Abstract
Objective
To evaluate temporal trends in pediatric type 1 diabetes (T1DM) management and resultant effects on outcomes.
Study design
Two pediatric T1DM cohorts were followed prospectively for 2 years and compared; Cohort 1 (N=299) was enrolled in 1997 and Cohort 2 (N=152) was enrolled in 2002. In both cohorts, eligible participants were identified and sequentially approached at regularly scheduled clinic visits until the target number of participants was reached. Main outcome measures were A1c, z-BMI, and incidence rates (IR, per 100 patient-years) of hypoglycemia, hospitalizations, and emergency room (ER) visits.
Results
At baseline, Cohort 2 monitored blood glucose more frequently than Cohort 1 (≥4 times/day: 72% vs. 39%, p<.001) and was prescribed more intensive therapy than Cohort 1 (≥3 injections/day or pump: 85% vs. 65%, p<.001). A1c was lower in Cohort 2 than Cohort 1 at baseline (8.4% vs. 8.7%, p=.03) and study’s end (8.7% vs. 9.0%, p=.04). The cohorts did not differ in z-BMI (0.83 vs. 0.79, p=.57) or IR of hospitalizations (11.2 vs. 12.9, p=.38). Cohort 2 had lower IR of total severe hypoglycemic events (29.4 vs. 55.4, p<.001) and ER visits (22.0 vs. 29.3, p=.02).
Conclusions
T1DM management intensified during the 5 years between cohorts and was accompanied by improved A1c and stable z-BMI. Along with improved control, IRs of severe hypoglycemia and ER visits decreased by almost 50% and 25%, respectively.
Keywords: diabetes, pediatrics, intensive, management, complications, BMI
The Diabetes Control and Complications Trial (DCCT) demonstrated that optimal glycemic control in type 1 diabetes mellitus (T1DM) delays the onset and slows the progression of microvascular complications (1). Current recommendations therefore mandate that youth with T1DM should be treated with intensive therapy to normalize glycemic control as early as possible (2). Intensive therapy involves multiple daily injections (MDI) of insulin or insulin pump therapy (continuous subcutaneous insulin infusion, CSII), as well as other essential components of diabetes care, such as self-monitoring of blood glucose (SMBG) (3–5).
Treatment of T1DM in children and adolescents is difficult. The combination of severe insulin deficiency and the physical and psychosocial changes that accompany normal growth and development present unique challenges to pediatric health care professionals (6–9). In the DCCT, the 195 adolescents, ages 13–17 years at entry, had significantly higher A1c values compared to their adult counterparts, and the investigators anticipated that worldwide translation of treatment recommendations for youth would be especially challenging (10). Recent data, however, suggest that clinicians have gained success in implementing DCCT standards in pediatric practice. Advances in technology, such as improved methods of SMBG, modern insulin infusion pumps, and new short- and long-acting insulin analogs, as well as innovative behavioral and educational approaches, have contributed to this success (11–14).
Maintenance of near-euglycemia, however, is not the only goal of intensive diabetes management. The prevention of excessive weight gain, previously associated with intensification of diabetes therapy (15–17), is desirable. In addition, acute diabetes-related complications [e.g., episodes of severe hypoglycemia, hospitalizations, and emergency room (ER) visits] should be minimized. Although early evidence from the DCCT showed that intensively treated adolescents had a greater risk of severe hypoglycemia, more recent data suggest that hypoglycemia does not inevitably accompany improved metabolic control (18–20).
Data regarding the impact of intensification of therapy on the occurrence of hospitalizations and ER utilization are limited. The purpose of this study was to examine whether intensive management and outcomes of diabetes care, as reflected by glycemic control, body mass index z-score (z-BMI), and acute complication rates have changed in the pediatric population in recent years.
METHODS
Participants
Two cohorts of youth with T1DM were followed longitudinally for two years and acute adverse event rates of the two groups were compared. Eligibility criteria for both cohorts included: age 8–16 years, duration of T1DM >6 months, stable living environment, no major psychiatric problems, and intention for routine follow-up care at the clinic. All youth had received insulin since diagnosis and had insulin requirements ≥0.5 U/kg/day at enrollment. The Committee on Human Studies of the Joslin Diabetes Center approved the study protocols.
Enrollment of Cohort 1 occurred between 1997–1998 (4 years post-DCCT). Enrollment of Cohort 2 occurred between 2002–2003 (9 years post-DCCT). The two time intervals allow for an examination of gradual trends in clinical care.
For Cohort 1, 413 eligible participants were identified and sequentially approached until the target number of 299 was reached. During enrollment, 35 families declined participation. Non-participants had a mean (standard deviation) age of 13.0(2.7) years, mean diabetes duration of 7.0(3.7) years, and mean A1c of 9.0(1.3)%. Non-participants were slightly older and had slightly longer diabetes duration than participants, but did not differ from participants with respect to glycemic control.
For Cohort 2, 462 eligible participants were identified and sequentially approached until the target number of 154 was reached. Two patients were subsequently removed from data analyses due to the occurrence of significant psychiatric problems in one patient and a revision of diagnosis from T1DM to maturity-onset diabetes of the young in the other patient. During enrollment, 20 families declined participation. Non-participants had a mean age of 13.2(1.8) years, mean diabetes duration of 6.5(3.2) years, and mean A1c of 8.5(1.6)%. There were no significant differences between participants and non-participants with respect to any of these characteristics.
The target number of participants in each cohort was in part determined by the availability of research staff to follow patients prospectively over a two-year period. Each research assistant (RA) followed between 70–75 patients. Fewer RAs were available in 2002, resulting in Cohort 2 being half the size of Cohort 1. Over 90% of patients in both cohorts were first seen, and subsequently followed, within 1.5 years of diagnosis and thus represent a community-based sample. Thirty-five patients were enrolled in both Cohort 1 and Cohort 2.
Procedures
Eligible families were enrolled at their regularly scheduled visits. RAs obtained written, informed consent from the parent and assent from the child.
During the two years of follow-up, families were encouraged by their medical providers to visit the clinic every 3 months. At each clinic visit, an RA conducted a 5–10 minute structured, joint child-parent interview to gather data pertaining to family demographics, diabetes management, and frequency and severity of recent hypoglycemia, hospitalizations, and ER utilization. The RA also extracted data, based on the interval history and physical examination performed by the medical provider, from the patient’s medical record. These data included measurements of height, weight, and blood pressure, as well as staging of sexual development by the method of Tanner. The inter-rater reliability of data extraction by chart review exhibited greater than 94% concordance in both studies. Medical providers (not RAs) formulated and implemented all management plans.
Measures of Outcomes of Care
We assessed outcomes of diabetes care using four separate measures: glycemic control (measured as A1c), z-BMI, frequency of hypoglycemia, and hospitalizations/ER utilization. A1c was measured at each visit using high-performance liquid chromatography standardized to the DCCT assay (reference range: 4–6%; Tosoh Medics, Inc, Foster City, CA). BMI was calculated from weight and height at each visit. Because normative values of BMI for children vary by sex and age, we calculated an age- and sex-adjusted BMI (z-BMI), which represents the number of standard deviations above or below the mean.
Severe hypoglycemia was defined, as in the DCCT, as any hypoglycemic event in which the patient required assistance from another person to recover (21). These events were divided into two mutually exclusive categories: (1) events requiring the help of another person for oral treatment, and (2) events, such as coma or seizure, requiring emergency medical response or treatment with glucagon and/or intravenous dextrose. The frequency of these events was ascertained by patient/family interviews, chart reviews, and interval questionnaires.
ER visits and hospitalizations included assessments and admissions for diabetic ketoacidosis, severe hypoglycemia, other diabetes-related problems (e.g., major treatment adjustments), and problems not directly related to, but complicated by, diabetes. As with hypoglycemic events, the frequency of these events was ascertained by patient/family interviews, chart reviews, and interval questionnaires.
Statistical Analysis
Statistical analysis of the data was performed with SAS, Version 8.2, for Windows (SAS Institute, Cary, NC). Means (SD) are presented unless otherwise indicated. Analyses included t tests, χ2 tests, and incidence rate (IR) calculations and comparisons. The numbers of months for which each patient contributed data were summed, and clinical outcomes were calculated as the number of events per 100 patient-years. An integrated A1c value was calculated for each patient by averaging all A1c values obtained during follow-up. Integrated A1c and outcome data were compared with previously published findings from the DCCT. An alpha level of .05 was used to determine statistical significance.
RESULTS
Patient Characteristics
The Table displays baseline characteristics for each cohort. The mean number of clinic visits per patient per year was 3.8(1.7) in Cohort 1 and 4.1(0.8) in Cohort 2 (p=.03). At study entry, only 39% of patients in Cohort 1 were performing SMBG ≥4 times/day, compared to 72% of Cohort 2 (χ2=48.0, df=3, p<.0001) (Figure 1, a). Cohort 2 had a significantly higher mean frequency of SMBG than Cohort 1 at both baseline [3.8(0.9) vs. 3.1(1.0), p<.0001] and after two years of follow-up [3.6(1.1) vs. 3.0(1.1), p<.0001].
Table 1.
Baseline Patient Characteristics
| Cohort 1 (1997–1998) N=299 | Cohort 2 (2002–2003) N=152 | p | |
|---|---|---|---|
| Age at entry (yrs) | 11.9 (2.5) | 12.9 (2.3) | <0.001 |
| Gender (% male) | 44% | 43% | 0.89 |
| Ethnicity (% Caucasian) | 92% | 90% | 0.52 |
| Developmental stage (%) | <0.001 | ||
| Prepubertal (Tanner stage I) | 41% | 21% | |
| Pubertal (Tanner stage II–IV) | 36% | 47% | |
| Postpubertal (Tanner stage V) | 23% | 32% | |
| Socioeconomic status (%)a | 0.32 | ||
| Major professional | 22% | 20% | |
| Minor professionals/skilled worker | 54% | 63% | |
| Semi-skilled worker | 17% | 14% | |
| Unskilled/unemployed/retired/student | 7% | 4% | |
| Family structure (% two-parent family) | 82% | 81% | 0.83 |
| BMI (kg/m2) | 21.1 (3.8) | 21.5 (3.8) | 0.19 |
| z-BMI | 0.72 (0.76) | 0.75 (0.73) | 0.50 |
| Duration of T1DM (yrs) | 5.2 (3.0) | 6.2 (3.5) | 0.001 |
| Insulin analog usage (%) | 45% | 82% | <0.001 |
| A1c (%) | 8.7 (1.4) | 8.4 (1.4) | 0.05 |
| A1c ≤ 8% (% of patients) | 32% | 48% | <0.001 |
Major professional = physician, lawyer, etc.; Minor professional = nurse, teacher, etc.; skilled worker = administrative personnel, etc.; semi-skilled worker = data entry personnel, etc.; unskilled worker = truck driver, etc.
Data reported as mean (SD) or percent.
Figure 1.
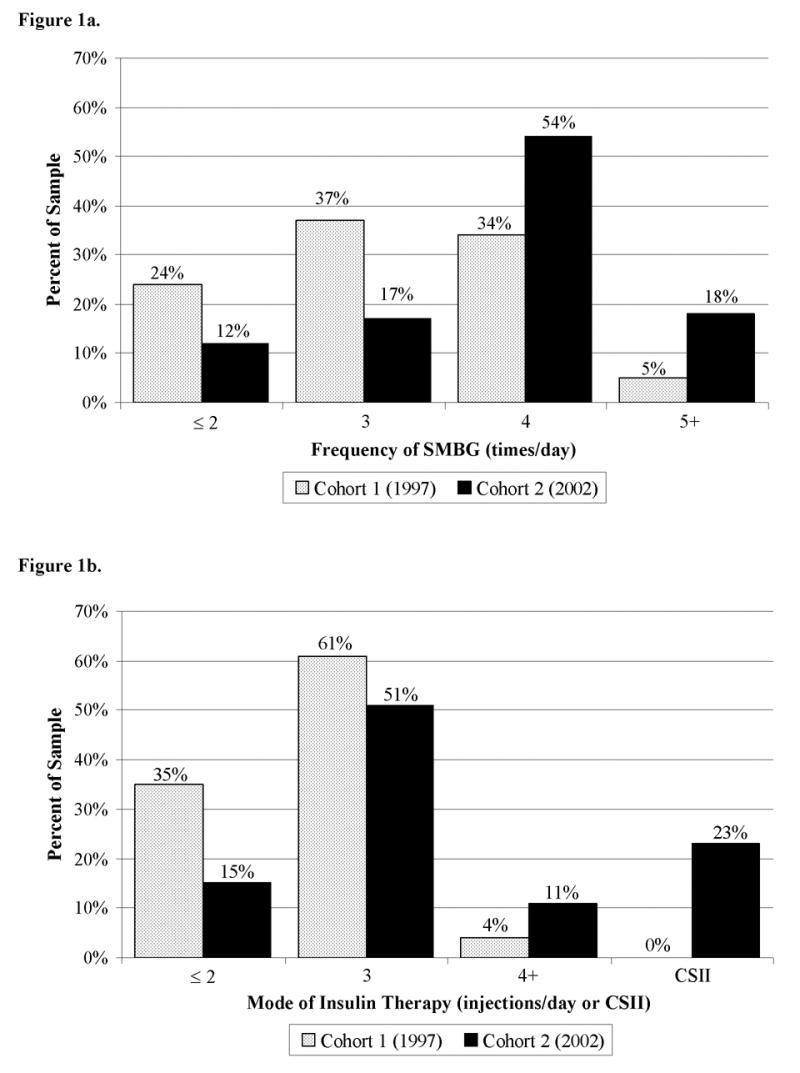
a, Baseline daily SMBG. Frequency of SMBG was higher in Cohort 2 than Cohort 1 at baseline (χ2=48.0, df=3, p<.0001). b, Baseline daily injection frequency. There was greater use of intensive treatment (≥ 3 injections/day or CSII) in Cohort 2 than Cohort 1 at baseline (χ2=90.2, df=3, p<.0001). Gray bar=cohort 1 (1997), black bar=cohort 2 (2002).
At both study entry and completion, Cohort 2 had a higher percentage of patients who were prescribed intensive insulin therapy (≥3 injections/day or CSII) compared to Cohort 1. At entry, 85% of Cohort 2 was prescribed intensive therapy (62% MDI, 23% CSII), compared to 65% of Cohort 1 (65% MDI, 0% CSII) (χ2=90.2, df=3, p<.0001, Figure 1, b). At study’s end, 94% of Cohort 2 was prescribed intensive therapy (59% MDI, 35% CSII), compared to 75% of Cohort 1 (73% MDI, 2% CSII) (χ2=132.5, df=3, p<.0001). Both cohorts transitioned to intensive therapy during follow-up at similar rates, with 14% of Cohort 1 and 12% of Cohort 2 changing to a more intense mode of therapy (i.e., ≤2 injections/day to MDI or CSII) (χ2=2.1, df=2, p=.34).
The type of insulin used at study entry was also examined. In Cohort 1, 133 patients (45%) used a rapid-acting insulin analog as part of their injection regimen. In Cohort 2, 125 patients (89 on injections; 36 on CSII) (82%) used a rapid-acting analog as part of their regimen at baseline.
Glycemic Control
After two years of follow-up, the mean A1c was 9.0(1.5)% in Cohort 1 and 8.7(1.4)% in Cohort 2 (p=.04). Thirty-five percent of Cohort 2 had an A1c ≤8.0% at study’s end, compared to 25% of Cohort 1 (p=.02). There were no significant differences between Cohorts 1 and 2 with regard to increase in A1c [0.3(1.3)% vs 0.3(1.1)%, p=1.0] or proportion of youth maintaining/improving their level of glycemic control (45% vs 40%, p=.39) over the course of the study. The integrated mean A1c values for Cohorts 1 and 2 over the two years of follow-up were 8.9(1.2)% and 8.6(1.3)%, respectively (p=.03).
z-BMI
After two years of follow-up, mean z-BMI was 0.79(0.72) in Cohort 1, an increase of 0.08(0.43) from baseline, and 0.83(0.69) in Cohort 2, an increase of 0.06(0.42) from baseline. Neither follow-up z-BMI (p=.57) nor change in z-BMI (p=.64) differed significantly between cohorts.
Frequency of Severe Hypoglycemia
The IR of severe hypoglycemic events requiring the help of another person for oral treatment was 47.1/100 patient-years in Cohort 1 and 18.5/100 patient-years in Cohort 2 (p<.001). The IR of severe hypoglycemic events requiring emergency medical response or treatment with glucagon and/or intravenous dextrose was 8.4/100 patient-years in Cohort 1 and 10.9/100 patient-years in Cohort 2 (p=.24). The IR of total severe hypoglycemic events was 55.4/100 patient-years in Cohort 1 and 29.4/100 patient-years in Cohort 2 (p<.001). Hence, the annual IR for any severe hypoglycemic event in Cohort 2 was almost half that of Cohort 1 (Figure 2, a).
Figure 2.
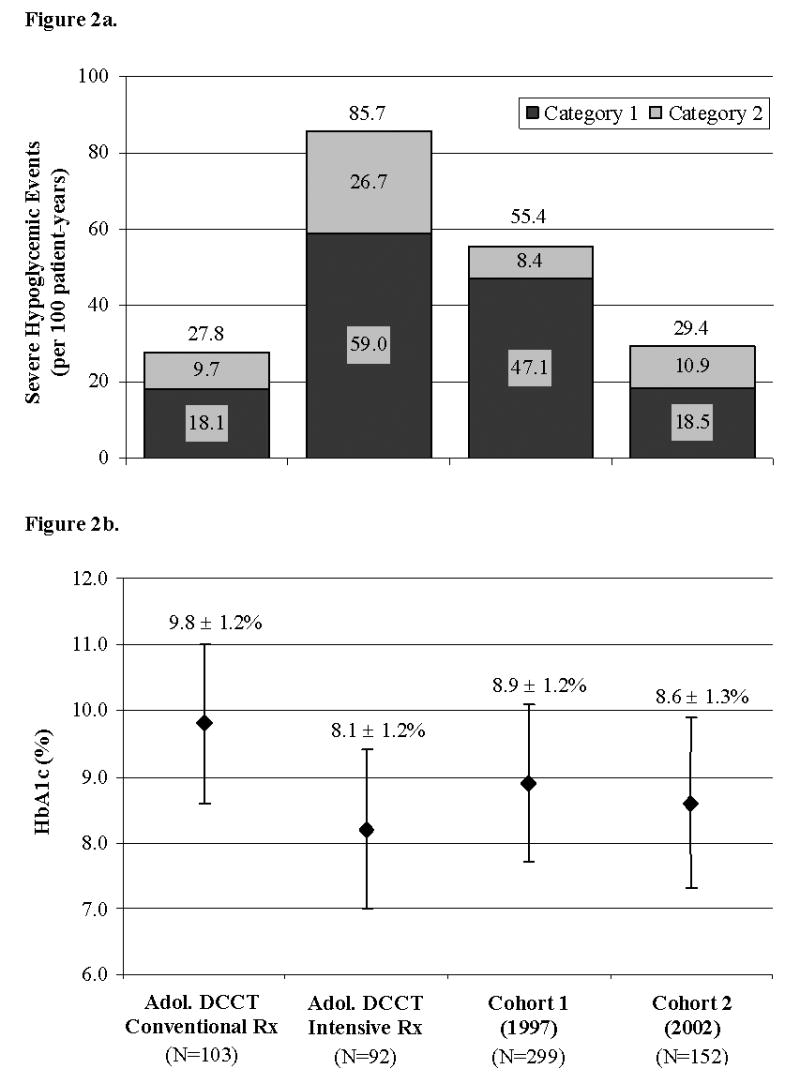
a, Incidence rate of severe hypoglycemia. Severe hypoglycemia was divided into two mutually exclusive categories. Dark gray area=events requiring the help of another person for oral treatment. Light gray area=events such as seizure or coma requiring emergency medical response or treatment with glucagon and/or intravenous dextrose. The total number of events is shown above each bar. Rates of severe hypoglycemia of adolescents in the DCCT are shown for comparison (10). b, Integrated A1c. An integrated A1c value was calculated for each patient by averaging all A1c values obtained during follow-up and compared with the adolescent cohort of the DCCT (10). Cohorts 1 and 2 had significantly lower A1c values than the conventionally treated adolescent DCCT cohort (p<.001) and significantly higher A1c values than the intensively treated adolescent DCCT cohort (p<.001) (10). The x-axis labels below Figure 2b apply to both Figures 2a and 2b.
Frequency of Hospitalizations and ER Utilization
The IR of hospitalizations was 12.9/100 patient-years in Cohort 1 and 11.2/100 patient-years in Cohort 2 (p=.38). The IR of ER visits was 29.3/100 patient-years in Cohort 1 and 22.0/100 patient-years in Cohort 2 (p=.02). Thus, the IR of ER visits was 24% lower in Cohort 2 than Cohort 1.
Comparison to adolescent cohort of the DCCT (10)
The integrated mean A1c values of the conventionally (N=103) and intensively (N=92) treated adolescents in the DCCT were 9.8(1.2)% and 8.1(1.2)%, respectively. Both Cohorts 1 and 2 had significantly lower A1c values compared to the conventionally treated adolescent DCCT cohort (p<.001) but significantly higher A1c values compared to the intensively treated adolescent DCCT cohort (p<.001) (Figure 2, b). The IR of total severe hypoglycemia for the conventionally and intensively treated adolescent DCCT groups were 27.8/100 patient-years and 85.7/100 patient-years, respectively. Cohorts 1 and 2 had significantly lower IR of total severe hypoglycemia than the intensively treated adolescent DCCT cohort (p<.001). Cohort 1 had a significantly higher IR of severe hypoglycemia than the conventionally treated adolescent DCCT cohort (p<.001), whereas no difference was observed between Cohort 2 and the conventionally treated adolescent DCCT cohort (p=.99) (Figure 2, a).
DISCUSSION
By comparing two pediatric cohorts with T1DM followed at the same center and separated in time by five years, we found that intensive insulin therapy and frequency of SMBG increased significantly in the post-DCCT era. A significant improvement in glycemic control coincided with these changes in treatment practices. Although the results are somewhat encouraging, the integrated mean A1c for the cohort enrolled in 2002 remained 0.5% higher than the intensively treated adolescent group in the DCCT.
Our A1c results are consistent with previous studies, including the large multicenter Hvidore study conducted in 17 European countries between 1995–1998, which documented the difficulty in improving individual clinic hemoglobin A1c values (22, 23). Only 40% of our 2002 cohort maintained or improved their level of glycemic control over the course of the study. This finding suggests that significant opportunities for improving blood glucose levels in youth remain. Unfortunately, although we currently have more tools (e.g., insulin analogs) than did the DCCT investigators, available resources, such as personnel required to perform monthly visits and weekly phone calls, remain fewer.
Although less frequent than the DCCT, the mean number of clinic visits per year in both cohorts was in keeping with current practice recommendations (24). However, patients in Cohort 2 had significantly more visits per year on average than patients in Cohort 1 (4.1 vs. 3.8). Previous reports have shown that patients with more frequent clinic visits demonstrate improved glycemic control (25). It is unclear, however, if increased exposure to care is the true mediator of improved glycemic control, or if increased exposure to care is better viewed as a marker of implementation of intensive diabetes management, which is the true mediator of improved glycemic outcomes (26, 27).
Perhaps more important than the number of clinic visits in improving glycemic control, however, was the increased frequency of SMBG performed by Cohort 2. Previous studies have shown that more frequent SMBG is associated with lower HbA1c levels (3, 5).
Both cohorts experienced equivalent deterioration in glycemic control during the two years of follow-up. Although disappointing, this finding was not entirely unexpected. At study entry, the mean age of participants in Cohorts 1 and 2 were 11.9 and 12.9 years, respectively. Numerous studies have shown that the transition to adolescence for children with T1DM is commonly associated with decreased adherence to diabetes management tasks leading to worse metabolic control (6, 28–30). Clearly, the design, implementation, and evaluation of affordable, efficacious, and translatable interventions aimed at improving glycemic control among adolescents with T1DM are still needed. The introduction of continuous glucose monitoring technologies into clinical practice could prove particularly useful in this patient population.
We did not find a significant difference in mean z-BMI between the two cohorts at baseline or after two years of follow-up. These results are encouraging since intensive insulin therapy has previously been associated with significant weight gain (10). Although concerns about weight gain should not deter intensive insulin therapy, forms of therapy that improve glycemic control without causing weight gain are desirable (31).
Hypoglycemia is the most frequent acute complication of T1DM. We observed a substantial decrease in the annual incidence of total severe hypoglycemic events between our two cohorts. Interestingly, this decrease occurred despite the fact that the 2002 cohort had diabetes of longer duration, a known risk factor for hypoglycemia (32, 33). We speculate that the cause of this declining incidence was multifactorial and likely due to more physiologic insulin replacement with MDI, CSII, and analog use. Previous reports evaluating pump therapy in children have shown significant reduction in risk of severe hypoglycemia despite improvements in A1c (20, 34, 35). Furthermore, other reports have suggested that severe hypoglycemia may be less common with insulin analog therapy (36–38). Although the relationship has not been as strong in pediatric studies (19, 20, 39), the fact that nearly twice as many patients were using insulin analogs as part of their treatment regimens in 2002 than in 1997 likely contributed to the decreased incidence of severe hypoglycemia in this cohort.
We also observed that the 2002 cohort experienced nearly 25% fewer ER visits than the 1997 cohort. Unfortunately, a similar trend was not observed in the rate of hospitalizations. When considering the direct and indirect costs of diabetes care in the US, it is well established that inpatient hospital care is a major cost driver, amounting to ~30% of the total costs (40). Therefore, the fact that we did not observe a decrease in the rate of hospitalizations for Cohort 2 at first glance suggests that intensification of therapy would not result in cost-savings for this population. One limitation of this study, however, is that duration of hospital stay was not evaluated. Recent studies involving pediatric patients have suggested that the mean length of hospital stay for non-DKA, as well as DKA, admissions is decreasing (41). If the same holds true for our patients, cost-savings may have been realized. The decrease in IR of ER visits likely translated into fewer missed school/work days for patients and family members. In addition, the better glycemic control that we observed would translate into cost-savings associated with the prevention or postponement of long-term complications and decreased inpatient hospital care in the future (42–44). Of course, these savings would be partially offset by increased outpatient charges and the increased cost of supplies needed for intensive diabetes management (e.g., insulin pump supplies). The lack of cost data is a weakness of this study; future studies addressing this issue are needed.
A few additional cautions must be made in discussing the results of this study. Although the prospective nature of data collection was designed to minimize underreporting of adverse events, it is possible that underreporting by parents and physicians resulted in underestimations of incidence rates in all categories. However, such underreporting would likely have occurred to the same extent in both cohorts, so that the differences observed between the cohorts would remain unchanged at a minimum. Next, these studies were conducted at a single tertiary care facility. Similar studies conducted at other centers involving different pediatric populations are needed (45). Nonetheless, these data are informative in showing that as we continue to pursue the goal of near-normal metabolic control in youth with diabetes, an increase in acute adverse events is not a necessary outcome.
Acknowledgments
We acknowledge contributions of the Pediatric Team at the Joslin Diabetes Center: Joan Mansfield, MD; Alyne Ricker, MD; Cindy Pasquarello, RN, BSN, CDE; Kathleen Walsh, RN, CDE; Deborah Holtorf, MPH, MSN, PNP; Kelly Dunkling, BSN, RN; Kerry Milaszewski, BSN, RN, CDE; Louise Crescenzi; and the pediatric endocrine fellows. We also acknowledge the research assistance of Samantha Huestis and Allison Maher.
ABBREVIATIONS
- A1c
hemoglobin A1c
- CSII
continuous subcutaneous insulin infusion
- DCCT
Diabetes Control and Complications Trial
- ER
emergency room
- IR
incidence rate
- MDI
multiple daily injections
- RA
research assistant
- SMBG
self-monitoring of blood glucose
- T1DM
type 1 diabetes mellitus
- z-BMI
body mass index z-score
Footnotes
This study was supported by grants from the NIH: RO1DK046887 (to LMBL) and 5T32DK063702 (to BMS and EM). Support was also received from the Charles H. Hood Foundation, the Katherine Adler Astrove Youth Education Fund, and the Maria Griffin Drury Fund.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Silverstein J, Klingensmith G, Copeland K, Plotnick L, Kaufman F, Laffel L, et al. Care of children and adolescents with type 1 diabetes: a statement of the American Diabetes Association. Diabetes Care. 2005;28:186–212. doi: 10.2337/diacare.28.1.186. [DOI] [PubMed] [Google Scholar]
- 3.Haller MJ, Stalvey MS, Silverstein JH. Predictors of control of diabetes: monitoring may be the key. J Pediatr. 2004;144:660–1. doi: 10.1016/j.jpeds.2003.12.042. [DOI] [PubMed] [Google Scholar]
- 4.Nathan DM, McKitrick C, Larkin M, Schaffran R, Singer DE. Glycemic control in diabetes mellitus: have changes in therapy made a difference? Am J Med. 1996;100:157–63. doi: 10.1016/s0002-9343(97)89453-3. [DOI] [PubMed] [Google Scholar]
- 5.Levine BS, Anderson BJ, Butler DA, Brackett J, Laffel L. Predictors of glycemic control and short-term adverse outcomes in youth with type 1 diabetes. J Pediatr. 2001;139:197–203. doi: 10.1067/mpd.2001.116283. [DOI] [PubMed] [Google Scholar]
- 6.Moreland EC, Tovar A, Zuehlke JB, Butler DA, Milaszewski K, Laffel LM. The impact of physiological, therapeutic and psychosocial variables on glycemic control in youth with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2004;17:1533–44. doi: 10.1515/jpem.2004.17.11.1533. [DOI] [PubMed] [Google Scholar]
- 7.Amiel SA, Sherwin RS, Simonson DC, Lauritano AA, Tamborlane WV. Impaired insulin action in puberty. A contributing factor to poor glycemic control in adolescents with diabetes. N Engl J Med. 1986;315:215–9. doi: 10.1056/NEJM198607243150402. [DOI] [PubMed] [Google Scholar]
- 8.Rydall AC, Rodin GM, Olmsted MP, Devenyi RG, Daneman D. Disordered eating behavior and microvascular complications in young women with insulin-dependent diabetes mellitus. N Engl J Med. 1997;336:1849–54. doi: 10.1056/NEJM199706263362601. [DOI] [PubMed] [Google Scholar]
- 9.Wysocki T, Taylor A, Hough BS, Linscheid TR, Yeates KO, Naglieri JA. Deviation from developmentally appropriate self-care autonomy. Association with diabetes outcomes. Diabetes Care. 1996;19:119–25. doi: 10.2337/diacare.19.2.119. [DOI] [PubMed] [Google Scholar]
- 10.The Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr. 1994;125:177–88. doi: 10.1016/s0022-3476(94)70190-3. [DOI] [PubMed] [Google Scholar]
- 11.Bui H, Perlman K, Daneman D. Self-monitoring of blood glucose in children and teens with diabetes. Pediatr Diabetes. 2005;6:50–62. doi: 10.1111/j.1399-543X.2005.00095.x. [DOI] [PubMed] [Google Scholar]
- 12.Tamborlane WV, Bonfig W, Boland E. Recent advances in treatment of youth with Type 1 diabetes: better care through technology. Diabet Med. 2001;18:864–70. doi: 10.1046/j.1464-5491.2001.00626.x. [DOI] [PubMed] [Google Scholar]
- 13.Laffel LM, Vangsness L, Connell A, Goebel-Fabbri A, Butler D, Anderson BJ. Impact of ambulatory, family-focused teamwork intervention on glycemic control in youth with type 1 diabetes. J Pediatr. 2003;142:409–16. doi: 10.1067/mpd.2003.138. [DOI] [PubMed] [Google Scholar]
- 14.Grey M, Boland EA, Davidson M, Li J, Tamborlane WV. Coping skills training for youth with diabetes mellitus has long- lasting effects on metabolic control and quality of life. J Pediatr. 2000;137:107–13. doi: 10.1067/mpd.2000.106568. [DOI] [PubMed] [Google Scholar]
- 15.The Diabetes Control and Complications Trial Research Group. Weight gain associated with intensive therapy in the Diabetes Control and Complications Trial. Diabetes Care. 1988;11:567–73. doi: 10.2337/diacare.11.7.567. [DOI] [PubMed] [Google Scholar]
- 16.Wing RR, Klein R, Moss SE. Weight gain associated with improved glycemic control in population-based sample of subjects with type I diabetes. Diabetes Care. 1990;13:1106–9. doi: 10.2337/diacare.13.11.1106. [DOI] [PubMed] [Google Scholar]
- 17.The Diabetes Control and Complications Trial Research Group. Adverse events and their association with treatment regimens in the diabetes control and complications trial. Diabetes Care. 1995;18:1415–27. doi: 10.2337/diacare.18.11.1415. [DOI] [PubMed] [Google Scholar]
- 18.Jones TW, Davis EA. Hypoglycemia in children with type 1 diabetes: current issues and controversies. Pediatr Diabetes. 2003;4:143–50. doi: 10.1034/j.1399-5448.2003.00025.x. [DOI] [PubMed] [Google Scholar]
- 19.Chase HP, Lockspeiser T, Peery B, Shepherd M, Mackenzie T, Anderson J, et al. The impact of the Diabetes Control and Complications Trial and humalog insulin on glycohemoglobin levels and severe hypoglycemia in type 1 diabetes. Diabetes Care. 2001;24:430–4. doi: 10.2337/diacare.24.3.430. [DOI] [PubMed] [Google Scholar]
- 20.Bulsara MK, Holman CD, Davis EA, Jones TW. The impact of a decade of changing treatment on rates of severe hypoglycemia in a population-based cohort of children with type 1 diabetes. Diabetes Care. 2004;27:2293–8. doi: 10.2337/diacare.27.10.2293. [DOI] [PubMed] [Google Scholar]
- 21.The Diabetes Control and Complications Trial Research Group. Epidemiology of severe hypoglycemia in the Diabetes Control and Complications Trial. Am J Med. 1991;90:450–9. [PubMed] [Google Scholar]
- 22.Mortensen HB, Hougaard P. Comparison of metabolic control in a cross-sectional study of 2,873 children and adolescents with IDDM from 18 countries. The Hvidore Study Group on Childhood Diabetes. Diabetes Care. 1997;20:714–20. doi: 10.2337/diacare.20.5.714. [DOI] [PubMed] [Google Scholar]
- 23.Danne T, Mortensen HB, Hougaard P, Lynggaard H, Aanstoot HJ, Chiarelli F, et al. Persistent differences among centers over 3 years in glycemic control and hypoglycemia in a study of 3,805 children and adolescents with type 1 diabetes from the Hvidore Study Group. Diabetes Care. 2001;24:1342–7. doi: 10.2337/diacare.24.8.1342. [DOI] [PubMed] [Google Scholar]
- 24.American Diabetes Association. Standards of medical care in diabetes--2006. Diabetes Care. 2006;29 (Suppl 1):S4–42. [PubMed] [Google Scholar]
- 25.Kaufman FR, Halvorson M, Carpenter S. Association between diabetes control and visits to a multidisciplinary pediatric diabetes clinic. Pediatrics. 1999;103:948–51. doi: 10.1542/peds.103.5.948. [DOI] [PubMed] [Google Scholar]
- 26.Svoren BM, Butler D, Levine BS, Anderson BJ, Laffel LMB. Reducing acute adverse outcomes in youths with type 1 diabetes: a randomized, controlled trial. Pediatrics. 2003;112:914–22. doi: 10.1542/peds.112.4.914. [DOI] [PubMed] [Google Scholar]
- 27.Laffel L, Brackett J, Ho J, Anderson BJ. Changing the process of diabetes care improves metabolic outcomes and reduces hospitalizations. Qual Manag Health Care. 1998;6:53–62. doi: 10.1097/00019514-199806040-00006. [DOI] [PubMed] [Google Scholar]
- 28.Daneman D, Wolfson DH, Becker DJ, Drash AL. Factors affecting glycosylated hemoglobin values in children with insulin-dependent diabetes. J Pediatr. 1981;99:847–53. doi: 10.1016/s0022-3476(81)80005-4. [DOI] [PubMed] [Google Scholar]
- 29.Jacobson AM, Hauser ST, Lavori P, Willett JB, Cole CF, Wolfsdorf JI, et al. Family environment and glycemic control: a four-year prospective study of children and adolescents with insulin-dependent diabetes mellitus. Psychosom Med. 1994;56:401–9. doi: 10.1097/00006842-199409000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Ingersoll GM, Orr DP, Herrold AJ, Golden MP. Cognitive maturity and self-management among adolescents with insulin- dependent diabetes mellitus. J Pediatr. 1986;108:620–3. doi: 10.1016/s0022-3476(86)80852-6. [DOI] [PubMed] [Google Scholar]
- 31.Palmer AJ, Roze S, Valentine WJ, Minshall ME, Lammert M, Nicklasson L, et al. Deleterious effects of increased body weight associated with intensive insulin therapy for type 1 diabetes: increased blood pressure and worsened lipid profile partially negate improvements in life expectancy. Curr Med Res Opin. 2004;20 (Suppl 1):S67–S73. doi: 10.1185/030079904X2033. [DOI] [PubMed] [Google Scholar]
- 32.Rosilio M, Cotton JB, Wieliczko MC, Gendrault B, Carel JC, Couvaras O, et al. Factors associated with glycemic control. A cross-sectional nationwide study in 2,579 French children with type 1 diabetes. The French Pediatric Diabetes Group. Diabetes Care. 1998;21:1146–53. doi: 10.2337/diacare.21.7.1146. [DOI] [PubMed] [Google Scholar]
- 33.Hepburn DA, Patrick AW, Eadington DW, Ewing DJ, Frier BM. Unawareness of hypoglycaemia in insulin-treated diabetic patients: prevalence and relationship to autonomic neuropathy. Diabet Med. 1990;7:711–7. doi: 10.1111/j.1464-5491.1990.tb01475.x. [DOI] [PubMed] [Google Scholar]
- 34.Pickup J, Mattock M, Kerry S. Glycaemic control with continuous subcutaneous insulin infusion compared with intensive insulin injections in patients with type 1 diabetes: meta-analysis of randomised controlled trials. BMJ. 2002;324:705. doi: 10.1136/bmj.324.7339.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pickup J, Keen H. Continuous subcutaneous insulin infusion at 25 years: evidence base for the expanding use of insulin pump therapy in type 1 diabetes. Diabetes Care. 2002;25:593–8. doi: 10.2337/diacare.25.3.593. [DOI] [PubMed] [Google Scholar]
- 36.Brunelle BL, Llewelyn J, Anderson JH, Jr, Gale EA, Koivisto VA. Meta-analysis of the effect of insulin lispro on severe hypoglycemia in patients with type 1 diabetes. Diabetes Care. 1998;21:1726–31. doi: 10.2337/diacare.21.10.1726. [DOI] [PubMed] [Google Scholar]
- 37.Heller SR, Amiel SA, Mansell P. Effect of the fast-acting insulin analog lispro on the risk of nocturnal hypoglycemia during intensified insulin therapy. UK Lispro Study Group Diabetes Care. 1999;22:1607–11. doi: 10.2337/diacare.22.10.1607. [DOI] [PubMed] [Google Scholar]
- 38.Davey P, Grainger D, MacMillan J, Rajan N, Aristides M, Gliksman M. Clinical outcomes with insulin lispro compared with human regular insulin: a meta-analysis. Clin Ther. 1997;19:656–74. doi: 10.1016/s0149-2918(97)80091-4. [DOI] [PubMed] [Google Scholar]
- 39.Heinemann L. Hypoglycemia and insulin analogues: is there a reduction in the incidence? J Diabetes Complications. 1999;13:105–14. doi: 10.1016/s1056-8727(99)00031-8. [DOI] [PubMed] [Google Scholar]
- 40.Hogan P, Dall T, Nikolov P. Economic costs of diabetes in the US in 2002. Diabetes Care. 2003;26:917–32. doi: 10.2337/diacare.26.3.917. [DOI] [PubMed] [Google Scholar]
- 41.Curtis JR, To T, Muirhead S, Cummings E, Daneman D. Recent trends in hospitalization for diabetic ketoacidosis in Ontario children. Diabetes Care. 2002;25:1591–6. doi: 10.2337/diacare.25.9.1591. [DOI] [PubMed] [Google Scholar]
- 42.The Diabetes Control and Complications Trial Research Group. Lifetime benefits and costs of intensive therapy as practiced in the Diabetes Control and Complications Trial. JAMA. 1996;276:1409–15. [PubMed] [Google Scholar]
- 43.Eastman RC, Javitt JC, Herman WH, Dasbach EJ, Copley-Merriman C, Maier W, et al. Model of complications of NIDDM. II. Analysis of the health benefits and cost-effectiveness of treating NIDDM with the goal of normoglycemia. Diabetes Care. 1997;20:735–44. doi: 10.2337/diacare.20.5.735. [DOI] [PubMed] [Google Scholar]
- 44.Stern Z, Levy R. Analysis of direct cost of standard compared with intensive insulin treatment of insulin-dependent diabetes mellitus and cost of complications. Acta Diabetol. 1996;33:48–52. doi: 10.1007/BF00571940. [DOI] [PubMed] [Google Scholar]
- 45.Rewers A, Chase HP, Mackenzie T, Walravens P, Roback M, Rewers M, et al. Predictors of acute complications in children with type 1 diabetes. JAMA. 2002;287:2511–8. doi: 10.1001/jama.287.19.2511. [DOI] [PubMed] [Google Scholar]


