Summary
Non-canonical Wnt signals control morphogenetic movements during vertebrate gastrulation. Casein kinase I epsilon (CKIε) is a Wnt-regulated kinase that regulates Wnt/β-catenin signaling and has β-catenin-independent role(s) in morphogenesis that are poorly understood. Here we report the identification of a CKIε binding partner, SIPA1L1/E6TP1, a GAP (GTPase activating protein) of the Rap small GTPases family. We show that CKIε phosphorylates SIPA1L1 to reduce its stability and thereby increase Rap1 activation. Wnt-8, which activates CKIε, enhances the CKIε-dependent phosphorylation and degradation of SIPA1L1. In early Xenopus or zebrafish development, inactivation of the Rap1 pathway results in abnormal gastrulation and a shortened anterior-posterior (AP) axis. Although CKIε also transduces Wnt/β-catenin signaling, inhibition of Rap1 does not alter β-catenin regulated gene expression. Our data demonstrate a role for CKIε in non-canonical Wnt signaling and indicate that Wnt regulates morphogenesis in part through CKIε-mediated control of Rap1 signaling.
Keywords: CKIε, Wnt signaling, convergent extension, Rap1, SIPA1L1
Introduction
Wnt proteins belong to a family of secreted hormones that regulate cell proliferation and embryonic development via several distinct signaling pathways (Logan and Nusse, 2004; Reya and Clevers, 2005). Aberrant regulation of these pathways can cause congenital malformation syndromes and cancer (Polakis, 2000; Xu et al., 2004). One well-studied consequence of Wnt signaling is stabilization of β-catenin, leading to changes in transcriptional programs that regulate cell proliferation and differentiation. Two additional ‘non-canonical’ Wnt pathways that are independent of β-catenin have been identified (Veeman et al., 2003). The Wnt/Ca2+ pathway activates protein kinase C and calcium/calmodulin-dependent protein kinase II and regulates ventral cell fate in Xenopus development (Kuhl et al., 2000; Saneyoshi et al., 2002; Westfall et al., 2003). The planar cell polarity (PCP) pathway, initially described in Drosophila, controls the orientation of wing hairs and ommatidia in eye disks. This pathway is activated by a subset of Wnts and is mediated through the intracellular protein Dishevelled (Dvl/Dsh). Downstream of Dvl, these pathways are controlled in part by monomeric GTPases including Rho and Rac (Fanto et al., 2000; Strutt et al., 1997).
During vertebrate embryogenesis, gastrulation requires cytoskeleton reorganizations that are regulated by a Wnt signaling cascade homologous to the PCP pathway in Drosophila (Keller, 2002; Solnica-Krezel, 2005; Wallingford et al., 2002). Through Rho and Rac dependent pathways, cytoskeletal changes drive cell intercalation, narrowing of the mediolateral axis (convergence) and elongation of the anterior-posterior (AP) axis (extension) (Habas et al., 2003; Habas et al., 2001). In Xenopus embryos, dominant negative proteins that inhibit non-canonical Wnt signaling disrupt convergent extension (CE) and result in embryos with a characteristic bent and shortened AP axis (Tada and Smith, 2000; Wallingford et al., 2001). Disruption of proper Rho or Rac activation produces comparable CE defects (Habas et al., 2003; Tahinci and Symes, 2003). In zebrafish, abrogation of non-canonical Wnt-mediated Rho kinase signaling impairs CE during gastrulation and embryos develop a shortened AP axis and in some cases cyclopia (Marlow et al., 2002).
Because of its ability to induce axis duplication and β-catenin accumulation, CKIε was identified as a positive regulator of the Wnt/β-catenin pathway (Peters et al., 1999; Sakanaka et al., 1999). CKIε also regulates CE during Xenopus gastrulation independent of its role in the Wnt/β-catenin pathway (McKay et al., 2001). Overexpression of a mutant CKIε protein (CKIε(MM2))—which contains 8 mutations in the CKIε autoinhibitory domain, making it an unregulated kinase—not only induces axis duplication, but also disrupts gastrulation in a β-catenin independent manner (Gietzen and Virshup, 1999; Swiatek et al., 2004) (I-C T and DMV, unpublished data). Similarly, the CKIε homolog in flies (double time/discs overgrown) positively regulates the Wnt/PCP pathway, controlling the arrangement of wing hairs (Klein et al., 2006; Strutt et al., 2006). However, the molecular mechanism by which CKIε regulates gastrulation remains unclear.
CKI is often found in signaling complexes, interacting with substrates either directly or via binding to a scaffolding protein (Eide et al., 2002; Price, 2006). The interaction between CKIε and various scaffolds allows CKIε to specifically phosphorylate pathway-specific substrates and restrict its downstream effects to a single signaling pathway (Knippschild et al., 2005; Price, 2006). To better understand the multiple roles of CKIε in Wnt signaling, a yeast two-hybrid screen was performed to find additional binding partners of CKIε. We report here that SIPA1L1/E6TP1 (Signal Induced Proliferation Associated protein 1 Like 1/HPV E6-Targeted Protein 1) (Gao et al., 1999) interacts with CKIε. SIPA1L1 is a GTPase activating protein (GAP) of the Rap small GTPases, originally identified as a target for degradation by the HPV E6 oncoprotein (Singh et al., 2003). The interaction between CKIε and SIPA1L1 suggests that CKIε may regulate gastrulation in part by activating the Rap pathway. The effects of Wnt and CKIε on SIPA1L1 and Rap1 activity were examined. We find that CKIε binds to, phosphorylates, and stimulates the degradation of SIPA1L1. Expression of CKIε alleviates SIPA1L1-mediated Rap1 inhibition. Wnt-8, known to activate CKIε, also promotes SIPA1L1 phosphorylation and reduces its abundance in a CKIε-dependent manner. Down-regulation of Rap1 in Xenopus embryos disrupts gastrulation in a β-catenin independent manner. Similar gastrulation defects are seen in zebrafish embryos expressing dominant negative Rap1 or SIPA1L1. Our data reveal a new function of Wnt-activated CKIε in the regulation of a small GTPase and indicate that the morphogenetic movements regulated by Wnt signaling require the coordinated regulation of multiple cytoskeletal regulators including the Rap1 GTPase.
Results
CKI ε binds to SIPA1L1, a GAP of the Rap small GTPase family
To identify CKIε interacting proteins, a yeast two-hybrid screen was performed with a HeLa cell cDNA library. Using an in vitro binding assay to confirm interactions found in the two-hybrid screen, ten CKIε binding proteins were identified. Of these, SIPA1L1α, a GTPase activating protein of Rap small GTPases, drew our attention since the regulation of small GTPases has been found to control convergent extension (Habas et al., 2003; Tahinci and Symes, 2003). The SIPA1L1 clone encoded amino acids 980–1783 from the GenBank nucleotide sequence AF090989. Reciprocal co-immunoprecipitation experiments (Figure 1A) confirmed the interaction between full length SIPA1L1 and CKIε. In addition, SIPA1L1 interacts with kinase-inactive CKIε, indicating that kinase activity is not required for the interaction (Figure 1A). Notably, Myc-SIPA1L1 abundance in transfected cell lysates was decreased when it was co-expressed with active, but not inactive, CKIε (compare the SIPA1L1 abundance in Figure 1A, lanes 5 and 6; lane 11 and 12) (discussed below). Myc-SIPA1L1 also co-precipitates endogenous CKIε (Figure 1B). We conclude that CKIε can bind to SIPA1L1 both in vitro and in vivo.
Figure 1. CKI ε binds to SIPA1L1, a GAP of Rap small GTPase.
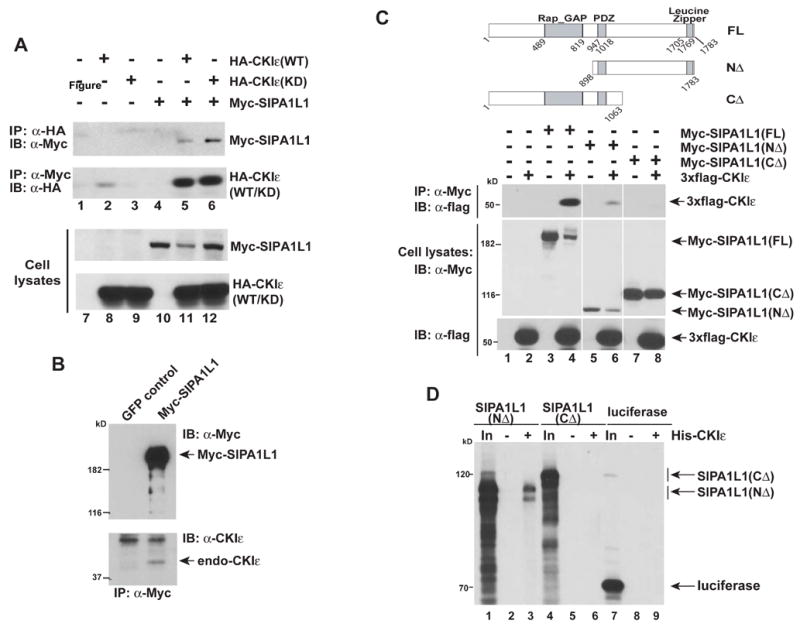
(A) In vivo interaction of SIPA1L1 and CKIε. Lanes 1–6 demonstrate the reciprocal co-immunoprecipitation (IP) of Myc-tagged SIPA1L1 and HA-tagged CKIε in HEK 293 cells. Lanes 7–12, immunoblot (IB) of whole cell lysates demonstrating the expression of Myc-SIPA1L1 and HA-CKIε. CKIε(WT): wild type CKIε; CKIε(KD): a kinase dead K38R mutant.
(B) SIPA1L1 binds to endogenous CKIε. Myc-SIPA1L1 was expressed in HEK293 cells and immunoprecipitated, followed by immunoblotting with a CKIε specific Ab. GFP was expressed as a negative control.
(C) The carboxyterminus of SIPA1L1 is required for interaction with CKIε. Upper and middle panels, co-IP of Myc-tagged SIPA1L1 truncated constructs and 3xflag-tagged CKIε. Bottom panel, expression of SIPA1L1 constructs. The figure is from a single exposure of the immunoblot; the lanes have been re-ordered for clarity.
(D) In vitro binding of SIPA1L1 to CKIε. Deletion constructs of SIPA1L1 were expressed in reticulocyte lysates. Recombinant 6x-His tagged CKIε was added where indicated. Luciferase was a negative control. In: Input; 20% of the amount used for binding assay.
To investigate the region of SIPA1L1 required for binding with CKIε, we tested the ability of truncated SIPA1L1 proteins to bind to CKIε in co-immunoprecipitation experiments. Consistent with the yeast two-hybrid screen results, deletion of the carboxy-terminus of SIPA1L1 (SIPA1L1(CΔ)) eliminated co-immunoprecipitation with CKIε (middle panel of Figure 1C, lane 2, 4, 6 and 8). For unknown reasons, SIPA1L1 lacking the amino-terminus (SIPA1L1(NΔ)) was expressed at low levels but precipitated proportionally much more CKIε than did SIPA1L1(CΔ). To further test the role of the carboxy-terminus of SIPA1L1 in CKIε binding, protein interactions were tested using in vitro translated proteins. We found that recombinant 6x His-tagged CKIε co-precipitated in vitro synthesized SIPA1L1(NΔ) but not SIPA1L1(CΔ) (Figure 1D). Taken together, these data indicate that SIPA1L1 is a binding partner of CKIε, and the interaction requires the carboxy-terminus of SIPA1L1 in a region distinct from the GAP and PDZ domains.
CKI ε phosphorylates SIPA1L1, facilitates its degradation and alleviates SIPA1L1-mediated Rap1 inhibition
The interaction between CKIε and SIPA1L1 suggested that SIPA1L1 could be a substrate of CKIε. Supporting this, full length SIPA1L1, but not SIPA1L1(CΔ), co-expressed with CKIε in HEK 293 cells shows a decrease in electrophoretic mobility (Figure 2A). The change in electrophoretic mobility is due to phosphorylation, since it is reversed by treatment of immunoprecipitated Myc-SIPA1L1 with calf intestinal alkaline phosphatase (CIP) (Figure 2B). Co-expression of wild type CKIε with SIPA1L1 also reduces the abundance of SIPA1L1 (Figure 1B and 2A), suggesting CKIε affects SIPA1L1 stability. To further examine this, we co-expressed SIPA1L1 with either CKIε(WT) or CKIε(KD) proteins. CKIε(WT) decreases SIPA1L1 abundance, whereas CKIε(KD) does not (upper panel of Figure 2C). In addition, inhibition of transfected and endogenous CKIε activity with the CKIε specific inhibitor IC261 (Mashhoon et al., 2001) increases Myc-SIPA1L1 protein levels (compare lane 5 to lane 3 and lane 6 to lane 2 in upper panel of Figure 2C). These data indicate that CKIε stimulates SIPA1L1 degradation in a kinase activity-dependent manner.
Figure 2. CKI ε phosphorylates SIPA1L1, promotes its degradation, and alleviates SIPA1L1-mediated Rap1 inhibition.
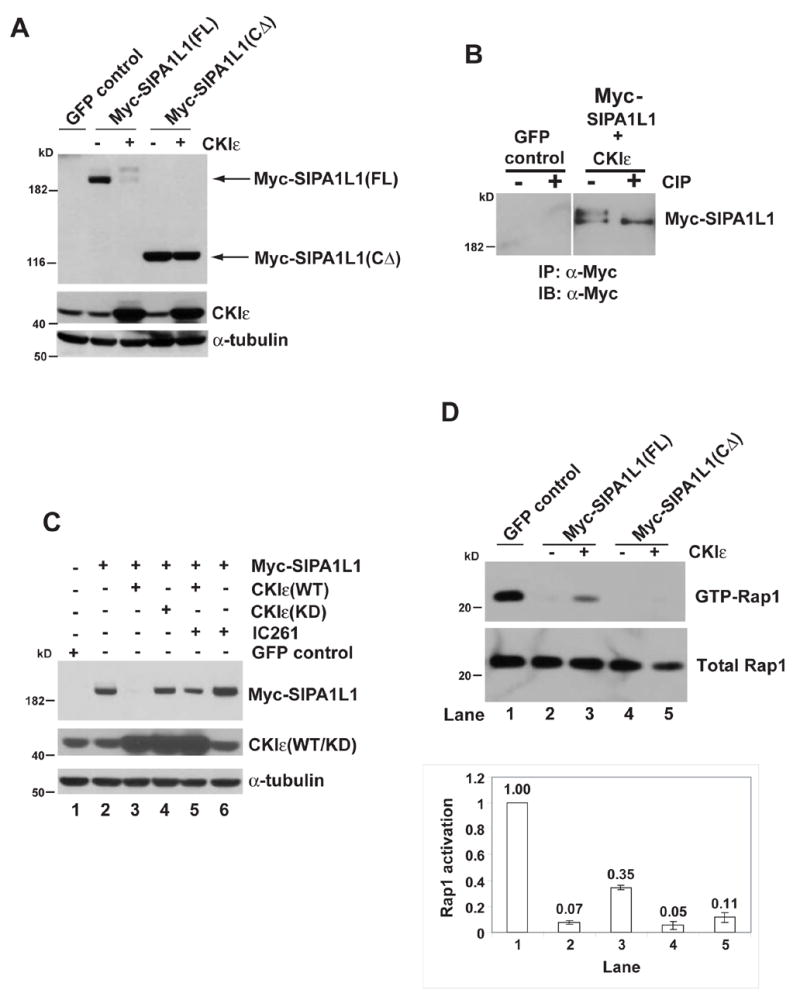
(A) CKIε expression causes an electrophoretic mobility shift of SIPA1L1(FL) but not SIPA1L1(CΔ). IB, Immunoblot of whole cell lysates from HEK 293 cells expressing the indicated proteins. CKIε and α-tubulin abundance are shown in the middle and bottom panels.
(B) SIPA1L1 is phosphorylated by CKIε. Myc-SIPA1L1 plus CKIε or GFP (as the negative control for IP) were expressed in HEK 293 cells. Myc-SIPA1L1 was precipitated and treated with or without CIP.
(C) CKIε stimulates SIPA1L1 degradation. Lanes 1–5, Myc-SIPA1L1 was co-expressed with CKIε(WT) or CKIε(KD) as indicated. Lane 5 and lane 6, cells expressing Myc-SIPA1L1 were treated with IC261 (50 μM) for 5 hr. IC261 treatment increased SIPA1L1 abundance (compare lane 5 to lane3 and lane 6 to lane 2). Expression of CKIε(WT/KD) and endogenous α-tubulin abundance are shown in middle and lower panels.
(D) CKIε rescues SIPA1L1-mediated Rap1 inhibition. Myc-SIPA1L1(FL) or Myc-SIPA1L1(CΔ) was co-expressed with or without CKIε in the CHO cells, followed by GST-RalGDS pull-down assay. The abundance of GTP-Rap1 and total Rap1 was quantified by ImageQuant. The ratio of GTP-Rap1 to total Rap1 is shown in the lower panel.
SIPA1L1 contains a highly conserved RapGAP domain (Gao et al., 2001) that stimulates the hydrolysis of GTP bound to Rap. To test if CKIε plays a role in the SIPA1L1-mediated regulation of Rap1, the abundance of GTP-Rap1 in cells was assessed by GTP-Rap pull-down with GST-RalGDS beads. As previously reported (Singh et al., 2003), expression of SIPA1L1 reduces the level of GTP-Rap1 (Figure 2D; compare lane 2 to lane 1). However, co-expression of CKIε with SIPA1L1 partially alleviates the SIPA1L1-mediated inhibition of Rap1 (Figure 2D; compare lane 3 to lane 2). This effect depends on the interaction between SIPA1L1 and CKIε, since CKIε does not alter Rap1 inhibition by SIPA1L1(CΔ), which contains the RapGAP domain but does not bind CKIε (Figure 2D; lanes 4–5). These results suggest CKIε regulates Rap1 through control of SIPA1L1 activity.
Wnt signaling affects SIPA1L1 phosphorylation and stability
CKIε is significantly activated by Wnt signaling (Swiatek et al., 2004). We therefore tested if Wnt signaling affects the Rap1 pathway via CKIε-mediated phosphorylation of SIPA1L1. Upon Wnt-8 treatment of HEK 293 cells, a significant amount of SIPA1L1 becomes more acidic, as expected after in vivo phosphorylation (compare Figure 3A, panel (i) and (ii)). Moreover, this Wnt-8 stimulated phosphorylation of SIPA1L1 is mediated by CKIε, because addition of the CKIε inhibitor IC261 inhibits the Wnt-8-induced shift (Figure 3A, panel (iii)). Since CKIε phosphorylates SIPA1L1 and reduces its abundance (Figure 2), we tested if Wnt-8 signaling affects the abundance of SIPA1L1. We found that Myc-SIPA1L1-expressing cells treated with Wnt-8 conditioned medium show reduced SIPA1L1 protein abundance, and that this effect is blocked by IC261 (Figure 3B). Taken together, our data indicate Wnt-8 signaling regulates SIPA1L1 phosphorylation and stability in a CKIε-dependent manner.
Figure 3. Wnt-8 regulates CKI ε-dependent phosphorylation and abundance of SIPA1L1.
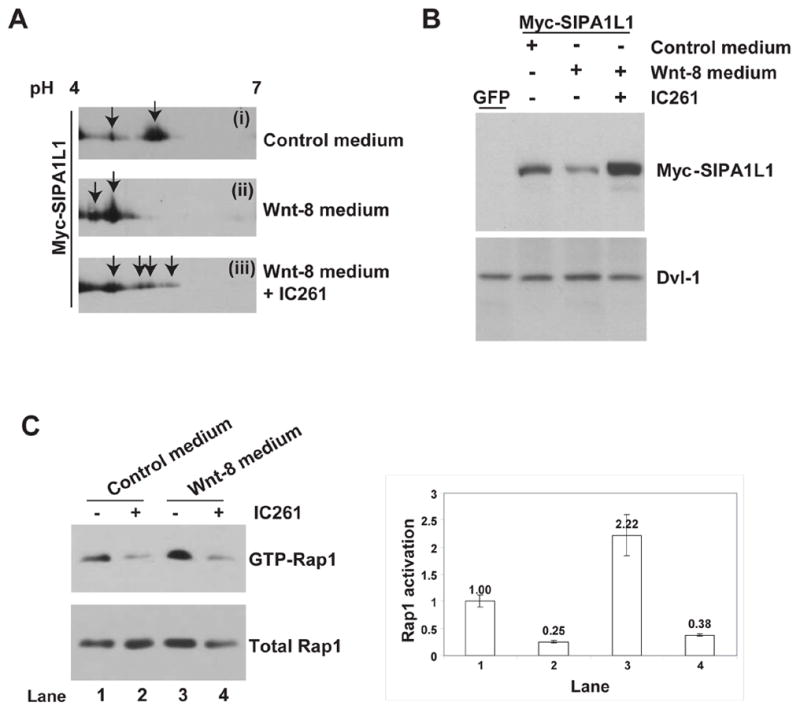
(A) Wnt-8 stimulates CKIε-dependent phosphorylation of SIPA1L1. Protein two-dimensional electrophoresis of Myc-SIPA1L1 was performed after treatment of HEK 293 cells with (i) control medium, (ii) Wnt-8 conditioned medium, or (iii) Wnt-8 conditioned medium plus IC261. Arrows indicate Myc-SIPA1L1 with several different isoelectric points.
(B) Wnt-8 conditioned medium reduces the abundance of SIPA1L1. Upper panel, after 6-hours of conditioned medium incubation and IC261 treatment, immunoblot analysis of Myc-SIPA1L1 was performed. Lower panel, endogenous Dvl-1 abundance (as a loading control).
(C) Wnt-8 conditioned medium increases Rap1 activation while IC261 blocks basal and Wnt-8 mediated Rap1 activation. HEK293 cells were incubated with control or Wnt-8 conditioned medium and treated with or without addition of IC261 as indicated. After 6 hours, cells were lysed, followed by GST-RalGDS pull-down assay. Quantification of each lane was performed as described in Figure 2D and shown in the right panel.
We next tested if Wnt-8, working via CKIε, elevates the abundance of GTP-Rap1 in cells. As shown in Figure 3C, Wnt-8 stimulates the activation of Rap1 (compare lane 3 to lane 1). Furthermore, the Wnt-8 mediated Rap1 activation is blocked by the addition of IC261 (compare lane 4 to lane 3) while IC261 alone also inhibits Rap1 activation (compare lane 2 to lane 1). Thus, Wnt-8 signaling affects Rap1 activation through a CKIε-dependent inhibition of SIPA1L1 activity.
Rap1 signaling is required for Wnt8/CKI ε-mediated gastrulation during embryogenesis
CKIε has been implicated in CE during gastrulation (McKay et al., 2001). Recent studies have shown Wnt signaling regulates both the Drosophila PCP pathway and vertebrate CE in part through activation of small GTPases including Rho and Rac (Habas et al., 2003; Habas et al., 2001). To test whether the Wnt-8 and CKIε-mediated regulation of SIPA1L1 and hence Rap1 activity plays a role in morphogenesis, we investigated the role of the Rap1 pathway during Xenopus embryonic development. Two approaches were used to abrogate Rap1 signaling. First, human dominant negative Rap1 (DN-Rap1), a S17N mutant that blocks the endogenous Rap pathway (Vossler et al., 1997) was expressed in embryos. Second, antisense Rap1 morpholino oligonucleotides (MO) were injected into Xenopus embryos at the one-cell stage to block the translation of Rap1 protein. Since Rap2, ~66% identical to Rap1, is involved in dorsalization events and induces axis duplication in Xenopus embryos (Choi and Han, 2005), we first asked if the Rap1 pathway is similarly involved in the CKIε- and Wnt-8- mediated axis duplication. Co-expression of Wnt-8 or CKIε mRNA with DN-Rap1 shows that DN-Rap1 does not block the initiation of ectopic axis. However, when DN-Rap1 was co-injected ventrally with Wnt-8 or CKIε RNA, gastrulation of the presumptive secondary axis was inhibited, resulting in an open yolk plug (Figure 4A, (iii) and (v); red arrowhead). Sox-2 expression pattern showed neural tube closure was initiated at the anterior end in both the primary and secondary axes, but convergence failed at the tail region of the presumptive secondary axis in the embryos co-expressing DN-Rap1 (red arrowheads in Figure 4A). Therefore, unlike Rap2, which is involved in dorsal cell fate specification, these results suggest the Rap1 pathway acts during gastrulation in Xenopus development.
Figure 4. Rap1 is required for Wnt-8 and CKI ε mediated axis development.
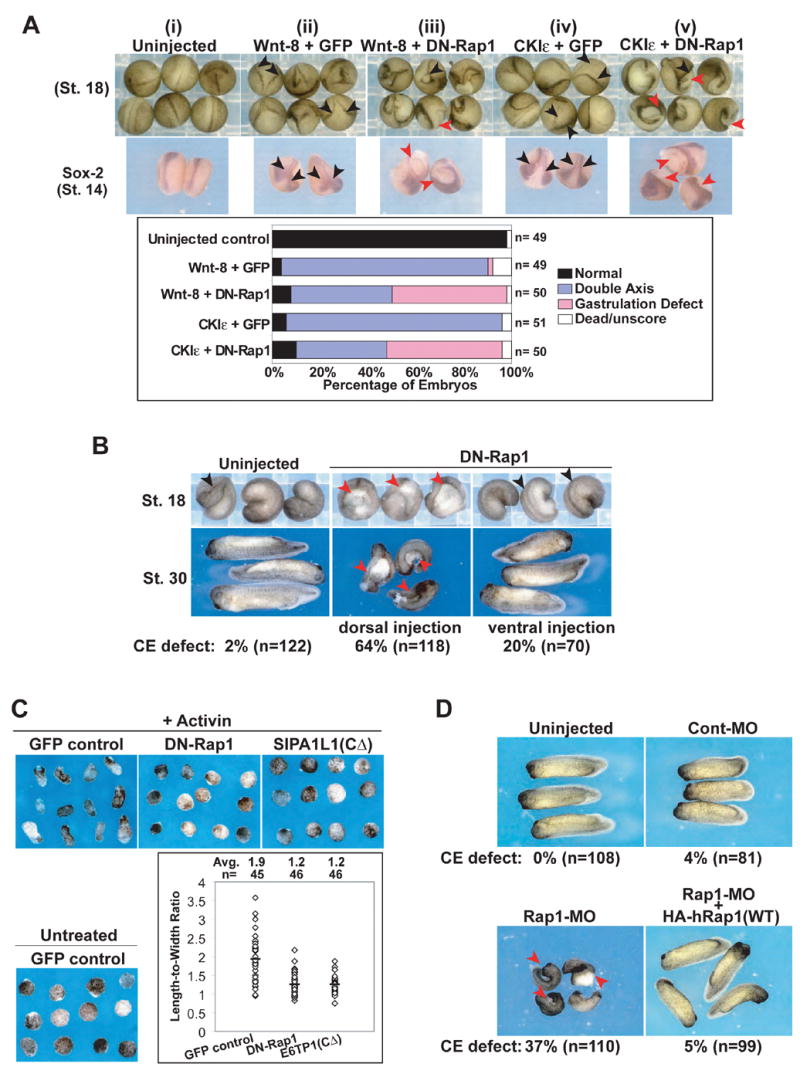
(A) Expression of DN-Rap1 in Xenopus embryos causes open yolk plug at the tail region of the presumptive secondary axis. mRNA of Wnt-8 (5 pg) or CKIε (1 ng) were co-injected with DN-Rap1 (100 pg) into a single ventral cell of 4-cell stage embryos. Embryos were scored and photographed at stage 18. Whole-mount In situ hybridization for Sox-2 expression was performed on stage 14 embryos. Black arrowhead: double axis. Red arrowhead: gastrulation defect. Bottom, scoring of stage 18 embryos.
(B) Dorsal expression of DN-Rap1 alone causes a gastrulation defect in Xenopus embryos, whereas ventral expression does not affect gastrulation in the majority of embryos. DN-Rap1 RNA (500 pg) was injected into a single dorsal or ventral cell of 4-cell stage embryos. Embryos were photographed at stages 18 and 30 as indicated. Black arrowhead: normal axis. Red arrowhead: blastopore closure defect.
(C) Disruption of Rap1 activity inhibits activin-induced convergent extension of animal cap. Convergent extension assays were performed at the Xenopus embryos, dorsally injected with RNA encoding GFP, DN-Rap1 or SIPA1L1(CΔ). Animal caps were photographed when the sibling embryos reach stage 18. The Length-to-Width ratios of the activin-treated animal caps were measured with MetaMorph software and shown in the lower-right panel. Individual results for each animal cap are denoted by ◇; and the average is shown in the top and denoted by — in the graph.
(D) Xenopus embryos injected with Rap1-MO display a defect in gastrulation. Black arrowhead: normal axis. Red arrowhead: defect in blastopore closure. The CE defect could be rescued by wild-type (WT) hRap1. Control MO (40 ng) or MO against XRap1A (40 ng) and XRap1B (40 ng) were injected together into the animal cap of Xenopus at the 1-cell stage. 2 to 4 pg of HA-tagged hRap1 were injected to two of dorsal cells at 4-cell stage to rescue the Rap1-MO caused defects. Only 5% (n= 99) of the embryos exhibit gastrulation defects after rescue. Red arrowhead: blastopore closure defect.
To further study the effects of Rap1 down-regulation in Xenopus development, DN-Rap1 RNA was injected into one of the dorsal or ventral cells of 4-cell stage Xenopus embryos. Dorsal expression of DN-Rap1 disrupts gastrulation. In these embryos, the blastopore fails to close, leading to embryos with an open yolk plug at the primary axis (Figure 4B, upper panel). The open blastopore of these embryos could still be seen at later stage and the embryos developed a bent and shortened AP body axis (Figure 4B, lower panel), reminiscent of defective non-canonical Wnt signaling (Tada and Smith, 2000; Wallingford et al., 2001). In contrast, ventral injection of DN-Rap1 alone in the absence of ectopic axis inducers produced no significant defect in gastrulation (Figure 4B).
Multiple pathways are required for blastopore closure. An independent and more direct assay of convergent extension is the activin-induced elongation of the animal cap of Xenopus (Sato et al., 2006). As shown in Figure 4C, down-regulation of Rap1 GTPase by expression of DN-Rap1 or SIPA1L1(CΔ), a constitutively active Rap GAP (described in more detail below), inhibited activin-induced convergent extension. These results indicate that Rap1 activity is required for proper convergent extension.
To further test the role of Rap1, we injected specific Rap1 antisense morpholino oligonucleotides (MO). Immunoblot of Xenopus Rap1 demonstrated effective MO knockdown (Supplementary Figure S1A). Rap1 knockdown caused gastrulation defects, leading to an open yolk plug and shortened AP axis in Xenopus embryos (Figure 4D, red arrowheads). This phenotype was similar to the DN-Rap1 phenotype, indicating that gastrulation defects are specific to Rap1 inhibition rather than other related pathways. Importantly, the defects in gastrulation caused by the Rap1 MO were specific to Rap1 knockdown, because these defects could be rescued by co-expression of wild-type human Rap1 (Figure 4D). Notably, the Rap1 MO phenotype is distinct from the hyper-ventralized phenotype caused by Rap2 knockdown (Choi and Han, 2005), indicating that Rap1 and Rap2 play distinct roles in Xenopus development. Taken together, the data demonstrate that Rap1 regulates gastrulation downstream of both endogenous Wnt signaling in the primary axis, and CKIε and Wnt-8 in the ectopic secondary axis.
CKI ε and the Rap1 GAP SIPA1L1 are upstream of Rap1
Our results show that Wnt-8 stimulates the CKIε-dependent phosphorylation and degradation of SIPA1L1 (but not SIPA1L1(CΔ)) in mammalian cells, and that SIPA1L1 regulates the Rap1 pathway. Similarly, we show a role for Rap1 in Wnt-8/CKIε mediated gastrulation. We therefore asked if CKIε regulates axis development in part by regulating the activity of SIPA1L1. Expression of SIPA1L1(FL) or SIPA1L1(CΔ) in dorsal cells in Xenopus embryos resulted in an open yolk plug and a short/bent AP axis (Figure 5A), similar to that seen in embryos injected with DN-Rap1 and Rap1 MO. Additionally, expression of SIPA1L1(CΔ) inhibits activin-induced convergent extension (Figure 4C). Since SIPA1L1 is a GAP of Rap1 that facilitates the accumulation of inactive GDP-Rap1, these data, taken together, are consistent with the model that down-regulation of Rap1 activity by SIPA1L1 disrupts gastrulation.
Figure 5. CKI ε and SIPA1L1 are upstream of Rap1.
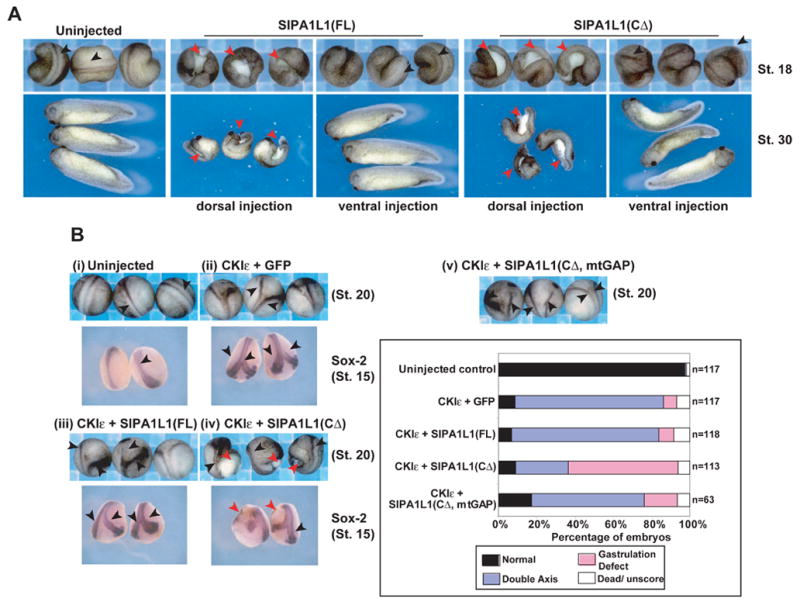
(A) Dorsal expression of SIPA1L1(FL) or SIPA1L1(CΔ) alone in Xenopus embryos causes a gastrulation defect in 23% (n= 65) and 73% (n= 70) of embryos respectively. Ventral expression does not induce an obvious phenotype in the majority of injected embryos (95% and 79% unaffected, n= 39 and 38 respectively). 4-cell stage embryos were injected with 1 ng of RNAs encoding SIPA1L1(FL) or SIPA1L1(CΔ) and photographed at stage 18 and 30. Black arrowhead: normal axis. Red arrowhead: blastopore closure defect.
(B) SIPA1L1(CΔ) but not SIPA1L1(FL) inhibits gastrulation at the presumptive secondary axis. (i) Uninjected control. (ii-iv) Xenopus 4-cell stage embryos were injected dorsally with mRNA encoding (ii) CKIε (1 ng) + GFP (250 pg); (iii) CKIε + SIPA1L1(FL) (250 pg); (iv) CKIε + SIPA1L1(CΔ) (250 pg); and (v) CKIε + SIPA1L1(CΔ, mtGAP) (250 pg). In situ hybridization for Sox-2 expression was performed in stage 15 embryos. Black arrowhead: double axis. Red arrowhead: gastrulation defect. Scoring data of stage 18 embryos are shown in the lower-right panel.
We next examined CKIε-mediated inactivation of SIPA1L1 in Xenopus embryos. Ventral expression of CKIε induces a secondary axis, which might require both β-catenin to initiate cell fate differentiation and Rap1 activation to control convergent extension. Co-expression of SIPA1L1(FL) with CKIε did not alter the CKIε-induced secondary axis formation (Figure 5B, (ii) and (iii)), consistent with the ability of CKIε to inactivate SIPA1L1. However, co-expression of CKIε and SIPA1L1(CΔ) (a non-regulated Rap GAP lacking the CKIε binding domain) caused a gastrulation defect at the presumptive secondary axis (Figure 5B, (iv)). Mutation of the GAP domain of SIPA1L1 (creating SIPA1L1(CΔ, mtGAP) (Pak et al., 2001)) decreased the frequency of the gastrulation defect (Figure 5B, (v)). Examination of Sox-2 expression at stage 15 in these embryos indicates that the hole caused by the defect of gastrulation is observed at the tail end of presumptive secondary axis (Figure 5B, red arrowhead). Thus, SIPA1L1 GAP activity regulates convergent extension, and its effect can be blocked by CKIε activity.
The role of the Rap1 pathway during convergent extension is conserved in zebrafish
As in Xenopus, non-canonical Wnt signaling through small GTPases has been implicated in CE during zebrafish gastrulation (Jopling and den Hertog, 2005; Marlow et al., 2002). To test whether Rap1 has a conserved role in CE, we used the three strategies we developed in the Xenopus system to abrogate Rap1 signaling in zebrafish embryos. As seen in Xenopus, injecting either DN-Rap1 or SIPA1L1(CΔ) RNA into zebrafish embryos resulted in a shortened and bent AP body axis at 2–3 days post-fertilization (dpf) that varies in severity (Figure 6A), resembling phenotypes previously shown to result from CE defects (Heisenberg et al., 2000; Marlow et al., 2002). Control injections using GFP RNA had little or no effect on development (Figure 6A). In addition, some zebrafish embryos expressing DN-Rap1 or SIPA1L1(CΔ) developed cyclopia (Figure 6B), which is associated with deficient convergent extension (Heisenberg et al., 2000; Marlow et al., 2002). To test whether these defects were specific to loss of Rap1 signaling, we injected MOs to block translation of the two zebrafish Rap1 genes, Rap1A and Rap1B. Similar to phenotypes observed in embryos injected with DN-Rap1 or SIPA1L1(CΔ), Rap1A-MO (data not shown) and Rap1B-MO (Figure 6A) injected embryos developed AP axis elongation defects. These defects were more severe in Rap1B MO embryos. In addition, cyclopia was observed in Rap1B-MO embryos (Figure 6A), but not Rap1A-MO embryos. Consistent with these results, immunoblotting of Rap1 protein levels in wild-type embryos, Rap1A MO embryos, Rap1B MO embryos and embryos co-injected with Rap1A+B MO suggested that Rap1B is the predominant isoform (Figure S1B) expressed during early zebrafish development. Rap1A+B MO injected embryos develop AP axis defects that were similar to Rap1B MO embryos, but slightly more severe (data not shown). Importantly, an independent MO designed to block splicing of Rap1B RNA (Rap1B-MO-2) reduced mRNA levels and correspondingly caused a similar shortened and bent AP axis phenotype (Figure S2), indicating these defects are specific to Rap1B knockdown.
Figure 6. Regulation of Rap1 GTPase in zebrafish development.
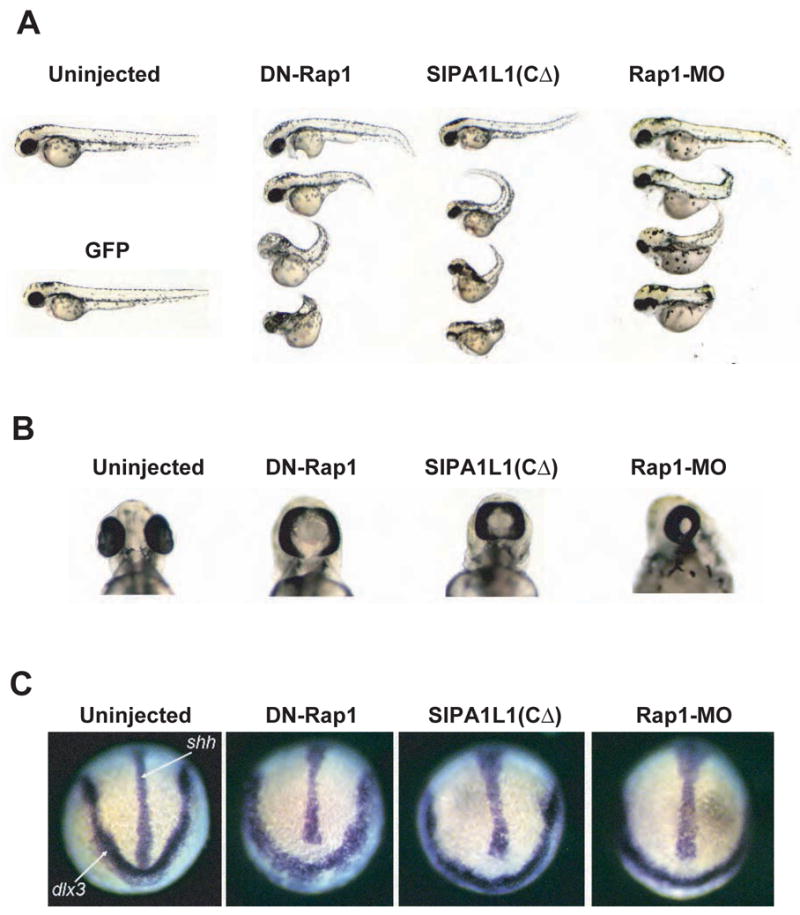
(A) Injection of DN-Rap1 RNA, SIPA1L1(CΔ) RNA or Rap1B-MO in zebrafish embryos results in a bent and shortened AP axis. RNAs encoding GFP (500 pg; as negative control), DN-Rap1 (500 pg) or SIPA1L1(CΔ) (250 pg) were injected into zebrafish embryos at the 1–4 cell stages. Between 2–3 dpf, 42% (n=150) of DN-Rap1, 37% (n=237) of SIPA1L1(CΔ) and 58% (n=63) of Rap1B-MO injected embryos displayed a shortened and bent AP axis that varied in severity. AP defects were seen in only 6% (n=143) of GFP injected embryos. Embryos injected with either 2, 2.5 or 3.4 ng of Rap1B-MO showed similar phenotypes and were pooled for analysis.
(B) Down-regulation of Rap1 signaling in zebrafish results in cyclopia. Cyclopia (fused eyes at the midline) was seen between 2–3 dpf in a small percentage of embryos injected with DN-Rap1 (15%, n=94), SIPA1L1(CΔ) (7%, n=115) or Rap1B MO (15%, n=34). Cyclopia was seen in less than 1% of wild-type (n=226) and GFP injected (n=111) controls..
(C) The ventral midline and neural plate are broadened in Rap1 deficient embryos. In situ analysis of the ventral midline by sonic hedgehog (shh) staining and the anterior boundary of the neural plate by distalless (dlx3) staining at the 1–3 somite stages revealed a broadening of the midline and neural plate in DN-Rap1 (34%, n=29), SIPA1L1(CΔ) (22%, n=9) and Rap1B MO (42%, n=24) injected embryos. Similar defects were seen in only 14% of uninjected controls (n=77).
To further determine whether these phenotypes are preceded by deficient CE during gastrulation, we examined embryos during early somite stages immediately following gastrulation. At these stages, the medio-lateral axis has begun to narrow (convergence) and the AP axis has started to elongate (extension). Embryos injected with DN-Rap1, SIPA1L1(CΔ) or Rap1 MO display normal epiboly, but had a shorter AP axis relative to uninjected controls at the 2 and 10 somite stages (Figure S3A). In situ analyses of neuroectoderm boundaries (marked by dlx3) and the ventral midline (marked by shh) between the 1–3 somite stages showed a broader neural plate and a midline that was shorter and broader in many DN-Rap1, SIPA1L1(CΔ) and Rap1 MO embryos relative to controls (Figure 6C). This results in a significant gap between the anterior end of the midline and the edge of the neural plate (Figure 6C). Furthermore, analyses of the notochord (marked by ntl) revealed a broader and shorter notochord in injected embryos (Figure S3B). These phenotypes are reminiscent of CE defects previously described in zebrafish; particularly the silberblick/Wnt-11 (non-canoncial wnt) mutant (Heisenberg and Nusslein-Volhard, 1997; Heisenberg et al., 2000). In order to control for variations in staging, we co-stained embryos with myoD to count somites, and quantified ‘convergence’ in individual embryos by measuring the width of the embryonic axis as marked by dlx3 at the 4 somite stage (Figure S3C). Embryos injected with DN-Rap1, SIPA1L1(CΔ) and Rap1B-MO developed broader neuroectoderm boundaries when compared to GFP control embryos (Figure S3C), suggesting that defects seen at earlier stages were not due to developmental delay, but rather result from deficient CE. Consistent with this, the prechordal plate was present but often misshapen in Rap1 compromised embryos at 2–3 somite stages (data not shown) and 6–8 somite stages (Figure S3D, upper panel). Further, the ventral marker Bmp4 was expressed normally at shield stage (Figure S3D, lower panel) and 6–8 somite stages (data not shown), indicating dorsal-ventral specification was not disturbed in these embryos. Taken together, these analyses indicate the role of the Rap1 pathway during CE is conserved between Xenopus and zebrafish.
CKI ε-mediated Rap1 pathway does not affect β-catenin dependent cell fate specificity and is not moderated by β-catenin overexpression
While CKIε was first placed in the Wnt/β-catenin pathway due to its ability to stabilize β-catenin and to induce axis duplication, it may play multiple roles. Inhibition of Rap1 by co-injection of DN-Rap1 or SIPA1L1 with CKIε leads to defects in gastrulation in Xenopus embryos (Figure 4A and 6C). Gastrulation is thought to be regulated via a β-catenin-independent mechanism. The Wnt-8 or CKIε-regulated Rap1 activation is also separated from the β-catenin-dependent dorsal mesodermal pattern, because co-expression of DN-Rap1or SIPA1L1(CΔ) with Wnt-8 or CKIε does not disrupt the duplicated expression of two dorsal organizers goosecoid (Gsc) and chordin (Chd) at gastrulation (Figure 7A). Nevertheless, these embryos display a wider distribution of gsc and chd expression at one of the axes, suggesting that CE inhibition caused by DN-Rap1 and SIPA1L1(CΔ) becomes apparent at the early gastrulation stage. This implies Wnt-8 or CKIε plays a role in non-β-catenin pathways via regulation of Rap1 activity. However, it has been reported that Rap2 functions positively in the Wnt/β-catenin pathway and β-catenin expression rescues Rap2 down-regulation (Choi and Han, 2005). To further characterize the relationship between the Rap1 pathway and β-catenin during Xenopus development, we asked if additional β-catenin expression is able to attenuate the gastrulation defect caused by DN-Rap1 or SIPA1L1(CΔ). While β-catenin was able to initiate ectopic axis formation (Figure 7B(ii)), it could not alleviate the gastrulation defect caused by DN-Rap1 and SIPA1L1(CΔ) (Figure 7B(iii) and 7B(iv)). Thus, distinct from what is reported regarding Rap2, β-catenin does not moderate Rap1-mediated gastrulation events.
Figure 7. DN-Rap1 and SIPA1L1(C Δ) do not interfere with β-catenin pathway.
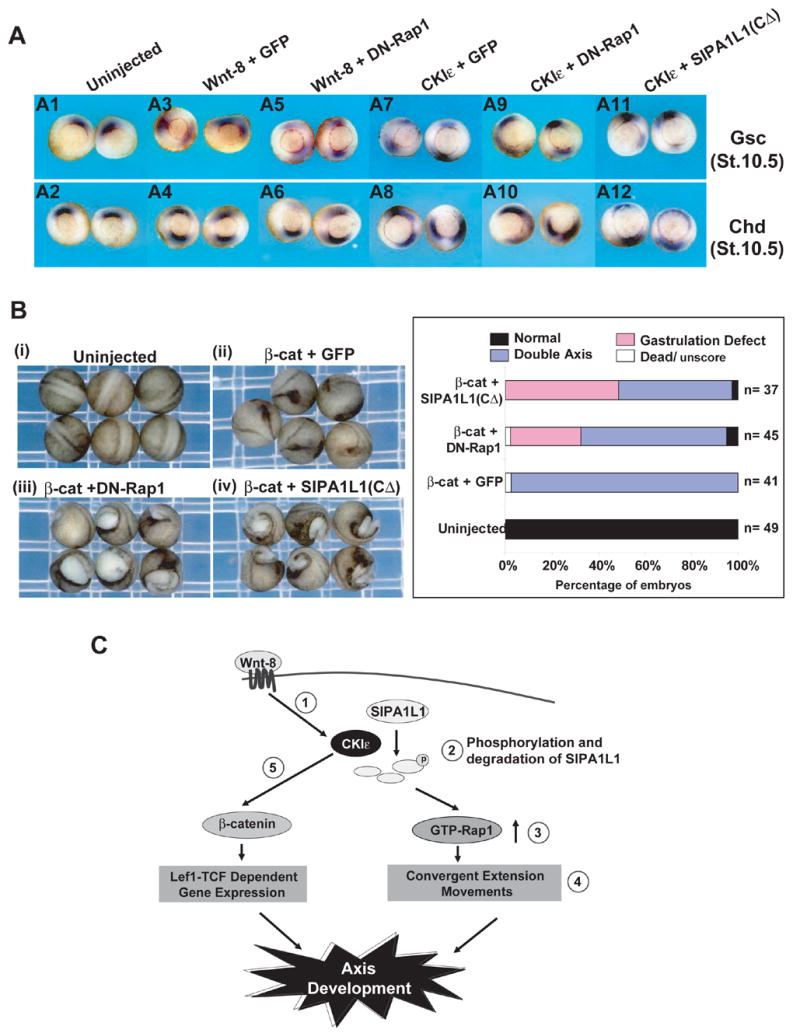
(A) DN-Rap1 and SIPA1L1(CΔ) do not block dorsal gene expression but cause a expanded field of expression of β-catenin target genes. (A1 and A2) Uninjected control. (A3 and A4) Wnt-8 +GFP; (A5 and A6) Wnt-8 + DN-Rap1; (A7 and A8) CKIε + GFP; (A9 and A10) CKIε + DN-Rap1; (A11 and A12) CKIε + SIPA1L1(CΔ) mRNA were injected into a single ventral cell of 4-cell stage embryos using the same quantities as in Fig 4A and 5B. In situ hybridization assays probing Goosecoid (Gsc) and Chordin (Chd) were performed on stage 10.5 embryos.
(B) DN-Rap1 and SIPA1L1(CΔ)-mediated gastrulation defects are not rescued by β-catenin. (i) Uninjected control. (ii–iii) Xenopus embryos injected with mRNAs encoding β-catenin (100 pg ) + GFP (250 pg), (ii); β-catenin + DN-Rap1 (100 pg), (iii); β-catenin + SIPA1L1(CΔ) (250 pg), (iv). Injection, photography and scoring were performed as Figure 4A and the scoring results were shown in the right panel.
(C) Schematic model of CKIε-mediated Wnt-8 pathway. Wnt-8 signaling activates CKIε (➀; and (Swiatek et al., 2004)), leading to phosphorylation and degradation of SIPA1L1 (➁; and see the result in Figure 2A) and accumulation of β-catenin (➄; and (Gao et al., 2002)). Degradation of SIPA1L1 results in the accumulation of GTP-Rap1 (➂; and see the result of Figure 2D), promoting convergent extension during gastrulation (➃; and the results in Figures 4–6). In a separate pathway, stabilized β-catenin activates Lef1-TCF dependent gene expression (➄). Both Rap1-mediated convergent extension and β-catenin regulated gene transcription contribute to the proper development of body axis.
Discussion
Wnt signaling plays crucial roles in cell proliferation, migration and morphogenetic movements that are important in both embryonic development and cancer progression. Here, we report a new role of Wnt-8 and CKIε in the regulation of a small GTPase, Rap1. The data reveals dual functions of CKIε in Wnt signaling. CKIε both regulates gastrulation by modulating Rap1 pathway, and activates Lef1-TCF dependent gene expression by stabilizing β-catenin (Figure 7C).
Although previous studies of CKIε in Xenopus development suggested it functions in non-canonical Wnt signaling, details of the downstream signal transduction pathways have been unclear (McKay et al., 2001). Non-canonical Wnt pathways affect cell movements rather than gene transcription, making them more difficult to assay. Second, single time-point assays in cell-based systems tend to miss dynamic effects of morphological progression. Here, we first utilized cell-based systems to determine how CKIε regulates the SIPA1L1-Rap1 pathway, and then tested the biological effects in Xenopus and zebrafish development. The data suggests CKIε is involved in multiple pathways downstream of Wnt. Prior to gastrulation, CKIε enhances β-catenin dependent gene expression, thus controlling cell fate specification (Figure 7A). However, during gastrulation, CKIε regulates convergent extension through the SIPA1L1-Rap1 pathway. Consistent with this, DN-Rap1 or CKIε-resistant SIPA1L1 (SIPA1L1(CΔ)) do not affect β-catenin-dependent gene expression in early gastrula stages, but inhibit convergent extension during gastrula and neurula stages of Xenopus development (Figure 7A, 4A and 5B).
Wnt-8 has been classified as a “canonical” Wnt because it can induce axis duplication in Xenopus embryos and β-catenin dependent gene expression (Christian and Moon, 1993). However, classifying Wnts by their ability to induce axis duplication or to activate a TCF-dependent promoter may be imprecise, since pathway activation also depends on receptor expression in target cells and cross-talk between canonical and non-canonical signaling has been reported (Kimura-Yoshida et al., 2005; Tao et al., 2005). Our data indicates a role for Wnt-8 in a non-canonical Wnt pathway. Consistent with previous studies that regulation of convergent extension does not require β-catenin, we found that β-catenin could not moderate the CE defect caused by downregulation of Rap1. We suggest that the CKIε/β-catenin and CKIε/RAP1 pathways together contribute to proper axis development. During axis development, Wnt-8 (and others) activates regional β-catenin distribution, initiating axis formation, and later activates the Rap1 pathway, facilitating morphogenic movements.
Rap GTPases include Rap1 and Rap2, which share about 60% amino acid identity. While both are involved in cell migration and adhesion, their functions in Xenopus development appear to be distinct. Although Rap2 affects β-catenin mediated dorsalization events (Choi and Han, 2005), we find that Rap1 is involved in a non-canonical Wnt pathway. Unlike Rap2, Rap1 knockdown causes a defect in gastrulation rather than a hyper-ventralized phenotype (Figure 4D). Inhibition of Rap1 does not change the expression of Gsc, Chd and Bmp4 (Figure 7A, S3D), suggesting that Rap1 signaling does not affect dorsal-ventral specification. Two prior observations are also consistent with the conclusion that a SIPA1L1-Rap1 pathway regulates morphogenetic movements in embryogenesis. First, Rap1 regulates cell migration in cell-based studies (McLeod et al., 2002), consistent with a role in morphological movements during development. Second, SIPA1L1 induces actin reorganization in rat neurons via its GAP activity (Pak et al., 2001). Although SIPA1L1 can facilitate GTP hydrolysis both in Rap1 and Rap2 (Singh et al., 2003), SIPA1L1 expression disrupts gastrulation rather than inducing a hyper-ventralized phenotype. SIPA1L1 may therefore preferentially affects Rap1 during development.
Two small GTPases, Rho and Rac, are known Wnt-regulated small GTPase required for proper gastrulation during Xenopus development (Habas et al., 2003; Habas et al., 2001). Biochemical analysis of Rho and Rac regulation indicates that Wnt signaling activates these pathways through parallel mechanisms (Habas et al., 2003). Both distinct and overlapping functions of Rho and Rac are essential for directing CE (Tahinci and Symes, 2003). Our study identifies Rap1 as an additional small GTPase downstream of Wnt. Rap1, Rho, and Rac may be cooperatively activated by a Wnt signal to mediate the complex series of movements involved in gastrulation. Rap1 has previously been found to be activated by changes in cytoskeletal tension (Tamada et al., 2004). We speculate that Rap1 activity facilitates changes in cell-cell adhesion required for proper morphogenetic movements.
Wnt signaling, via CKIε and SIPA1L1, regulates Rap1, and Rap1 plays an essential role in embryogenesis. SIPA1L1/E6TP1 is also a target of the oncogenic human papillomavirus protein E6, suggesting its inactivation contributes to cancer development (Gao et al., 2001). E6-mediated SIPA1L1/E6TP1 degradation correlates with the immortalization of epithelial cells (Gao et al., 2001). In addition, mutations of SIPA1, a SIPA1L1 related protein, correlates with efficiency of breast cancer metastasis (Crawford et al., 2006; Park et al., 2005). The fact that Wnt signaling and CKIε activity also decrease SIPA1L1, combined with the fact that some Wnts are oncogenes, is consistent with the hypothesis that SIPA1L1 downregulation contributes to malignant transformation. The role of Rap1 in cell migration suggests Wnt signaling may therefore play a role not only in proliferation but in tumor invasion and metastasis as well.
Experimental Procedures
Cell culture, plasmid preparation and protein 2-dimentional gel analysis
Cell culture, transfections and immunoblotting was performed as described (Gao et al., 2000; Swiatek et al., 2004). CKIε(WT), pCS2-CKIε(KD) pCEP4-4HA-CKIε(WT) and pCEP4-4HA-CKIε(KD) have been described (Gao et al., 2002; Gietzen and Virshup, 1999). pCS2-MT-SIPA1L1(FL) and pCS2-MT-SIPA1L1(CΔ) were constructed by inserting a PCR product of SIPA1L1(FL) and SIPA1L1(CΔ; 1–1063) into pCS2-MT. The pCS2+XWnt-8 plasmid was the gift of Randall T. Moon. pCS2–3xHA-Rap1(S17N) was obtained by inserting 3xHA-Rap1(S17N) (purchased from UMR cDNA Resource Center) into pCS2. Anti-Rap1 Ab was from BD Transduction Laboratories. Protein two-dimensional electrophoresis was performed as described (Swiatek et al., 2004) except that cells were harvested directly into 200 μl of IEF buffer and analyzed by 7% SDS- PAGE and immunoblotting for Myc-SIPA1L1.
In vitro binding assay
Biotin-labeled SIPA1L1 proteins were prepared by T7 Coupled Transcription and Translation (TnT) System (Promega) and biotinylated tRNA (Promega) and binding assays performed as described (Gao et al., 2000) except the precipitated product was detected by strepavidin-coupled HRP.
In vivo binding assay
Tagged SIPA1L1 or CKIε were expressed in HEK293 cells. Cells were harvested in RIPA buffer (50 mM HEPES pH7.6, 1% NP-40, 0.1% SDS, 50 mM NaCl, 0.5% Na-Deoxycholate, 1 mM NaF, and 0.02 mM DTT), followed by immunoprecipitation (IP) with the antibodies indicated in the figures. The precipitates were then washed four times with RIPA buffer and analyzed by immunoblot.
Microinjections of Xenopus and zebrafish embryos
Xenopus and zebrafish embryo injections were performed and scored as described (Essner et al., 2005; Gao et al., 2002).
GTP-Rap1 pull-down assay
The GTP-Rap1 pull-down assay was adapted from (Franke et al., 2000). After transfection, CHO cells were harvested with lysis buffer (10% glycerol, 50 mM Tris pH7.5, 2.5 mM MgCl2, 1% NP-40, and 200 mM NaCl) and incubated with GST-RalGDS beads for 1 hour at 4°C. The beads were then washed three times with lysis buffer, and analyzed by immunoblotting with Rap1 Ab. 6% of the lysates was analyzed in parallel for total Rap1.
Whole-mount in situ hybridization
Antisense probes were generated from the linear templates of Gsc (obtained from Dr. Eddy DeRobertis), Chd, Sox-2, ntl, shh, myoD and dlx3 using dig-labeling kit (Roche), following manufacturer’s instructions. In situ hybridization was performed as previously described (Essner et al., 2000; Harland, 1991), using a Biolane HTI in situ machine (Huller and Huttner AG). After color development, Xenopus embryos were soaked in the bleaching solution (1% H2O2, 5% formamide, 0.5x SSC) to remove the black pigment and zebrafish embryos were cleared in 70% glycerol in PBST, followed by photography.
Convergent extension assay
The convergent extension assays followed published procedures (Sokol, 1996) with animal caps excised using a Gastromaster (Xenotek Engineering) with a 400-micron tip. The animal caps were then incubated in 0.6 X MMR with or without activin treatment (10 ng/ml). The Length-Width-Ratios were measured as described (Tahinci and Symes, 2003).
Supplementary Material
Acknowledgments
We thank R. Dorsky and M. Vetter for helpful discussions and J. L. Bos, E. M. DeRobertis and R. T. Moon for providing plasmids used in this study. We also thank J. Brewer and M. Karthikeyan for technical assistance. These studies were funded by NIH P01 CA73992, R01 HL057840, P30 CA42014, the Huntsman Cancer Foundation, and the Willard Snow Hansen Chair in Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Choi SC, Han JK. Rap2 is required for Wnt/beta-catenin signaling pathway in Xenopus early development. Embo J. 2005;24:985–996. doi: 10.1038/sj.emboj.7600571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian JL, Moon RT. Interactions between Xwnt-8 and Spemann organizer signaling pathways generate dorsoventral pattern in the embryonic mesoderm of Xenopus. Genes Dev. 1993;7:13–28. doi: 10.1101/gad.7.1.13. [DOI] [PubMed] [Google Scholar]
- Crawford NP, Ziogas A, Peel DJ, Hess J, Anton-Culver H, Hunter KW. Germline polymorphisms in SIPA1 are associated with metastasis and other indicators of poor prognosis in breast cancer. Breast Cancer Res. 2006;8:R16. doi: 10.1186/bcr1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide EJ, Vielhaber EL, Hinz WA, Virshup DM. The circadian regulatory proteins BMAL1 and cryptochromes are substrates of casein kinase Iepsilon. J Biol Chem. 2002;277:17248–17254. doi: 10.1074/jbc.M111466200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essner JJ, Amack JD, Nyholm MK, Harris EB, Yost HJ. Kupffer's vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left-right development of the brain, heart and gut. Development. 2005;132:1247–1260. doi: 10.1242/dev.01663. [DOI] [PubMed] [Google Scholar]
- Essner JJ, Branford WW, Zhang J, Yost HJ. Mesendoderm and left-right brain, heart and gut development are differentially regulated by pitx2 isoforms. Development. 2000;127:1081–1093. doi: 10.1242/dev.127.5.1081. [DOI] [PubMed] [Google Scholar]
- Fanto M, Weber U, Strutt DI, Mlodzik M. Nuclear signaling by Rac and Rho GTPases is required in the establishment of epithelial planar polarity in the Drosophila eye. Curr Biol. 2000;10:979–988. doi: 10.1016/s0960-9822(00)00645-x. [DOI] [PubMed] [Google Scholar]
- Franke B, van Triest M, de Bruijn KM, van Willigen G, Nieuwenhuis HK, Negrier C, Akkerman JW, Bos JL. Sequential regulation of the small GTPase Rap1 in human platelets. Mol Cell Biol. 2000;20:779–785. doi: 10.1128/mcb.20.3.779-785.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Singh L, Kumar A, Srinivasan S, Wazer DE, Band V. Human papillomavirus type 16 E6-induced degradation of E6TP1 correlates with its ability to immortalize human mammary epithelial cells. J Virol. 2001;75:4459–4466. doi: 10.1128/JVI.75.9.4459-4466.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Srinivasan S, Boyer SN, Wazer DE, Band V. The E6 oncoproteins of high-risk papillomaviruses bind to a novel putative GAP protein, E6TP1, and target it for degradation. Mol Cell Biol. 1999;19:733–744. doi: 10.1128/mcb.19.1.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZH, Metherall J, Virshup DM. Identification of casein kinase I substrates by in vitro expression cloning screening. Biochem Biophys Res Commun. 2000;268:562–566. doi: 10.1006/bbrc.2000.2168. [DOI] [PubMed] [Google Scholar]
- Gao ZH, Seeling JM, Hill V, Yochum A, Virshup DM. Casein kinase I phosphorylates and destabilizes the beta-catenin degradation complex. Proc Natl Acad Sci USA. 2002;99:1182–1187. doi: 10.1073/pnas.032468199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietzen KF, Virshup DM. Identification of inhibitory autophosphorylation sites in casein kinase I epsilon. J Biol Chem. 1999;274:32063–32070. doi: 10.1074/jbc.274.45.32063. [DOI] [PubMed] [Google Scholar]
- Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003;17:295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- Harland RM. In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Nusslein-Volhard C. The function of silberblick in the positioning of the eye anlage in the zebrafish embryo. Dev Biol. 1997;184:85–94. doi: 10.1006/dbio.1997.8511. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- Jopling C, den Hertog J. Fyn/Yes and non-canonical Wnt signalling converge on RhoA in vertebrate gastrulation cell movements. EMBO Rep. 2005;6:426–431. doi: 10.1038/sj.embor.7400386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–1954. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- Kimura-Yoshida C, Nakano H, Okamura D, Nakao K, Yonemura S, Belo JA, Aizawa S, Matsui Y, Matsuo I. Canonical Wnt signaling and its antagonist regulate anterior-posterior axis polarization by guiding cell migration in mouse visceral endoderm. Dev Cell. 2005;9:639–650. doi: 10.1016/j.devcel.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Klein TJ, Jenny A, Djiane A, Mlodzik M. CKIepsilon/discs overgrown promotes both Wnt-Fz/beta-catenin and Fz/PCP signaling in Drosophila. Curr Biol. 2006;16:1337–1343. doi: 10.1016/j.cub.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Knippschild U, Gocht A, Wolff S, Huber N, Lohler J, Stoter M. The casein kinase 1 family: participation in multiple cellular processes in eukaryotes. Cell Signal. 2005;17:675–689. doi: 10.1016/j.cellsig.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Kuhl M, Sheldahl LC, Park M, Miller JR, Moon RT. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet. 2000;16:279–283. doi: 10.1016/s0168-9525(00)02028-x. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Marlow F, Topczewski J, Sepich D, Solnica-Krezel L. Zebrafish Rho kinase 2 acts downstream of Wnt11 to mediate cell polarity and effective convergence and extension movements. Curr Biol. 2002;12:876–884. doi: 10.1016/s0960-9822(02)00864-3. [DOI] [PubMed] [Google Scholar]
- Mashhoon N, Carmel G, Pflugrath JW, Kuret J. Structure of the unliganded cAMP-dependent protein kinase catalytic subunit from Saccharomyces cerevisiae. Arch Biochem Biophys. 2001;387:11–19. doi: 10.1006/abbi.2000.2241. [DOI] [PubMed] [Google Scholar]
- McKay RM, Peters JM, Graff JM. The casein kinase I family: roles in morphogenesis. Dev Biol. 2001;235:378–387. doi: 10.1006/dbio.2001.0307. [DOI] [PubMed] [Google Scholar]
- McLeod SJ, Li AH, Lee RL, Burgess AE, Gold MR. The Rap GTPases regulate B cell migration toward the chemokine stromal cell-derived factor-1 (CXCL12): potential role for Rap2 in promoting B cell migration. J Immunol. 2002;169:1365–1371. doi: 10.4049/jimmunol.169.3.1365. [DOI] [PubMed] [Google Scholar]
- Pak DT, Yang S, Rudolph-Correia S, Kim E, Sheng M. Regulation of dendritic spine morphology by SPAR, a PSD-95-associated RapGAP. Neuron. 2001;31:289–303. doi: 10.1016/s0896-6273(01)00355-5. [DOI] [PubMed] [Google Scholar]
- Park YG, Zhao X, Lesueur F, Lowy DR, Lancaster M, Pharoah P, Qian X, Hunter KW. Sipa1 is a candidate for underlying the metastasis efficiency modifier locus Mtes1. Nat Genet. 2005;37:1055–1062. doi: 10.1038/ng1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM, McKay RM, McKay JP, Graff JM. Casein kinase I transduces Wnt signals. Nature. 1999;401:345–350. doi: 10.1038/43830. [DOI] [PubMed] [Google Scholar]
- Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- Price MA. CKI, there's more than one: casein kinase I family members in Wnt and Hedgehog signaling. Genes Dev. 2006;20:399–410. doi: 10.1101/gad.1394306. [DOI] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Sakanaka C, Leong P, Xu L, Harrison SD, Williams LT. Casein kinase iepsilon in the wnt pathway: regulation of beta-catenin function. Proc Natl Acad Sci U S A. 1999;96:12548–12552. doi: 10.1073/pnas.96.22.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saneyoshi T, Kume S, Amasaki Y, Mikoshiba K. The Wnt/calcium pathway activates NF-AT and promotes ventral cell fate in Xenopus embryos. Nature. 2002;417:295–299. doi: 10.1038/417295a. [DOI] [PubMed] [Google Scholar]
- Sato A, Khadka DK, Liu W, Bharti R, Runnels LW, Dawid IB, Habas R. Profilin is an effector for Daam1 in non-canonical Wnt signaling and is required for vertebrate gastrulation. Development. 2006;133:4219–4231. doi: 10.1242/dev.02590. [DOI] [PubMed] [Google Scholar]
- Singh L, Gao Q, Kumar A, Gotoh T, Wazer DE, Band H, Feig LA, Band V. The high-risk human papillomavirus type 16 E6 counters the GAP function of E6TP1 toward small Rap G proteins. J Virol. 2003;77:1614–1620. doi: 10.1128/JVI.77.2.1614-1620.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol SY. Analysis of Dishevelled signalling pathways during Xenopus development. Curr Biol. 1996;6:1456–1467. doi: 10.1016/s0960-9822(96)00750-6. [DOI] [PubMed] [Google Scholar]
- Solnica-Krezel L. Conserved patterns of cell movements during vertebrate gastrulation. Curr Biol. 2005;15:R213–228. doi: 10.1016/j.cub.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Strutt DI, Weber U, Mlodzik M. The role of RhoA in tissue polarity and Frizzled signalling. Nature. 1997;387:292–295. doi: 10.1038/387292a0. [DOI] [PubMed] [Google Scholar]
- Strutt H, Price MA, Strutt D. Planar polarity is positively regulated by casein kinase Iepsilon in Drosophila. Curr Biol. 2006;16:1329–1336. doi: 10.1016/j.cub.2006.04.041. [DOI] [PubMed] [Google Scholar]
- Swiatek W, Tsai IC, Klimowski L, Pepler A, Barnette J, Yost HJ, Virshup DM. Regulation of casein kinase I epsilon activity by Wnt signaling. J Biol Chem. 2004;279:13011–13017. doi: 10.1074/jbc.M304682200. [DOI] [PubMed] [Google Scholar]
- Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–2238. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- Tahinci E, Symes K. Distinct functions of Rho and Rac are required for convergent extension during Xenopus gastrulation. Dev Biol. 2003;259:318–335. doi: 10.1016/s0012-1606(03)00206-9. [DOI] [PubMed] [Google Scholar]
- Tamada M, Sheetz MP, Sawada Y. Activation of a signaling cascade by cytoskeleton stretch. Dev Cell. 2004;7:709–718. doi: 10.1016/j.devcel.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, Asashima M, Wylie CC, Lin X, Heasman J. Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell. 2005;120:857–871. doi: 10.1016/j.cell.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent. Wnt signaling Dev Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- Vossler MR, Yao H, York RD, Pan MG, Rim CS, Stork PJ. cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell. 1997;89:73–82. doi: 10.1016/s0092-8674(00)80184-1. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Fraser SE, Harland RM. Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev Cell. 2002;2:695–706. doi: 10.1016/s1534-5807(02)00197-1. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Vogeli KM, Harland RM. Regulation of convergent extension in Xenopus by Wnt5a and Frizzled-8 is independent of the canonical Wnt pathway. Int J Dev Biol. 2001;45:225–227. [PubMed] [Google Scholar]
- Westfall TA, Brimeyer R, Twedt J, Gladon J, Olberding A, Furutani-Seiki M, Slusarski DC. Wnt-5/pipetail functions in vertebrate axis formation as a negative regulator of Wnt/beta-catenin activity. J Cell Biol. 2003;162:889–898. doi: 10.1083/jcb.200303107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, Kelley MW, Jiang L, Tasman W, Zhang K, Nathans J. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116:883–895. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


