contained ORFs consisting of the melittin signal sequence followed, in frame, by the entire A27L ORF or the A33 ectodomain (codons 58–185) and then 6 histidine codons. The resulting pVT-Bac plasmids were co-transfected into Sf9 insect cells with baculovirus DNA (BaculoGold, BD-Pharmingen) and after several days, recombinant virus was obtained. The viruses were plaque purified and high titer stocks were prepared. Recombinant A27 or A33 protein was secreted into the culture medium of baculovirus-infected cells and was purified using nickel-NTA agarose. The purified recombinant proteins were analyzed by SDS-PAGE followed by silver staining and western blotting. The purified A33, B5, L1, and A27 proteins were stored frozen in PBS at 5 mg/ml final concentrations. Proteins (each used at 2 μg/mouse) were added to alum, in the form of aluminum hydroxide (Alhdrogel, Accurate Chemical, Westbury, NY). The amount of alum used in the vaccine formulation was based on 25 μg of aluminum ion/μg of total protein. A wholly phosphorothioate B-class mouse specific CpG ODN (sequence #1826, TCC ATG ACG TTC CTG ACG TT) (Coley Pharmaceutical Group, Wellesley, MA) was used at 50 μg/mouse. Sterile PBS was added such that the final volume injected into each mouse was 50 μl. On the morning of vaccinations, the vaccine formulations were prepared, mixed at room temperature for 2 to 3 hours on a rotation mixer, and then loaded into syringes.
2.2. Mice and vaccinations
Female BALB/c mice, purchased from Charles River and housed in microisolator cages, were vaccinated when they were approximately six weeks old. Groups of 5 to 12 mice were vaccinated by priming on day 0 and boosting 2 weeks later using an intramuscular (i.m.) route. We used i.m. injections instead of subcutaneous (s.q.) because work with CpG and other antigens revealed that i.m. administration was superior to s.q. (H. Davis, unpublished observation). For all immunizations, the hair of the right hind leg of anesthetized mice was removed with a depillary and 50 μl of the vaccine formulation was injected i.m. into the gastrocnemius muscle of each mouse using a 0.3 ml insulin syringe with a 29-gauge needle (Becton Dickinson). Control groups were vaccinated with antigen formulations with and without CpG and/or alum. A positive control involved vaccination of mice once at day 0 with vaccinia virus (106 plaque forming units (pfu)) inoculated by tail scarification. As a negative control, a group of naïve-unvaccinated mice was included with each experiment.
2.3. Vaccinia virus and ectromelia virus challenge
Vaccinia virus (strain WR) was grown in BSC-1 cells in culture media (MEM containing 2.5% FBS and antibiotics/antimycotics) and pelleted twice through a 36% sucrose cushion (10 ml) at ~24,000 g for 60 min. Ectromelia virus (virulent Moscow strain) was grown in BSC-1 cell monolayers from seed virus kindly provided by R. M. Buller (St. Louis University, St. Louis, MO). Virus was released from infected cell pellets by homogenization, then low-speed centrifugation to remove cell debris, followed by pelleting the virus through a 36% sucrose cushion (17 ml) at ~33,000 g for 80 min. Titers were determined by plaque formation in BSC-1 cells. Ectromelia virus LD50 determination (by the intranasal route) were carried out in 12–14 wk old susceptible BALB/c mice with LD50 values of ~5 pfu. Depending on the experiment, ~3 to 5 weeks after the second vaccination, anesthetized mice were challenged intranasally with ~6 x 106 pfu of vaccinia virus (strain WR) or 103 pfu of ectromelia virus (Moscow strain) in a 20 μl volume. Mice were weighed each day and morbid mice or mice that had reached >30% weight loss were humanely sacrificed. In some experiments, several mice were sacrificed at day 3 and day 5 after challenge and the lungs and spleens were harvested. Harvested organs were placed in pre-weighed microcentrifuge tubes containing 0.5 ml of culture media and stored at −70 °C. The organs were homogenized using a pellet pestle system (Kontes), frozen and thawed three times, and then sonicated prior to titration. After low-speed centrifugation, the clarified supernatants were serially diluted and titrated on monolayers of BSC-1 cells and viral titers were quantitated by crystal violet staining of the monolayer 48 hrs after infection and counting the resulting plaques.
2.4. Determination of antibody responses by ELISA, virus neutralization, and comet inhibition assays
Mice were bled prior to vaccination, a day prior to boost, and a day prior to challenge. Equal volumes of heat-inactivated serum from individual mice in each group were combined and assayed at a 1:550 dilution by direct ELISA on plates coated with the baculovirus expressed B5, L1, and A27 purified proteins at 5 μg/ml. Because all protein vaccines contained both EV proteins, we only tested responses against B5. In addition, based on initial pilot tests of sera reactivity by ELISA, we selected a 1:550 dilution serum dilution to examine all sera. After 1 hr incubation at 20 °C, plates were washed, probed with anti-mouse secondary antibody, and the absorbance determined as described previously [38]. Standard MV plaque reduction neutralization assays were carried out in duplicate by mixing ~200 pfu of MV with heat-inactivated sera from groups of mice at a concentration of 1:100, 1:1000, or 1:10,000 in a 100 μl final volume of culture media. After 1 to 2 hr incubation at 37 °C, a portion of the incubated mixture was then added to 1 ml culture media in a well of a 6-well plate containing a confluent monolayer of BSC-1 cells. After 2 hours at 37 °C in a 5% CO2 atmosphere, the media was removed and the well overlaid with culture media containing 1% carboxymethylcellulose. The plates were incubated for ~2 days and then plaques were visualized by crystal violet staining. Comet inhibition assays were carried out on confluent monolayers of BSC-1 cells in 6-well plates initially infected for 2 hr with 20 to 30 pfu of vaccinia virus (strain IHDJ). The infecting inoculum was removed and 1.5 ml of fresh culture media containing heat-inactivated sera from groups of mice at 1:50 final concentration was added to each well. The plates were incubated for ~36 hr and then plaques were visualized by crystal violet staining.
3. RESULTS
3.1. Mice are protected from lethal challenge after only two vaccinations
Prior work with the vaccinia virus protein components in the Ribi adjuvant system (MPL TDM) or the saponin adjuvant QS-21 required 3 to 4 vaccinations given at 3-week intervals to confer optimal protection [17]. We elected to study vaccinations with the adjuvant CpG given 2-weeks apart, the goal being to confer protection after a prime and a short-interval single boost. The prior study had shown that a mixture of 3 proteins, A33, B5, and L1 was superior to individual proteins or a combination of two proteins, so we used this as the basis of proteins to study in the new vaccine formulation. Second, we examined if an alternative MV target, A27, to which MV neutralizing antibody can be generated [26,41], could substitute for the MV L1 protein or if there was a benefit to adding this fourth protein to the vaccine cocktail. We also used the proteins at 2 μg/mouse, which represents 5-times less protein than the prior study.
As shown in Figure 1, mice vaccinated i.m. with A33, B5, L1 in adjuvant (ABL/CpG/alum), boosted 2 weeks later, and then intranasally challenged with ~15LD50 of vaccinia virus strain WR, conferred excellent protection against challenge as witnessed by survival (Figure 1A). These animals also experienced less weight loss after challenge than the group that received live vaccinia virus vaccination by scarification 5 weeks prior to challenge (Figure 1B). In this experiment the 3-protein formulation of ABL/CpG/alum performed as well as the 4-protein (A33, B5, L1, and A27) formulation in CpG/alum. The inclusion of both CpG and alum were key components of the vaccine formulations that conferred protection since the formulation of 4-proteins plus alum alone (Figure 1) or the combination of 4-proteins plus CpG alone (data not shown) provided no protection. Groups of mice vaccinated with these suboptimal formulations fared no better than the challenged unvaccinated naïve mice (Figure 1A & B). We also determined that the ability of CpG to stimulate the innate immune system [34] had no residual non-specific effect on vaccinia virus pathogenesis at the time of challenge because mice injected with CpG/alum without antigens had no protection (Figure 1A & B).
Figure 1.
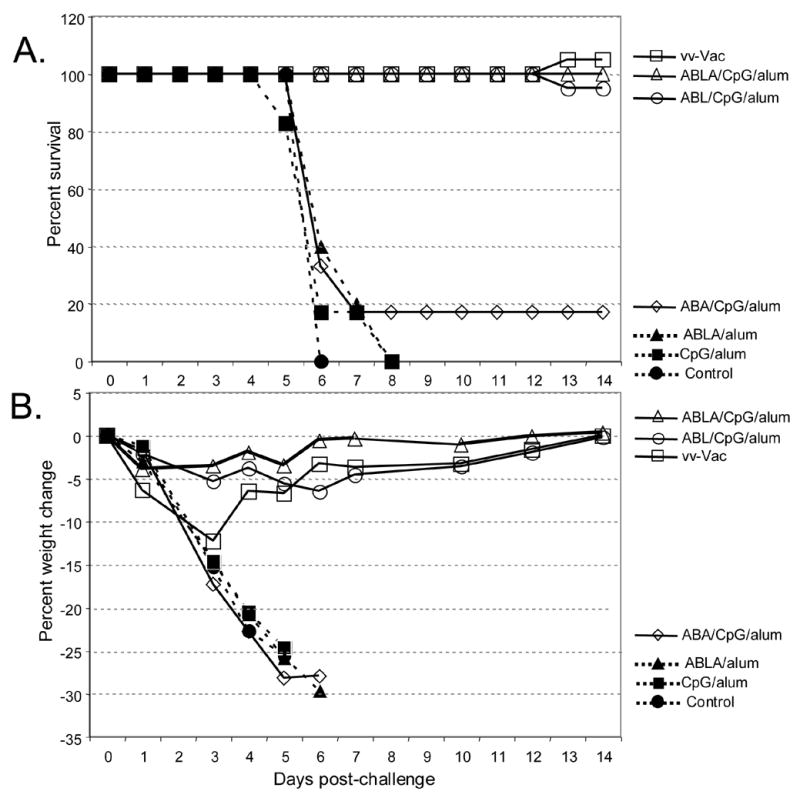
Survival and weight loss after vaccinia virus challenge. Six mice/group were vaccinated with vaccinia virus by scarification (VV-vac) or with vaccine formulations containing A33, B5, L1 along with CpG and alum (ABL/CpG/alum), A33, B5, L1, A27 along with CpG and alum (ABLA/CpG/alum) or A33, B5, A27 along with CpG and alum (ABA/CpG/alum). Control groups included unvaccinated naïve mice (Control), CpG and alum (CpG/alum), and the 4-proteins and alum (ABLA/alum). Three weeks after the boost vaccination, groups were challenged intranasally with ~15LD50 (~6 x 106 pfu) of vaccinia virus (strain WR). A. Survival curve. B. Weight loss during the course of infection.
Finally, in this experiment we also determined if the MV protein A27 could substitute for L1 in the 3-protein vaccine formulation that included two EV targets (A33 and B5). We found that mice vaccinated with A33, B5, and A27 (ABA/CpG/alum), boosted 2 weeks later, and then intranasally challenged with ~15LD50 of vaccinia virus strain WR, were not protected against challenge as measured by survival (Figure 1A) and weight loss (Figure 1B). As discussed earlier, the 3-protein formulation containing A33, B5, and L1 (ABL/CpG/alum) conferred much better protection with all mice surviving challenge. Table 1 summarizes the data for multiple experiments where these vaccine formulations were used. While the ABA/CpG/alum formulation consistently provided the poorest protection with the greatest weight loss and only <50% survival of challenged mice, there was more variability of the ABL formulation when compared to the 4-protein (ABLA) formulation in experiments that were done months apart. This discrepancy may represent a combination of the variability of the protein preparations used for vaccinations, the challenge virus preparations, and in the mice and vivarium conditions.
Table 1.
Summary of survival and maximum weight loss after challenge
| Experimenta | ||||
|---|---|---|---|---|
| Vaccination strategyb | #1 | #2 | #3 | |
| VV-vac | Survival
Max weight loss |
100%
12% |
100%
10% |
100%
10% |
| ABLA | Survival
Max weight loss |
100%
4% |
100%
0% |
100%
16% |
| ABL | Survival
Max weight loss |
100%
6% |
100%
15 |
83%
22% |
| ABA | Survival
Max weight loss |
17%
c |
50%
c |
33%
c |
Each column represents an separate experiment with the following number of mice/group and vaccinia virus challenge:
#1 6 mice/group. Virus challenge ~6 x 106 pfu
#2 10 mice/group. Virus challenge ~7 x 106 pfu
#3 6 mice/group. Virus challenge ~6 x 106 pfu
Vaccination strategies shown are live vaccinia virus vaccination by scarification (VV-vac), 4-proteins in CpG and alum (ABLA), 3-proteins consisting of A33, B5, L1 in CpG and alum (ABL), and, 3-proteins consisting of A33, B5, A27 in CpG and alum (ABA).
Since these groups had ≤ 50% survival, average weight loss of the group is not reported since some mice were sacrificed when they had 30% weight loss or died prior to reaching this degree of weight loss.
We next determined if vaccination with the vaccinia virus proteins would protect mice against challenge with a heterologous orthopoxvirus, the pathogenic mousepox virus (ectromelia virus) [42]. Since BALB/c mice are highly susceptible to ectromelia virus infection, we felt that this challenge model would also amplify differences in the effectiveness of the various vaccines. This was important because as discussed above, in some vaccinia virus challenge experiments the 4-protein ABLA/CpG/alum formulation had enhanced protection compared to the 3-protein ABL/CpG/alum formulation. To our surprise, mice were fully protected from a 200LD50 intranasal challenge of ectromelia virus after vaccination with either ABLA/CpG/alum or ABL/CpG/alum formulations (Figure 2A). All protected groups experienced <5% weight loss (Figure 2B). In pilot studies, we found that some control unvaccinated mice could initially survive an intranasal challenge with a lower dose of ectromelia (1 to 5 pfu) for as long as 30 days before succumbing to infection. Therefore, we followed the vaccinated groups for 40 days and found that no late deaths occurred in any vaccinated mouse groups (Figure 2B).
Figure 2.
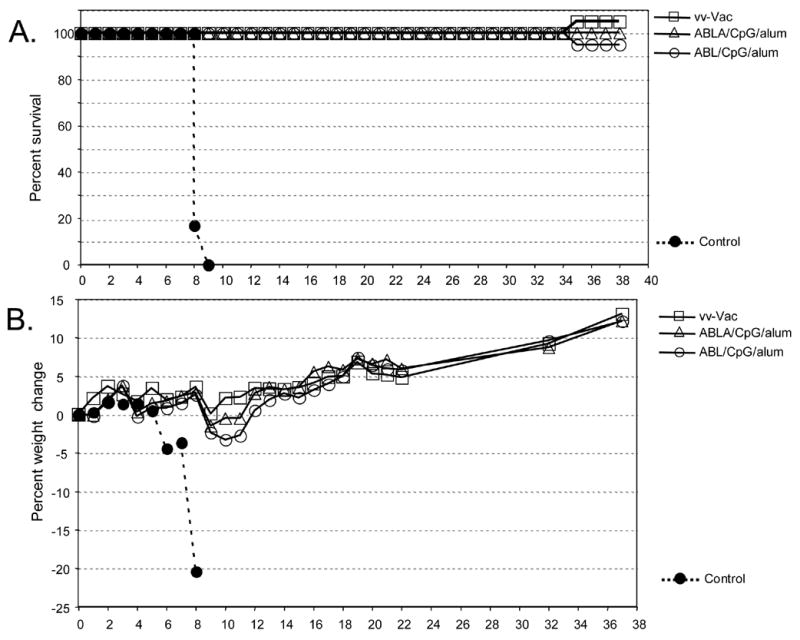
Ectromelia virus challenge after prime and boost vaccinations. Six mice/group were vaccinated with vaccinia virus by scarification (VV-vac) or with vaccine formulations containing A33, B5, L1 along with CpG and alum (ABL/CpG/alum) or A33, B5, L1, A27 along with CpG and alum (ABLA/CpG/alum). A control group of unvaccinated naïve mice (Control) was included. Four to five weeks after the boost vaccination, groups were challenged intranasally with ~200LD50 (~1 x 103 pfu) of ectromelia virus (Moscow strain). The experiment was performed twice with identical results. A. Survival curve. B. Weight loss during the course of infection.
Finally, we examined if the subunit vaccination strategy would provide any long-term protection against lethal vaccinia virus challenge. In this experiment, mice that received the standard primary vaccination followed by a single boost 2 weeks later were challenged with vaccinia virus 6 months after the last vaccination. As shown in Figure 3, mice vaccinated by scarification with live vaccinia performed the best with 100% survival and ~12% weight loss that occurred early after challenge. Mice immunized with the adjuvanted subunit vaccines, either the 3-protein (ABL/CpG/alum) or 4-protein (ABLA/CpG/alum) formulations were partially protected when challenged 6 months after the boost. In both of these groups, 5 out of 6 mice survived lethal challenge (Figure 3A) and although they experienced ~ 25% weight loss by week 1, they recovered to nearly their starting weights by 2 weeks post challenge (Figure 3B). Similar to the experiments with mice challenged 3 weeks after the boost vaccination (Figure 1), all mice challenged 6 months after receiving the suboptimal formulations (ABA/CpG/alum, ABLA/alum, or CpG/alum) either died or needed to be humanely sacrificed.
Figure 3.
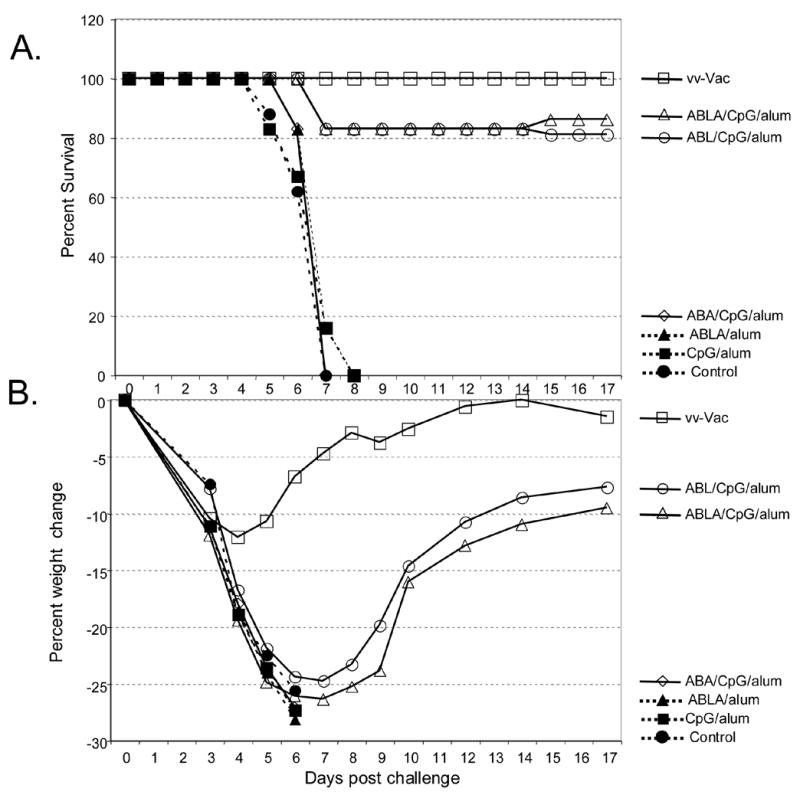
Long-term protection after vaccination. Six mice/group were vaccinated with vaccinia virus by scarification (VV-vac) or with vaccine formulations containing A33, B5, L1 along with CpG and alum (ABL/CpG/alum), A33, B5, L1, A27 along with CpG and alum (ABLA/CpG/alum), and A33, B5, A27 along with CpG and alum (ABA/CpG/alum). Control groups included unvaccinated naïve mice (Control), CpG and alum (CpG/alum), and the 4-proteins and alum (ABLA/alum). Six months after the boost vaccination, groups were challenged intranasally with ~15LD50 (~6 x 106 pfu) of vaccinia virus (strain WR). A. Survival curve. B. Weight loss during the course of infection. The unvaccinated control group, as well as mice that received the suboptimal vaccine formulations: ABA/CpG/alum, CpG/alum, and ABLA/alum all rapidly lost weight and either died or were humanely sacrificed.
3.2. Viral titers are lower in organs of challenged mice after effective vaccination
To determine if the vaccination strategy conferred “sterilizing” immunity, we examined viral titers in organs at day 3 and day 5 after challenge. To do this, mice were immunized with each preparation and challenged in parallel to the protection studies shown in Figure 1 and Table 1. These mice were sacrificed at day 3 or day 5 post challenge and the lungs and spleens were harvested for virus titration. Mice immunized with live vaccinia virus showed the lowest titers in the lungs at both time points (Figure 4A) and virus was below the level of detection in the spleen (Fig. 4B). In all cases where protein vaccines were used, some virus was detected in the spleen at 3 days post challenge, indicating that virus had spread systemically. However, titers were below the level of detection by day 5 (Figure 4B). Viral titers in both organs paralleled the relative protection of each of the vaccination strategies. Mice that were immunized with ABLA/CpG/alum or ABL/CpG/alum prior to virus challenge had viral titers in their lungs that were nearly 1,000-fold lower than the lung titers of unvaccinated controls. In both groups the lung and spleen titers continued to drop between days 3 and 5 (in fact the spleen titer was below the level of detection by day 5) indicating that these animals were controlling spread of the infection. In contrast, mice immunized with ABA/CpG/alum, which conferred little to no protection after challenge (Figure 1), had nearly the same lung titers as the unvaccinated controls at day 3 and day 5 (Figure 4A). Viral titers in the spleens of vaccinated mice (Figure 4B) were nearly 2-logs lower than those of control unvaccinated mice at day 3 after challenge, indicating that vaccination altered virus spread. However, even the mice vaccinated with ABA/CpG/alum, which were poorly protected from death and had high titers of virus in the lungs, showed less virus in the spleen compared to the control unvaccinated mice.
Figure 4.
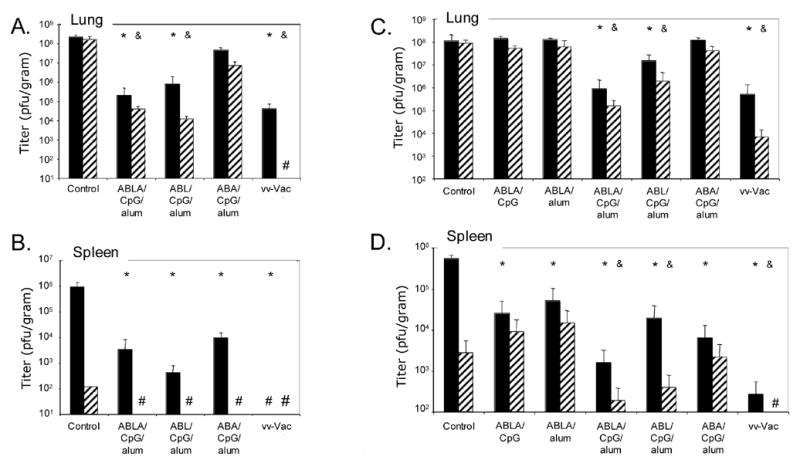
Viral titers in organs at various times after challenge. Viral titers in lungs (A and C) and spleens (B and D) from groups of 3 mice sacrificed at day 3 (black bars) and day 5 (striped bars) post-challenge. Data are from two separate vaccination and challenge experiments where additional mice were included at the start of the experiment. A and B correspond to mice vaccinated and challenged in parallel to those shown in Figure 1 (Table 1, experiment #1); C and D correspond to parallel mice summarized in Table 1, experiment #3. “#” in the figure stands for measurements below the level of detection. Statistical differences (p ≤ 0.02 by student’s t-test) between organ titers obtained from the vaccination groups and the control unvaccinated group at day 3 (*) and day 5 (&) are shown above the bars.
Figures 4C and 4D depict viral titers in the lungs and spleens of mice vaccinated and challenged at a different time. In this set of data, all groups of mice followed in parallel for survival and weight loss were more acutely sick after vaccinia virus challenge (Table 1, column #3). Groups of mice receiving vaccinations that conferred 100% protection (ABLA/CpG/alum and vaccinia virus) showed >2.5-logs lower viral titers in the lungs (Figure 4C) and had lower titers in the spleen (Figure 4D) at 3 or 5 days post-challenge than unvaccinated controls or groups receiving sub-optimal vaccine formulations that showed no protection (ABLA/CpG, ABLA/alum, and ABA/CpG/alum) (Figure 4C). At day 3 post-challenge, all vaccinated groups, even those receiving suboptimal formulations, had at least 10-fold lower titers in the spleen relative to the control unvaccinated mice, but by day 5 post-challenge, only groups of mice that had good protection (vaccinia virus vaccinated, ABLA/CpG/alum, and ABL/CpG/alum) had significantly lower titers of virus in the spleen (Figure 4D).
3.3. Sera from vaccinated groups have anti-MV and EV activity
We obtained sera from groups of mice obtained 2 weeks after the initial vaccination (prior to the boost) and 3 weeks after the boost (just prior to lethal challenge) and tested them by ELISA for reactivity to the purified proteins. Sera from all groups taken 2 weeks after the first vaccination showed little to no reactivity (data not shown). Sera of mice vaccinated with proteins along with CpG and alum taken 3 weeks after the boost showed good reactivity to the proteins that were part of their particular formulation (Figure 5A). Sera from mice vaccinated with the 4-proteins plus CpG (without alum) showed no measurable antibody titers to any of the proteins (data not shown). Interestingly, reactivity to A27 in mice vaccinated with 4-proteins plus alum (without CpG) was quite strong (Figure 5A). Despite the high level of anti-A27 antibody generated by vaccination with ABLA/alum (no CpG) or ABA/CpG/alum, these vaccine formulations offered little to no protection (Figure 1 and Table 1).
Figure 5.
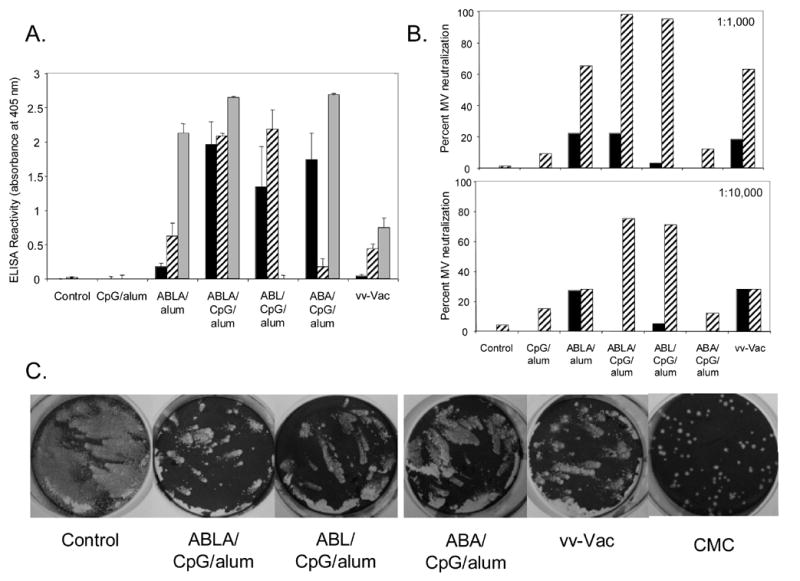
Antibody responses after vaccination. Groups of mice were bled prior to vaccination, 2 weeks after the first vaccination, and 3 weeks after the boost (just prior to challenge). Equal volumes of heat-inactivated sera from groups of mice were combined and studied as described in the Materials and Methods. A. Anti-B5, L1, and A27 reactivity by ELISA. Shown is the reactivity of sera (obtained 3 weeks after the boost) at a 1:550 dilution to B5 (black bars), L1 (striped bars), and A27 (grey bars). B. MV neutralization activity. Shown are MV-neutralization assays with sera, obtained 2 weeks after the prime (black bars) and 3 weeks after the boost (striped bars). Sera were used at a dilution of 1:1000 (top) or 1:10,000 (bottom). C. Comet inhibition. Shown are infected wells that were stained with crystal violet after about 40 hr incubation in liquid media containing sera from the indicated vaccination group at a 1:50 final concentration. To show an example of complete comet inhibition, included is an infected well that was overlaid with 1% carboxymethylcellulose (CMC). This semi-solid overlay prevents EV spreading distant from the primary site of infection.
To try to understand why, despite good anti-A27 ELISA responses, mice vaccinated with ABA/CpG/alum fared worse in terms of protection than mice vaccinated with ABL/CpG/alum, we examined the ability of sera from these vaccinated mice to neutralize MV. Since A27 and L1 are the only MV protein targets that are part of each of these 3-protein vaccine formulations, MV-neutralizing activity of the sera should be due to antibody responses to each of these proteins. As shown in Figure 5B, sera taken 2 weeks after vaccination had little to no MV-neutralizing activity. However, for mice immunized with ABL/CpG/alum or with ABLA/CpG/alum, sera taken 3 weeks after the boost had close to 100% MV-neutralizing activity (at 1:1000 dilution). Even when these sera were diluted at 1:10,000, they had 80% MV-neutralizing activity. In contrast, sera from mice immunized with ABA/CpG/alum exhibited little to no MV-neutralizing activity. Thus, even though A27 was highly immunogenic, it did not result in potent neutralizing antibodies after two-dose vaccinations in combination with CpG and alum. Sera from the group that received vaccinia virus vaccination had much lower anti-L1 and A27 reactivity by ELISA (Figure 5A), but it still was able to neutralize ~70% of MV. This good neutralization activity despite low ELISA activity to L1 and A27 is likely due to the multiple potential targets of MV-neutralizing activity that are generated by live vaccinia virus vaccination.
Finally, to assess the anti-EV activity of the mouse sera from vaccinated mice, we performed a comet inhibition assay of the sera on cells infected with the IHDJ strain of vaccinia virus, which generates comet-type plaques when grown in liquid media. As shown in Figure 5C, similar to sera from mice vaccinated with live vaccinia virus, sera from mice vaccinated with the various protein combinations along with CpG and alum reduced comet formation when compared to the unvaccinated controls. This indicates that these vaccinated groups developed functional antibodies that altered comet formation, while groups vaccinated with ABLA/alum (no CpG) or CpG/alum (no proteins) had not effect on comet formation (data not shown).
4. DISCUSSION
The ability to rapidly confer protection with a minimum number of vaccinations is an important part of a preparedness equation against potential bioterrorism with an agent like smallpox. Here we report a step toward such a goal using the purified ectodomains of vaccinia virus envelope proteins prepared from a baculovirus expression system. We chose to test these antigens together with CpG and alum as vaccine adjuvants, since this adjuvant combination has been shown to work well with a subunit vaccine for hepatitis B virus (HBV) in animal models for quickly inducing high levels of antibody with less reactogenicity than other adjuvants [43], to allow lower doses of antigen to be used [44], and to overcome the immature [45] or genetically hyporesponsive immune system [46]. Furthermore, in a phase I human study in healthy volunteers, addition of CpG to a commercial HBV vaccine that also contains alum induced significantly faster and higher levels of HBV-specific antibodies [37] of significantly enhanced avidity [47]. A subsequent phase II trial in HIV-infected subjects who had failed to respond adequately to previous HBV vaccination produced similar results [36]. All of these features made the CpG/alum adjuvant combination of interest to test with the vaccinia proteins.
In the present study we found that a vaccine formulation containing MV and EV proteins with CpG and alum conferred complete protection in mice within 5 weeks of initiating the protein vaccinations. This level of protection was similar to that provided by vaccination with live vaccinia virus by tail scarification. Partial protection from the protein vaccinations was still present 6 months after the initial vaccinations. While the long-term protection with the subunit vaccine was definitely not as robust as the protection conferred by live vaccinia virus vaccination, it does show that some protective immunity after subunit vaccinations is present long after the initial vaccinations. These findings may be important in the setting of either an actual monkeypox or variola virus outbreak. A prophylactic subunit vaccination could be an initial strategy to safely provide a significant degree of baseline immunity against orthopoxvirus infections and might also improve the safety profile of a subsequent live vaccinia virus vaccination.
While sterilizing immunity was not found with either live vaccinia virus vaccination or the subunit vaccines, virus titers in organs from mice sacrificed at various times after lethal challenge showed that groups that were protected from challenge had lower titers of challenge virus than groups with little or no protection. This indicates that vaccination provides protection against lethal challenge by allowing the host time to clear the infection. In the setting of ectromelia challenge of highly susceptible mice, vaccination protected from early and late death. The exact immunologic correlates of protection to orthopoxvirus infections are not known. Because the 3-protein vaccination strategy with ABA was not protective, it is clear that potent EV targets (A33 and B5) are not sufficient for optimal immunity. This is similar to the findings of the prior work with these proteins in different adjuvants [17]. As seen in Figure 5A, A27 was very immunogenic. When A27 was included in vaccine formulations, high sera reactivity was seen against it both in the presence of CpG (ABA/CpG/alum) and in the absence of CpG (ABLA/alum). However despite this high anti-A27 antibody, little to no protection was seen when compared to a similar 3-protein formulation containing L1 (ABL/CpG/alum). A potential explanation for this finding is seen when sera was examined for its ability to neutralize MV in a plaque reduction assay. Despite the high antibody titers to A27, sera from ABA vaccinated mice showed little to no MV neutralization activity when compared to sera from ABL vaccinated mice. Of note, this baculovirus-produced A27 protein when given repeatedly to mice and rabbits (initially in complete Freund’s adjuvant and then in incomplete Freund’s adjuvant), induced excellent neutralizing polyclonal antibody (data not shown). Thus, a prime and single boost vaccination with A27 appears to be insufficient to induce a good neutralizing antibody response to the protein.
ABL/CpG/alum provided a similar level of protection as ABLA/CpG/alum in two out of three short-term vaccinia virus challenges. Additionally, both formulations offered some long-term protection as seen when mice were challenged 6 months after initial vaccinations. Whether the addition of A27 to the cocktail containing A33, B5, and L1 afforded enhanced protection is difficult to say, since it appeared to help in one case where vaccinia was used for challenge but not in two others (Table 1). Furthermore, ABL/CpG/alum provided a similar level of protection as ABLA/CpG/alum in ectromelia challenge experiments indicating that the three proteins were sufficient to protect against this heterologous challenge. However there may still be a need for an additional protein(s) to protect against monkeypox and variola infections in humans. In addition, while we focused on the humoral responses to protein vaccination in this report, future studies will also evaluate the contribution of T-cell responses to the CpG/alum-adjuvanted poxvirus protein vaccines.
Finally, it is worth emphasizing that the ectromelia challenge experiments are a good indication of the ability of the subunit vaccine strategy to protect against a heterologous virus challenge. There are 17 amino acid differences between the vaccinia virus (strain WR) A33 ectodomain and its ortholog in ectromelia virus (Moscow strain) [48]. For B5 and L1 ectodomains, there are 13 and 3 amino acid differences between the vaccinia virus and ectromelia virus orthologs, respectively [48]. Clearly, these differences had no significant effect on the protection afforded by the subunit vaccination strategy we used. That being said, it is worth considering using proteins based on the variola virus sequence for a subunit vaccine strategy to protect against smallpox. While there is only 1 amino acid difference between the vaccinia virus (strain WR) L1 ectodomain and its ortholog in variola virus (Bangladesh-1975) [48], the A33 and B5 ectodomains have 10 and 21 amino acid differences between the vaccinia virus and variola virus orthologs, respectively [48]. Using the variola proteins would potentially prevent missing critical antibody and cellular epitopes and we are currently pursuing this approach.
Acknowledgments
Authors would like to thank Drs. Myron Levine for advice on the adjuvants and vaccination strategies and R. Mark Buller for providing a seed stock of ectromelia virus. This work was funded by the NIH NIAID Middle Atlantic Regional Center of Excellence (MARCE) grant U54 AI057168.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shalala DE. Smallpox: setting the research agenda. Science. 1999;285(5430):1011. doi: 10.1126/science.285.5430.1011. [DOI] [PubMed] [Google Scholar]
- 2.Enserink M. Biodefense. Smallpox vaccines: looking beyond the next generation. Science. 2004;304(5672):809. doi: 10.1126/science.304.5672.809a. [DOI] [PubMed] [Google Scholar]
- 3.Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. Smallpox and its eradication. World Health Organization; Geneva: 1988. p. 1460. [Google Scholar]
- 4.Hammarlund E, Lewis MW, Hansen SG, et al. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9(9):1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 5.Fulginiti VA, Papier A, Lane JM, Neff JM, Henderson DA. Smallpox vaccination: a review, part II. Adverse events. Clin Infect Dis. 2003;37(2):251–271. doi: 10.1086/375825. [DOI] [PubMed] [Google Scholar]
- 6.Poland GA, Grabenstein JD, Neff JM. The US smallpox vaccination program: a review of a large modern era smallpox vaccination implementation program. Vaccine. 2005;23(17–18):2078–2081. doi: 10.1016/j.vaccine.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Wyatt LS, Earl PL, Eller LA, Moss B. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc Natl Acad Sci U S A. 2004;101(13):4590–4595. doi: 10.1073/pnas.0401165101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Earl PL, Americo JL, Wyatt LS, et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428:182–185. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- 9.Stittelaar KJ, van Amerongen G, Kondova I, et al. Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. J Virol. 2005;79 (12):7845–7851. doi: 10.1128/JVI.79.12.7845-7851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Empig C, Kenner JR, Perret-Gentil M, et al. Highly attenuated smallpox vaccine protects rabbits and mice against pathogenic orthopoxvirus challenge. Vaccine. 2006;24 (17):3686–3694. doi: 10.1016/j.vaccine.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 11.Saijo M, Ami Y, Suzaki Y, et al. LC16m8, a highly attenuated vaccinia virus vaccine lacking expression of the membrane protein B5R, protects monkeys from monkeypox. J Virol. 2006;80(11):5179–5188. doi: 10.1128/JVI.02642-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hooper JW, Custer DM, Schmaljohn CS, Schmaljohn AL. DNA vaccination with vaccinia virus L1R and A33R genes protects mice against a lethal poxvirus challenge. Virology. 2000;266:329–339. doi: 10.1006/viro.1999.0096. [DOI] [PubMed] [Google Scholar]
- 13.Hooper JW, Custer DM, Thompson E. Four-gene-combination DNA vaccine protects mice against a lethal vaccinia virus challenge and elicits appropriate antibody responses in nonhuman primates. Virology. 2003;306(1):181–195. doi: 10.1016/S0042-6822(02)00038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hooper JW, Thompson E, Wilhelmsen C, et al. Smallpox DNA Vaccine Protects Nonhuman Primates against Lethal Monkeypox. J Virol. 2004;78(9):4433–4443. doi: 10.1128/JVI.78.9.4433-4443.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pulford DJ, Gates A, Bridge SH, Robinson JH, Ulaeto D. Differential efficacy of vaccinia virus envelope proteins administered by DNA immunisation in protection of BALB/c mice from a lethal intranasal poxvirus challenge. Vaccine. 2004;22(25–26):3358–3366. doi: 10.1016/j.vaccine.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 16.Galmiche MC, Goenaga J, Wittek R, Rindisbacher L. Neutralizing and protective antibodies directed against vaccinia virus envelope antigens. Virology. 1999;254:71–80. doi: 10.1006/viro.1998.9516. [DOI] [PubMed] [Google Scholar]
- 17.Fogg C, Lustig S, Whitbeck JC, Eisenberg RJ, Cohen GH, Moss B. Protective immunity to vaccinia virus induced by vaccination with multiple recombinant outer membrane proteins of intracellular and extracellular virions. J Virol. 2004;78(19):10230–10237. doi: 10.1128/JVI.78.19.10230-10237.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies DH, McCausland MM, Valdez C, et al. Vaccinia virus H3L envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice. J Virol. 2005;79(18):11724–11733. doi: 10.1128/JVI.79.18.11724-11733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang M, Cheng H, Dai Z, Bu Z, Sigal LJ. Immunization with a single extracellular enveloped virus protein produced in bacteria provides partial protection from a lethal orthopoxvirus infection in a natural host. Virology. 2006;345(1):231–243. doi: 10.1016/j.virol.2005.09.056. [DOI] [PubMed] [Google Scholar]
- 20.Smith GL, Vanderplasschen A, Law M. The formation and function of extracellular enveloped vaccinia virus. J Gen Virol. 2002;83(12):2915–2931. doi: 10.1099/0022-1317-83-12-2915. [DOI] [PubMed] [Google Scholar]
- 21.Moss B. Poxvirus entry and membrane fusion. Virology. 2006;344(1):48–54. doi: 10.1016/j.virol.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 22.Moss B. Poxviridae: The Viruses and Their Replication. In: Knipe DM, Howley PM, editors. Fields Virology. Vol. 2. Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 2849–2883. [Google Scholar]
- 23.Ichihashi Y, Takahashi T, Oie M. Identification of a vaccinia virus penetration protein. Virology. 1994;202:834–843. doi: 10.1006/viro.1994.1405. [DOI] [PubMed] [Google Scholar]
- 24.Wolffe EJ, Vijaya S, Moss B. A myristylated membrane protein encoded by the vaccinia virus L1R open reading frame is the target of potent neutralizing monoclonal antibodies. Virology. 1995;211:53–63. doi: 10.1006/viro.1995.1378. [DOI] [PubMed] [Google Scholar]
- 25.Su HP, Garman SC, Allison TJ, Fogg C, Moss B, Garboczi DN. The 1.51-Angstrom structure of the poxvirus L1 protein, a target of potent neutralizing antibodies. Proc Natl Acad Sci U S A. 2005;102(12):4240–4245. doi: 10.1073/pnas.0501103102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez JF, Janeczko R, Esteban M. Isolation and characterization of neutralizing monoclonal antibodies to vaccinia virus. J Virol. 1985;56:482–488. doi: 10.1128/jvi.56.2.482-488.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gong SC, Lai CF, Esteban M. Vaccinia virus induces cell fusion at acid pH and this activity is mediated by the N-terminus of the 14-kDa virus envelope protein. Virology. 1990;178(1):81–91. doi: 10.1016/0042-6822(90)90381-z. [DOI] [PubMed] [Google Scholar]
- 28.Vazquez MI, Rivas G, Cregut D, Serrano L, Esteban M. The vaccinia virus 14-kilodalton (A27L) fusion protein forms a triple coiled-coil structure and interacts with the 21-kilodalton (A17L) virus membrane protein through a C-terminal alpha-helix. J Virol. 1998;72(12):10126–10137. doi: 10.1128/jvi.72.12.10126-10137.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roper RL, Payne LG, Moss B. Extracellular vaccinia virus envelope glycoprotein encoded by the A33R gene. J Virol. 1996;70:3753–3762. doi: 10.1128/jvi.70.6.3753-3762.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engelstad M, Howard ST, Smith GL. A constitutively expressed vaccinia gene encodes a 42-kDa glycoprotein related to complement control factors that forms part of the extracellular virus envelope. Virology. 1992;188:801–810. doi: 10.1016/0042-6822(92)90535-w. [DOI] [PubMed] [Google Scholar]
- 31.Isaacs SN, Wolffe EJ, Payne LG, Moss B. Characterization of a vaccinia virus-encoded 42-kilodalton class I membrane glycoprotein component of the extracellular virus envelope. J Virol. 1992;66:7217–7224. doi: 10.1128/jvi.66.12.7217-7224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halloran ME, Longini IM, Jr, Nizam A, Yang Y. Containing bioterrorist smallpox. Science. 2002;298(5597):1428–1432. doi: 10.1126/science.1074674. [DOI] [PubMed] [Google Scholar]
- 33.Bozzette SA, Boer R, Bhatnagar V, et al. A model for a smallpox-vaccination policy. N Engl J Med. 2003;348(5):416–425. doi: 10.1056/NEJMsa025075. [DOI] [PubMed] [Google Scholar]
- 34.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5(6):471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 35.Kim SK, Ragupathi G, Cappello S, Kagan E, Livingston PO. Effect of immunological adjuvant combinations on the antibody and T-cell response to vaccination with MUC1-KLH and GD3-KLH conjugates. Vaccine. 2000;19(4–5):530–537. doi: 10.1016/s0264-410x(00)00195-x. [DOI] [PubMed] [Google Scholar]
- 36.Cooper CL, Davis HL, Angel JB, et al. CPG 7909 adjuvant improves hepatitis B virus vaccine seroprotection in antiretroviral-treated HIV-infected adults. Aids. 2005;19 (14):1473–1479. doi: 10.1097/01.aids.0000183514.37513.d2. [DOI] [PubMed] [Google Scholar]
- 37.Cooper CL, Davis HL, Morris ML, et al. CPG 7909, an immunostimulatory TLR9 agonist oligodeoxynucleotide, as adjuvant to Engerix-B HBV vaccine in healthy adults: a double-blind phase I/II study. J Clin Immunol. 2004;24(6):693–701. doi: 10.1007/s10875-004-6244-3. [DOI] [PubMed] [Google Scholar]
- 38.Aldaz-Carroll L, Whitbeck JC, Ponce de Leon M, et al. Epitope-mapping studies define two major neutralization sites on the vaccinia virus extracellular enveloped virus glycoprotein B5R. J Virol. 2005;79(10):6260–6271. doi: 10.1128/JVI.79.10.6260-6271.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aldaz-Carroll L, Whitbeck JC, Ponce de Leon M, et al. Physical and immunological characterization of a recombinant secreted form of the membrane protein encoded by the vaccinia virus L1R gene. Virology. 2005;341(1):59–71. doi: 10.1016/j.virol.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 40.Tessier DC, Thomas DY, Khouri HE, Laliberte F, Vernet T. Enhanced secretion from insect cells of a foreign protein fused to the honeybee melittin signal peptide. Gene. 1991;98(2):177–183. doi: 10.1016/0378-1119(91)90171-7. [DOI] [PubMed] [Google Scholar]
- 41.Ramirez JC, Tapia E, Esteban M. Administration to mice of a monoclonal antibody that neutralizes the intracellular mature virus form of vaccinia virus limits virus replication efficiently under prophylactic and therapeutic conditions. J Gen Virol. 2002;83 (Pt 5):1059–1067. doi: 10.1099/0022-1317-83-5-1059. [DOI] [PubMed] [Google Scholar]
- 42.Esteban DJ, Buller RM. Ectromelia virus: the causative agent of mousepox. J Gen Virol. 2005;86(Pt 10):2645–2659. doi: 10.1099/vir.0.81090-0. [DOI] [PubMed] [Google Scholar]
- 43.Weeratna RD, McCluskie MJ, Xu Y, Davis HL. CpG DNA induces stronger immune responses with less toxicity than other adjuvants. Vaccine. 2000;18(17):1755–1762. doi: 10.1016/s0264-410x(99)00526-5. [DOI] [PubMed] [Google Scholar]
- 44.Weeratna R, Comanita L, Davis HL. CPG ODN allows lower dose of antigen against hepatitis B surface antigen in BALB/c mice. Immunol Cell Biol. 2003;81(1):59–62. doi: 10.1046/j.1440-1711.2003.01135.x. [DOI] [PubMed] [Google Scholar]
- 45.Kovarik J, Bozzotti P, Love-Homan L, et al. CpG oligodeoxynucleotides can circumvent the Th2 polarization of neonatal responses to vaccines but may fail to fully redirect Th2 responses established by neonatal priming. J Immunol. 1999;162(3):1611–1617. [PubMed] [Google Scholar]
- 46.Davis HL, Suparto II, Weeratna RR, et al. CpG DNA overcomes hyporesponsiveness to hepatitis B vaccine in orangutans. Vaccine. 2000;18(18):1920–1924. doi: 10.1016/s0264-410x(99)00443-0. [DOI] [PubMed] [Google Scholar]
- 47.Siegrist CA, Pihlgren M, Tougne C, et al. Co-administration of CpG oligonucleotides enhances the late affinity maturation process of human anti-hepatitis B vaccine response. Vaccine. 2004;23(5):615–622. doi: 10.1016/j.vaccine.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 48.Poxvirus Bioinformatics Resource Center. [(Accessed September 2006)]; http://www.poxvirus.org/.2006.


