Abstract
Despite numerous aphasia and functional imaging studies, the exact correlation between cortical language areas and subcomponents of the linguistic system has not been established. Here, we used functional MRI to identify cortical areas specifically involved in syntactic processing. An experimental design contrasted sentences containing grammatical errors with sentences containing spelling errors. The ungrammatical sentences produced more activation in cortical language areas than did the sentences with spelling errors, and the difference in activation was significantly greater in Broca's area than in Wernicke's area or in the angular gyrus/supramarginal gyrus. The present findings provide direct evidence of a syntactic specialization for Broca's area and establish the existence of distinct modules for our knowledge of language.
Linguistic theory divides human linguistic ability into distinct modules responsible for distinct aspects of our knowledge of language. For instance, the syntactic component governs the hierarchical organization of words and phrases in sentences, whereas the phonological component is responsible for the sound structure of a language. The identification of specialized cortical areas responsible for these distinct aspects of linguistic competence is a first step toward understanding how language is instantiated in the brain. This study uses functional MRI (fMRI) to identify cortical areas involved specifically in syntactic processing. The focus is on the role of Broca's area in syntax, an issue that has been debated from a number of different perspectives.
Event-related brain potential (ERP) studies have identified a component, the P600, which correlates with a subject's recognition of syntactic ill formedness (1). However, the language specificity of this component has been challenged (2), and the specific cortical generator of the component has not been localized. A second component, the left anterior negativity, has also been associated with error detection in grammatical processing (3, 4); its source is once again unclear, but its detection by left-anterior electrodes is suggestive of the involvement of Broca's area. In the imaging literature, activation in Broca's area has been found for some linguistic tasks, whereas other studies have argued for the involvement of Broca's area in decidedly nonsyntactic tasks, such as in the phonological processing of words or letters (5). Two prior imaging studies by Stromswold et al. (6) and Just et al. (7) have studied syntactic complexity and made specific claims about Broca's area. In these studies, increased sentential complexity correlates with an increase in activity in Broca's area. Activity in Broca's area in these experiments either may stem from the fact that Broca's area is specifically involved in syntactic processing or may reflect a general increase in demands on the linguistic system or on verbal short-term memory rather than syntactic processing per se. Just et al. (7), noting an increase in activated voxels in both Broca's and Wernicke's areas that correlated with sentential complexity, concluded that increased sentential complexity resulted in the recruitment of more neural tissue in each of a network of cortical areas; i.e., that increases were caused by an increase in complexity, with no region more specifically involved in sentential processing than any other. Two recent fMRI studies (8, 9) have claimed to have evoked differential activity in Broca's area in syntactic as opposed to semantic processing. However, syntactic and semantic processing were involved in both contrasting experimental conditions in these studies; moreover, neither provides any independent support for their claims about the processing behind the experimental tasks, as the tasks lack an experimental history in both cases.
Research on aphasia also provides contradictory hypotheses about whether Broca's area is specifically involved in syntax. After Broca's initial insight, Broca's area was first regarded as a speech production area, because patients diagnosed with Broca's aphasia were relatively or grossly inarticulate. Subsequent work on aphasic patients argued that Broca's area is the locus of syntactic processing or of the grammatical system because of the agrammatism of Broca's aphasics (10, 11). More recent studies, however, have argued that there is dissociation between Broca's aphasics' problems with syntactic processing/comprehension and lesions in Broca's area (12, 13), although dissenting views continue to be voiced (14).
Given this uncertainty about the role played by Broca's area in syntax, the present study seeks to identify brain areas associated specifically with syntactic processing, independent of error detection requiring verbal short-term memory and of error detection in a linguistic context. Our study combines a version of the error-detection paradigm well exemplified in ERP studies of syntactic anomaly (15) with a linguistic control condition that involves both normal sentence processing and error detection of misspelled words, comparable in difficulty to syntactic error detection.
Methods
Tasks.
Sentences were divided into two conditions, grammar (GR) and spelling (SP). The sentential stimuli use the same lexical material across conditions and differ only in the types of errors they contain (Table 1). Sentences of the GR type contained one or two errors in word order; sentences of the SP type contained one or two errors in spelling. In each condition, stimuli were presented visually, and the task was to respond whether the sentence contained one or two errors by pressing one of two buttons attached to pneumatic switches. By using this one- vs. two-error design, we were able to place stimuli of the same error type in blocks, while maintaining a task that requires the processing of each stimulus. Subjects were instructed to read every sentence slowly and carefully before responding. In an additional control (CO) condition, subjects viewed rows of colored Ls and Ts, with a basic association between a single color and a letter, for instance, purple Ts and yellow Ls. In this case, the task was to find matches for a target combination in the upper left-hand corner, where the letter/color violated the basic pattern; the subject, scanning the further rows of letters, responded as to whether there was a single match to this combination or two matches. Each of the SP and GR conditions combines linguistic processing with an error-detection task. The difference between the two lies in the different types of errors: grammatical errors, which induce syntactic violations, and spelling errors, which concern orthographic representations. The CO condition controls for both serial search and short-term memory of targets.
Table 1.
Sample stimuli used in GR and SP conditions
| Error | GR | SP |
|---|---|---|
| 1 | Bill wrote paper a about the discussion of the treaty. | Bill wrote a papger about the discussion of the treaty. |
| 1 | Mary wanted to read about the destruction the of city | Mary waanted to read about the destruction of the city. |
| 1 | John drove to store the in a very fast car two weeks ago. | John drove to the store in a very fasvt car two weeks ago. |
| 2 | Mary asked question a about theorem the in class. | Mary askepd a question about the theorem in cllass. |
| 2 | The editor read article the with revisions Anne's after lunch. | The editor read the artilce with Anne's rezvisions after lunch. |
| 2 | Tom drove the to beach on Will's fast extremely motorcycle. | Tom drove to tfe beach on Will's extremely fqast motorcycle. |
Stimuli.
We presented each sentence in three or four rows of yellow letters against a dark background. Each visual stimulus (maximum visual angle: 5° × 15°; presentation time, 5,500 ms) was back projected onto a translucent screen near the subject's feet with a liquid crystal projector (XV-E300, Sharp, Osaka), driven by a PC-Linux system. The subjects viewed the stimuli through prism glasses in front of eyes and were asked to fixate on a red central cross, where the stimuli appeared, and to read sentences silently. Both accuracy and reaction times (RTs) were measured on line, and the stimulus presentation and behavioral data collection were controlled by a PC-LabVIEW system (National Instruments, Austin, TX), which was synchronized with the PC-Linux system. Stimuli were presented in blocks of CO-SP-CO-GR-CO… Each block contained three one-error stimuli and three two-error stimuli in a random sequence. Each run consisted of five CO blocks, two SP blocks, and two GR blocks.
Subjects.
Eight native speakers of English (6 male and 2 female, ages: 20 − 35) participated in the present study. All subjects showed right handedness (laterality quotients: 54 − 100) by the Edinburgh inventory (16). During the study, the subject wore earplugs and was in a supine position in the magnet, while the subject's head was immobilized with padding inside the radio-frequency coil. Informed consent from each subject was obtained after the nature and possible consequences of the studies were explained. Approval for these experiments was obtained from the institutional review boards of the University of Tokyo, Komaba.
fMRI Data Acquisition and Analysis.
The fMRI scans were conducted on a conventional 1.5T scanner (Stratis II, Hitachi Medical Corporation, Tokyo), by using a steady-state acquisition with rewinded gradient echo sequence (repetition time = 70 ms, echo time = 40 ms, respectively, flip angle = 30°, field of view = 256 × 256 mm2, resolution = 2 × 2 mm2) for four horizontal slices (thickness = 8 mm), covering z = 0 ≈ + 32 from the horizontal bicommissural plane (up to 40 in the case of two subjects). In a single scanning session, we acquired each image every 6 s. During the same session as fMRI scanning, we obtained structural images by using a spin echo sequence (repetition time = 150 ms, echo time = 15 ms, flip angle = 90°, field of view = 256 × 256 mm2, resolution = 1 × 1 × 8 mm3) at the same slice positions. For anatomical localization, a three-dimensional structural image of a whole brain of each subject was obtained by using a gradient echo sequence (repetition time = 30 ms, echo time = 8 ms, flip angle = 60°, field of view = 256 × 256 mm2, resolution = 1 × 1 × 3 mm3) in a separate session.
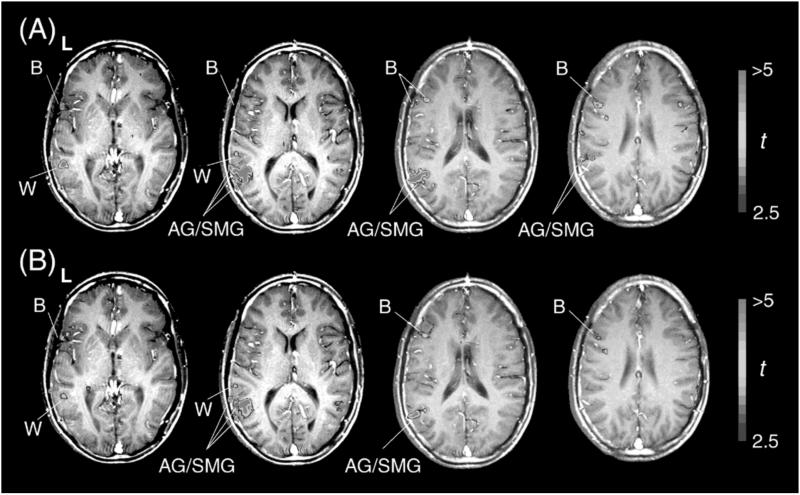
For the analysis of fMRI time-series data, we used in-house software developed with Hitachi Seibu Software (Tokyo) (17). Time-series data of each voxel were converted to percent signal changes from the initial target block and corrected for baseline by using linear fitting to data throughout all target periods with a hemodynamic delay of 6 s. They were then averaged for multiple sessions after correction for head movements between scans with no spatial or temporal smoothing. Regions consisting of four contiguous voxels with the highest t-values above a threshold of 2.57 (P < 0.005, one-tailed; P < 0.02 after Bonferroni correction) were selected, thus excluding the spatial extents of activated regions. We compared signal changes in GR and SP for each region defined in the t-map of GR vs. CO; the location of an activated region in the t-map of GR vs. CO matched with that in SP vs. CO when activation was observed in both conditions (Fig. 1). When two or more clusters were identified in the t-map as separate but still within the same predefined anatomical area, signal changes were averaged among these clusters. These clusters might be candidates for further functional parcellation, but it is possible that slicing undulate gyri resulted in apparently separated clusters. Points of activation in each subject were identified anatomically by using three-dimensional structural images of each subject's brain. Separation of angular gyrus/supramarginal gyrus (AG/SMG) and Wernicke's area followed criteria reported previously (18). For each subject, the data included values for each of the two SP and GR blocks per activated area.
Figure 1.
Cortical activation in GR and SP conditions. Thresholded fMRI images in t-maps were superimposed on structural images showing z = 4, 12, 20, and 28 horizontal slices (see Table 3) for a single representative subject. The left side (L) of the brain is shown left. (A) GR vs. CO; (B) SP vs. CO. The color bars at right indicate the t-values of the comparison. Activation in the GR condition is generally greater in language areas than activation in the SP condition; this difference is particularly prominent in Broca's area (B), compared with Wernicke's area (W) and AG/SMG.
Results
According to an ANOVA on behavioral data (Table 2), there was a main effect of conditions (CO, SP, and GR) in accuracy (P < 0.05), but RT showed such a main effect only under the condition with two errors (P < 0.05; with one error, P > 0.1). According to a post-hoc test (Fisher's protected least significant difference), the difference between SP and CO was not significant in either RT or accuracy (P > 0.1). As to the comparison between GR and SP, there was no consistent significant difference in both RT and accuracy across conditions with one error and two errors; whereas RT showed a significant difference between GR and SP only under the condition with two errors (P < 0.05), accuracy showed such a difference only under the condition with one error (P < 0.05). Overall, there was a strong main effect of errors (one and two) in both RT and accuracy (P < 0.0005). Conditions with one error showed longer RTs and higher accuracy rates than conditions with two errors, indicating that the subject paid more attention when checking for an additional (absent) error.
Table 2.
Behavioral data and similarity in task difficulty between CO, SP, and GR conditions
| Condition | Error | RT, ms | Accuracy, % |
|---|---|---|---|
| CO | 1 | 4,069 ± 280 | 97.5 ± 1.7 |
| SP | 1 | 4,266 ± 454 | 96.1 ± 2.1 |
| GR | 1 | 4,293 ± 384 | 92.2 ± 4.5 |
| CO | 2 | 3,421 ± 416 | 93.2 ± 2.3 |
| SP | 2 | 3,610 ± 446 | 89.6 ± 5.1 |
| GR | 2 | 4,109 ± 400 | 84.4 ± 7.5 |
Data are shown in mean ± SD.
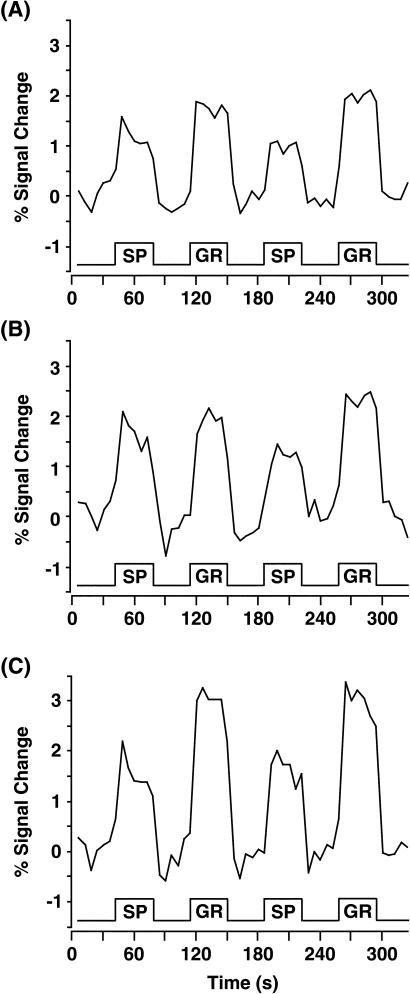
We found that each of the cortical language areas, i.e., AG/SMG, Wernicke's area, and Broca's area (Table 3), showed significant increases in signal change in both GR and SP when each condition was compared with CO (Fig. 1). In addition, the GR condition produced significantly more activation than SP across language areas. As the time courses show, activation in the language conditions was stimulus locked and did not show an effect of habituation or repetition for the different language blocks (Fig. 2). Neither the main effect of blocks nor the interaction between conditions and blocks was significant (P > 0.1).
Table 3.
Activated regions and differences between GR and SP conditions
| Region | BA | Hemisphere | n | x | y | z | GR vs. SP |
|---|---|---|---|---|---|---|---|
| AG/SMG | 39/40 | L | 7 | −49 ± 7 | −51 ± 8 | 16 ± 10 | 7.1* |
| Wernicke | 22 | L | 6 | −58 ± 5 | −33 ± 16 | 10 ± 5 | 6.9* |
| Broca | 44/45 | L | 7 | −51 ± 4 | 11 ± 7 | 17 ± 5 | 22.9** |
| R | 5 | 46 ± 4 | 17 ± 3 | 14 ± 11 | 5.7* |
Identification of regions is described in Methods. BA denotes Brodmann's area and n is the number of subjects showing significant activation in the region in GR vs. CO. Columns x, y, and z show coordinates (mean ± SD) of the centers of active regions in each subject, presented in terms of millimeters from the anterior end of the bicommissural line. Column GR vs. SP shows F-values of the main effect of tasks in an ANOVA (condition × block) for each region. *, P < 0.05; **, P < 0.0001.
Figure 2.
Mean-time series for each region of interest in the study. The ordinate indicates percent signal change; the abscissa is time, with stimulus blocks indicated and labeled. The time courses show stimulus-locked activation for each of the three language areas in the left hemisphere: AG/SMG (A), Wernicke (B), and Broca (C).
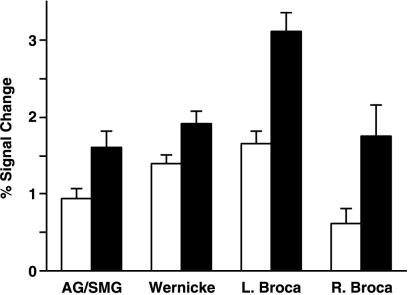
An ANOVA for the left hemisphere (region × condition × block) showed significant main effects of regions and conditions (P < 0.0001) but no main effect of blocks (P = 0.8). Moreover, there was a significant interaction between regions and conditions (P < 0.05), but there were no other significant interactions (P > 0.1). These results suggest that the cortical language areas were not uniformly activated under the GR and SP conditions; rather, there was a clear dissociation among language-related regions. The differential response between GR and SP was significantly greater in Broca's area than in Wernicke's area, AG/SMG, and the right homolog of Broca's area (Fig. 3 and Table 3). A post-hoc test by using Fisher's protected least significant difference revealed that pairs consisting of the signal changes in Broca's area and those in each other region were significant: (Broca, AG/SMG) P < 0.0001, (Broca, Wernicke) P < 0.01, (Broca, right Broca) P < 0.0001, whereas comparisons of other regions did not show significant differences (AG/SMG, Wernicke) P = 0.1, (AG/SMG, right Broca) P = 0.7, (Wernicke, right Broca) P = 0.07.
Figure 3.
The effect of two language conditions in the language areas. Histogram comparing the values for percent signal change (mean ± SEM of subjects and blocks) for each language condition vs. CO (white = SP, black = GR) is shown in each region of interest. Note the prominent condition difference in left Broca's area (L. Broca), which is larger than in other language areas and the right homolog of Broca's area (R. Broca).
Discussion
These data indicate that Broca's area is specifically involved in syntactic processing. They also preclude an interpretation according to which general computational complexity is the only difference between the GR and SP conditions, as the behavioral data did not reveal a consistent complexity contrast between these two conditions. Differences in activation found between GR and SP are instead attributable to the different linguistic systems involved in each condition: syntactic error detection in GR, as opposed to orthographic error detection and normal syntactic processing in SP. The source of the differences between GR and SP in Wernicke's area and AG/SMG is less clear. It could be that error detection focused in Broca's area induces an increase in activation in other language areas through feedback projections as the subject attempts reanalysis of the apparently deviant sentence. Or it could be that a certain amount of syntactic processing also takes place in Wernicke's area and AG/SMG. Finally, by claiming that Broca's area is involved in syntax in a special way, we are making no claims about exclusivity; whether Broca's area is directly associated with other linguistic and/or nonlinguistic modules is still an open question. The involvement of Broca's area in the process of speech production is another issue for future study (19).
The idea that Broca's area is a locus of syntax stems initially from the aphasia literature (10, 11). The imaging literature on syntax has attributed activation in Broca's area in syntactic tasks to an increase in general complexity, rather than to a specific role for this area in syntax (6, 7). Recent fMRI studies reported that a part of Broca's area (BA 44) is implicated in processing syntactic information as opposed to semantic information (8, 9). However, each contrastive experimental condition in these studies involved both syntactic and semantic processing under the same task instructions, and in neither study is there any experimental history or independent evidence to support the authors' claims about implicit and conflicting task demands across conditions. In addition, different sets of sentences were used for two conditions, thus involving uncontrolled cognitive factors. In contrast, the present study used an explicit syntactic paradigm with an established experimental history and cognitive theory of the task and further used the same lexical material across conditions. Our results suggest a correlation with results from the ERP literature (3, 4) in the possibility that ERP components observed in syntactic error-detection experiments result from enhanced activation in Broca's area. Future work combining electrophysiological (ERP, magnetoencephalography) and hemodynamic (e.g., fMRI, positron emission tomography) methods should clarify the relationship between temporal response components like the left anterior negativity/P600 and the activation in Broca's area observed in this experiment. Such work will allow us to move beyond the functional anatomy of the brain to ask questions about how module-specific brain areas actually perform linguistic computations.
Acknowledgments
We thank F. Homae for help with data analysis and H. Matsuda for administrative assistance. This work was supported by an International Cooperative Research Project grant from Japan Science and Technology Corporation (JST) (D.E., A.M., Y.M., and W.O.) and a Core Research for Evolutional Science and Technology grant from JST (K.L.S.).
Abbreviations
- AG/SMG
angular gyrus/supramarginal gyrus
- CO
control
- fMRI
functional MRI
- GR
grammar
- RT
reaction time
- SP
spelling
- ERP
event-related brain potential
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.100098897.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.100098897
References
- 1.Osterhout L, Holcomb P J. J Mem Lang. 1992;31:785–806. [Google Scholar]
- 2.Patel A D, Gibson E, Ratner J, Besson M, Holcomb P J. J Cognit Neurosci. 1998;10:717–733. doi: 10.1162/089892998563121. [DOI] [PubMed] [Google Scholar]
- 3.Münte T F, Heinze H-J, Mangun G R. J Cognit Neurosci. 1993;5:335–344. doi: 10.1162/jocn.1993.5.3.335. [DOI] [PubMed] [Google Scholar]
- 4.Friederici A D, Pfeifer E, Hahne A. Cognit Brain Res. 1993;1:183–192. doi: 10.1016/0926-6410(93)90026-2. [DOI] [PubMed] [Google Scholar]
- 5.Poeppel D. Brain Lang. 1996;55:317–351. doi: 10.1006/brln.1996.0108. [DOI] [PubMed] [Google Scholar]
- 6.Stromswold K, Caplan D, Alpert N, Rauch S. Brain Lang. 1996;52:452–473. doi: 10.1006/brln.1996.0024. [DOI] [PubMed] [Google Scholar]
- 7.Just M A, Carpenter P A, Keller T A, Eddy W F, Thulborn K R. Science. 1996;274:114–116. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- 8.Dapretto M, Bookheimer S Y. Neuron. 1999;24:427–432. doi: 10.1016/s0896-6273(00)80855-7. [DOI] [PubMed] [Google Scholar]
- 9.Kang A M, Constable R T, Gore J C, Avrutin S. Neuroimage. 1999;10:555–561. doi: 10.1006/nimg.1999.0493. [DOI] [PubMed] [Google Scholar]
- 10.Geschwind N. Science. 1965;170:940–944. doi: 10.1126/science.170.3961.940. [DOI] [PubMed] [Google Scholar]
- 11.Goodglass H. In: Studies in Neurolinguistics. Whitaker H, Whitaker H A, editors. Vol. 1. New York: Academic; 1976. pp. 237–260. [Google Scholar]
- 12.Dronkers N F, Ludy C A. In: Handbook of Neurolinguistics. Stemmer B, Whitaker H A, editors. New York: Academic; 1998. pp. 173–187. [Google Scholar]
- 13.Caplan D, Hildebrandt N, Makris N. Brain. 1996;119:933–949. doi: 10.1093/brain/119.3.933. [DOI] [PubMed] [Google Scholar]
- 14.Grodzinsky Y, Finkel L. J Cognit Neurosci. 1998;10:281–292. doi: 10.1162/089892998562708. [DOI] [PubMed] [Google Scholar]
- 15.Neville H, Nicol J L, Barss A, Forster K I, Garrett M F. J Cognit Neurosci. 1991;3:151–165. doi: 10.1162/jocn.1991.3.2.151. [DOI] [PubMed] [Google Scholar]
- 16.Oldfield R C. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 17.Sakai K, Watanabe E, Onodera Y, Itagaki H, Yamamoto E, Koizumi H, Miyashita Y. Magn Reson Med. 1995;33:736–743. doi: 10.1002/mrm.1910330521. [DOI] [PubMed] [Google Scholar]
- 18.Rademacher J, Galaburda A M, Kennedy D N, Filipek P A, Caviness V S., Jr J Cognit Neurosci. 1992;4:352–374. doi: 10.1162/jocn.1992.4.4.352. [DOI] [PubMed] [Google Scholar]
- 19.Levelt W J M. Trends Cognit Sci. 1999;3:223–232. doi: 10.1016/s1364-6613(99)01319-4. [DOI] [PubMed] [Google Scholar]