Abstract
Objective
To evaluate the impact of an interconceptional antibiotic regimen on the endometrial microbial flora and histology.
Study Design
Secondary analysis of a double-blind randomized placebo-controlled trial of prophylactic metronidazole + azithromycin given to 241 women (118 on antibiotics and 123 on placebo) with a prior preterm delivery to prevent recurrent preterm delivery. Endometrial cultures and histology were obtained at randomization and 2 weeks after treatment. The prevalence of either the new acquisition or the resolution of individual microbes, categories of microbes and plasma cell endometritis were compared using Chi square or Fisher exact tests.
Results
Overall, antibiotics were associated with lower acquisition and higher resolution of microbes. Of women without Gardnerella vaginalis at baseline, 14% on antibiotics vs. 34% on placebo had positive endometrial culture for the organism after treatment (p<0.05), while of those with Gardnerella at baseline, 57% vs. 33% (p<0.05) had a negative follow-up culture. Other Gram-negative rods, especially aerobes in general also manifested similar patterns. The impact on anaerobes and plasma cell endometritis was not definitive but there was a trend toward the increased resolution of the former (77% vs. 55%) and reduced acquisition of the latter (28% vs. 50%).
Conclusion
The antibiotic regimen prevented acquisition and promoted resolution, but not eradication, of Gram-negative rods such as Gardnerella vaginalis and the aerobic sub-category.
Keywords: Endometrium, Microbial flora & Antibiotics
Introduction
There is an accumulating body of evidence suggesting that the human endometrium is not sterile but harbors a microbial flora [1]. Indeed, findings from a large observational study revealed that 82% of women had endometrial cultures positive for at least one microorganism approximately 3 months postpartum [2]. It is thought that microbial colonization of the upper genital tract leading to infection of the chorioamnion and amniotic fluid might play a key role in the etiology of spontaneous preterm birth [3–9]. Results of antibiotic intervention studies, focusing frequently on bacterial vaginosis, for the prevention of preterm birth have been mixed and do not support the routine use of antibiotics [10]. However, in all of these studies antibiotics were administered after the first trimester and investigators have hypothesized that this may be too late in the disease process as preexisting chronic endometrial colonization may be a determinant [2]. In a recent double-blind randomized clinical trial, a one-week course of interconceptional prophylactic antibiotics (metronidazole plus azithromycin) or placebo was administered every 4 months to 241 women at high risk for preterm delivery (PTD) [11]. These women had endometrial cultures and histology evaluated at randomization and two weeks after initial treatment. Antibiotics did not reduce the prevalence of subsequent PTD among the 124 women who conceived; instead there was a trend towards lower birth weight and gestational age. Overall among the 241 women randomized, 49% of antibiotic treated women demonstrated a decrease in the number of culture-isolated microbial species compared to only 31% of women on placebo. Only 22% vs. 29% respectively demonstrated a resolution in plasma cell endometritis (p=0.4). We hypothesized that the impact of this regimen on the endometrium may vary depending on baseline demographic, microbial or histologic characteristics of individual study women. Therefore, our primary objective was to comprehensively evaluate the impact of the antibiotic regimen on the endometrial microbial flora and histology. As a secondary objective, we evaluated whether the impact of antibiotics was modified by individual baseline characteristics.
Material and Methods
We performed secondary analyses of data obtained as part of a double-blind placebo-controlled randomized clinical trial conducted at the Center for Women’s Reproductive Health (CWRH) at the University of Alabama at Birmingham. The detailed methodology has been previously described [2, 11]. Briefly, CWRH research personnel recruited women with singleton pregnancies ending in a spontaneous preterm birth or pregnancy loss between 16 and 34 weeks prior to discharge from the hospital. Spontaneous preterm birth was defined as birth following spontaneous preterm labor or premature rupture of membranes. Women who were not at risk of becoming pregnant (had a cesarean hysterectomy or sterilization, or had Norplant or IUD placement) were excluded from the study, as were those with a multiple gestation or fetal anomaly in the index pregnancy. The Institutional Review Board approved the study and all participating women provided written informed consent. Enrollment occurred between January 1998 and August 2001.
At approximately four months postpartum, study candidates were evaluated and after excluding pregnancy, baseline endometrial specimens were obtained and cultures performed for aerobic and anaerobic bacteria, as well as Ureaplasma urealyticum, Mycoplasma species, Neisseria gonorrhoeae, group B Streptococcus, Trichomonas vaginalis, and a ligase chain reaction test for Chlamydia trachomatis. Histopathology was also performed on the endometrial specimens. The methods for cultures, chlamydia ligase chain reaction, histology, and method of endometrial sampling have all been previously described [2]. During this initial visit, these non-pregnant women were randomized in a double-blind fashion to an active-drug group versus placebo group using a computerized random number sequence with a block size of 4 and stratified according to gestational age at delivery of the index pregnancy (≤ 23 weeks or 23 weeks), and time from index delivery to randomization ( 4months or > 4 months). The active-drug group received 2 doses of azithromycin 1.0 g given 4 days apart plus sustained-release metronidazole 750 mg daily for 7 days. The control group received identical-appearing placebos that contained the same fillers and coatings as the active-drug medications. The same treatment was repeated every 4 months until conception or until the study was terminated. Study women were re-evaluated 2 weeks after randomization at which time repeat endometrial cultures and histopathology were performed. Plasma cell endometritis was defined as the presence of any plasma cells, seen using 40 X magnification.
Follow-up continued through March 2003 during which time 134 women out of 241 conceived. Seven women electively terminated their pregnancies while 3 had no outcome data. The outcomes of the remaining 124 pregnancies and the impact of antibiotics have been reported [11]. In the current study we focused on the impact of antibiotics on the larger randomized population of 241 women.
The primary outcomes were the proportions of women who acquired a microbe, category of microbes or plasma cell endometritis when these were not present at baseline (% Acquisition). Alternatively, if a microbe, category of microbes or plasma cell endometritis was present at baseline, we determined the proportions of women with a corresponding change to a negative culture or histology status at follow-up evaluation (% Resolution). The secondary outcomes involved proportions of acquisition and resolution stratified by the baseline characteristics.
Baseline demographic comparisons were performed using Chi square test or student t test for categorical and continuous variables respectively. Chi square or Fisher’s exact tests were used as appropriate to compare the outcome measures (proportions of acquisition and resolution) for women on antibiotics vs. those on placebo. Chi-square test for homogeneity was utilized to test for effect modification by baseline characteristics. For all comparisons p-value < 0.05 indicated statistical significance. Data management and statistical analyses were performed using SAS version 9.1 software (Cary, NC).
Results
Out of 736 women screened and the total of 241 women studied, 118 were randomized to antibiotics and 123 to placebo (Figure). Women in both arms had similar baseline socio-demographic characteristics (Table I) including age, race, marital status, smoking, education, prior miscarriage, gestational age at prior delivery and time from randomization to index delivery. Culture results were available for 95 women (80.5%) on antibiotics vs. 104 (84.6%) on placebo (p-value = 0.41). Histology results were available for 54 (45.8%) of those on antibiotics vs. 55 (44.7%) on placebo (p-value = 0.87). In addition, when restricted to the sub-samples of women with available culture results (n=199) or those with histology (n=109), both treatment arms still maintained a similar socio-demographic profile (results not shown).
Figure.
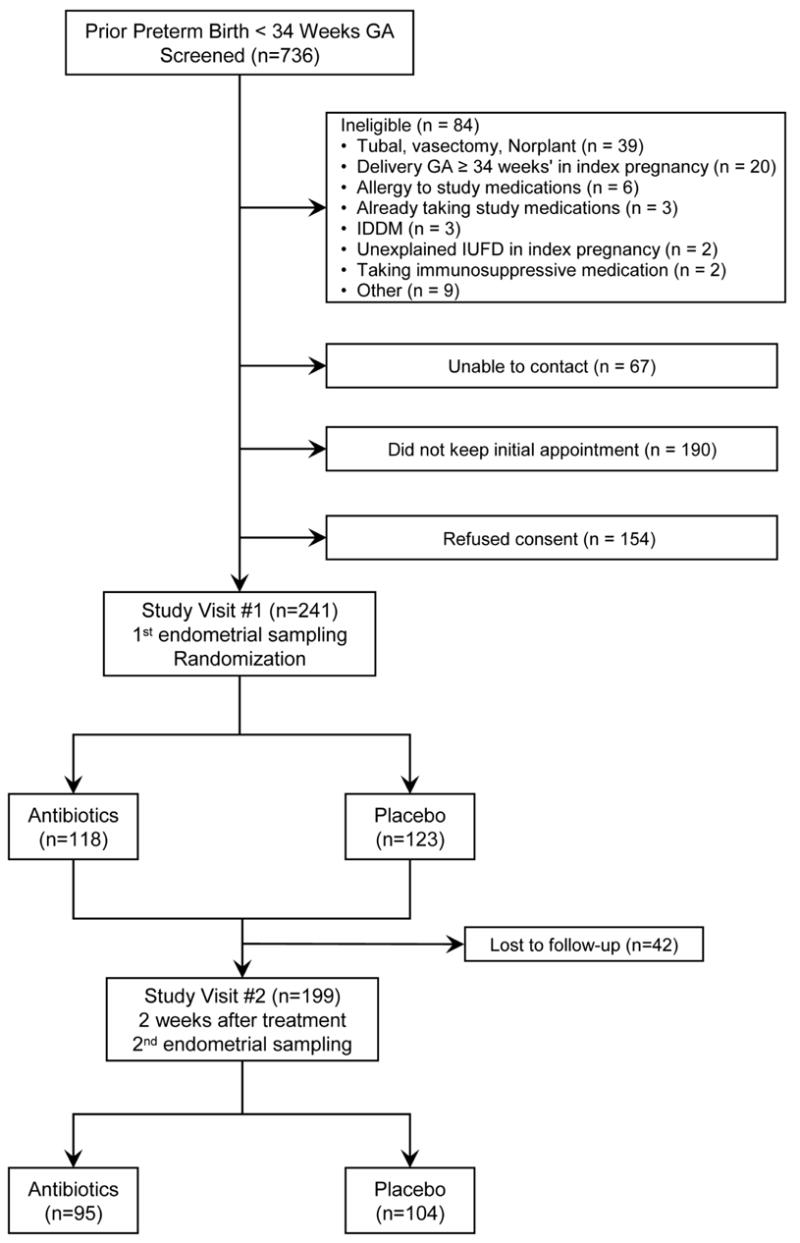
Flow chart of screening, randomization to antibiotics vs. placebo, and endometrial sampling. GA, Gestational age; IDDM, Insulin-dependent diabetes mellitus; IUFD, intrauterine fetal demise.
Table I.
Baseline characteristics of study population (N=241)
| Characteristic | Antibiotics (n=118) | Placebo (n=123) | p-value |
|---|---|---|---|
| Maternal age (years)* | 23.8 ± 5.7 | 23.6 ± 5.6 | 0.839 |
| African American (vs. White) | 73.7 | 72.4 | 0.811 |
| Unmarried | 74.4 | 65.0 | 0.117 |
| Smoker | 27.1 | 31.7 | 0.435 |
| Education <12 years | 29.7 | 30.9 | 0.835 |
| History of spontaneous abortion | 30.5 | 27.6 | 0.624 |
| Gestational age of index pregnancy (weeks)* | 25.9 ± 4.7 | 26.3 ± 4.7 | 0.450 |
| Previous delivery to randomization (days)* | 153.3 ± 205 | 165.5 ± 198 | 0.638 |
Results reported in mean ± standard deviation, all others are percentages
In Table II we present primary outcome results corresponding to the prevalence of acquisition or resolution of individual microbes and categories of microbes after treatment. In general, acquisition of microbes was lower and their resolution higher among antibiotic-treated women compared to those on placebo. The acquisition of Gram negative rods such as the aerobic subgroup and Gardnerella vaginalis in particular was significantly lower in the antibiotic group compared to the placebo group while the prevalence of their resolution was significantly higher among women on antibiotics. There was no definite impact on acquisition or resolution of lactobacillus species. There were too few women with Gram-negative cocci for a meaningful analysis to be performed. In addition, the resolution of aerobic microbes in general was significantly higher in the antibiotic group but the impact of antibiotics on acquisition was negligible. Though not statistically significant, antibiotics were associated with a potentially clinically relevant increase in the resolution (but not acquisition) of anaerobes.
Table II.
Prevalence (%) of acquisition and resolution of the most commonly isolated endometrial microbes by treatment group
| Prevalence of Acquisition* | Prevalence of Resolution** | |||||
|---|---|---|---|---|---|---|
| Antibiotic§ %(n/N) | Placebo§ %(n/N) | p-value | Antibiotic§ %(n/N) | Placebo§ %(n/N) | p-value | |
| Any microbe | 80(8/10) | 83(5/6) | 1.00 | 17(14/85) | 8(8/98) | 0.08 |
| Gardnerella vaginalis | 14(7/49) | 34(17/50) | 0.02 | 57(26/46) | 33(18/54) | 0.02 |
| Lactobacillus species | 44(22/50) | 45(23/51) | 0.91 | 44(20/45) | 34(18/53) | 0.29 |
| Streptococcus viridans | 11(10/87) | 5(4/88) | 0.09 | 100(8/8) | 69(11/16) | 0.13 |
| Ureaplasma urealyticum | 4(4/90) | 7(7/97) | 0.42 | 80(4/5) | 71(5/7) | 0.74 |
| Mycoplasma hominis | 7(6/86) | 7(7/97) | 0.95 | 100(9/9) | 57(4/7) | 0.06 |
| Peptostreptococcus | 13(10/80) | 18(15/85) | 0.36 | 87(13/15) | 74(14/19) | 0.43 |
| Aerobic Gram(−) rods | 16(8/49) | 37(18/49) | 0.02 | 54(25/46) | 33(18/55) | 0.03 |
| Aerobic Gram(+) cocci | 26(18/69) | 20(15/76) | 0.36 | 65(17/26) | 57(16/28) | 0.53 |
| Aerobic Gram(+) rods | 41(19/46) | 45(23/51) | 0.70 | 41(20/49) | 30(16/53) | 0.26 |
| Anaerobic Gram(−) rods | 5(4/86) | 8(8/95) | 0.31 | 78(7/9) | 56 (5/9) | 0.62 |
| Anaerobic Gram(+) rods | 6(5/82) | 8(8/96) | 0.57 | 100(13/13) | 88(7/8) | 0.38 |
| Any Gram (−) rod‡ | 13(6/47) | 34(16/47) | 0.01 | 50(24/48) | 32(18/57) | 0.05 |
| Any Aerobe | 71(10/14) | 73(8/11) | 0.94 | 21(17/81) | 9(8/93) | 0.02 |
| Any Anaerobe | 21(13/61) | 25(18/73) | 0.65 | 77(26/34) | 55(17/31) | 0.07 |
Acquisition: Negative at baseline & Positive on follow-up;
Resolution: Positive at baseline & Negative on follow-up;.
Figures in columns are percentage(Numerator/denominator). N.B. All percentages are rounded to nearest integer; statistically significant findings are in bold.
Acquisition required being negative for both aerobic and anaerobic Gram (−) rods at 1st sampling.
Primary outcome results indicated a non-significant reduction in the acquisition of plasma cell endometritis in the antibiotic group with a prevalence of 28% (9/32), compared to 50% (14/28) in the placebo group (p=0.11). Resolution was similar in both groups (p=0.78): 55% (12/22) for women on antibiotics vs. 59% (16/27) for those on placebo.
Stratification of the prevalence of acquisition and resolution of Gardnerella vaginalis, aerobic Gram negative rods and Gram negative rods in general by the different baseline characteristics revealed a similar overall pattern of lower acquisition and higher resolution of microbes in the antibiotic group regardless of the characteristic of the subgroup. In Table III, we present the stratified relative risks and 95% confidence intervals of acquisition and of resolution of Gram-negative rods (relative risk = prevalence of acquisition or resolution on antibiotics ÷ prevalence of acquisition or resolution on placebo). All relative risks for microbial acquisition were less than one - consistent with a protective impact of antibiotics. In addition, all relative risks for resolution were greater than one, suggesting antibiotics promote resolution. The relative impact of antibiotics on the acquisition of Gram-negative rods appeared higher in whites (5-fold reduction) than in blacks (2.2-fold reduction). Similarly, the impact appeared to be higher in women whose index preterm delivery occurred prior to 28 weeks (7-fold reduction) than in those whose deliveries occurred from 28–34 weeks (1.7-fold reduction). Statistical testing for heterogeneity was however negative. The protective effect against acquisition of Gram negative rods in the sub-groups of unmarried women, non-smoking women, and women without spontaneous abortion, and increased resolution in African American women were statistically significant. However, corresponding tests for heterogeneity were negative. Further stratification of index gestational age into 16–23 and 24–28 and >28 weeks was limited by small cell sizes, but indicated the same tendency for reduced acquisition and increased resolution of Gram-negative rods due to antibiotics for each smaller sub-group. Results for Gardnerella vaginalis and aerobic Gram-negative rods (not shown) presented an identical pattern.
Table III.
Relative impact of Antibiotics on Acquisition and Resolution of Gram-negative rods stratified by baseline characteristics
| Characteristic | Acquisition
†RR (95% CI) |
Resolution
†RR (95% CI) |
|---|---|---|
| African American | 0.45 (0.18, 1.16)* | 1.82 (1.01, 3.28)* |
| White | 0.20 (0.03, 1.53)* | 1.10 (0.50, 2.38)* |
|
| ||
| Unmarried | 0.33 (0.12, 0.93)* | 1.62 (0.92, 2.88) |
| Married | 0.43 (0.10, 1.88)* | 1.42 (0.55, 3.65) |
|
| ||
| Smoker | 0.57 (0.19, 1.76)* | 1.71 (0.73, 4.00) |
| Non-smoker | 0.30 (0.09, 0.99)* | 1.52 (0.86, 2.70) |
|
| ||
| History of spontaneous abortion | 0.63 (0.04, 8.91)* | 1.51 (0.68, 3.36) |
| No spontaneous abortion | 0.40 (0.16, 0.97)* | 1.62 (0.90, 2.91) |
|
| ||
| Index Preterm birth < 28 weeks | 0.14 (0.02, 1.10)* | 1.41 (0.79, 2.53) |
| Index Preterm birth 28–34 weeks | 0.58 (0.24, 1.40)* | 1.94 (0.86, 4.39) |
Test for heterogeneity between subgroup strata was not significant (p-value > 0.05) though RRs appeared different or statistically significant in one but not in the other subgroup.
Relative risk (and 95% CI) comparing prevalence of acquisition or resolution in antibiotic group relative to placebo group (referent)
Comment
Our findings indicate that the interconceptional antibiotic regimen of azithromycin and metronidazole prevents the acquisition and promotes the resolution, but not eradication, of endometrial microbial organisms and plasma cell endometritis when tested 2 weeks after treatment. This dual impact was statistically significant for Gram-negative rods such as the aerobic subgroup and Gardnerella vaginalis species, and was supported by the trends observed after stratification by baseline sociodemographic characteristics. Though the magnitude of this impact appeared to be higher for whites (compared to blacks) and for index preterm delivery prior to 28 weeks gestation (vs. 28–34 weeks), statistical tests did not confirm an effect modification. Similarly, statistical tests were not suggestive of effect modification in acquisition or resolution in spite of significant associations in isolated sub-categories within socio-demographic groups. There was no impact on the acquisition or resolution of lactobacillus (second most frequent species) and the impact on anaerobes in general and plasma cell endometritis was not definitive. Importantly, we did not ascertain clinically or statistically significant overgrowth of any one organism due to antibiotics.
The assessment of the impact of prophylactic interconceptional treatment of genital tract infection on pregnancy outcomes used in our primary study represented a novel approach. The current study comprehensively addresses the impact of antibiotics on endometrial microbial colonization and provides useful information about the impact on histology. The randomized design and intent-to-treat analysis obviously enhance the validity of our findings. Nonetheless, we acknowledge some limitations. The sample size was inadequate for a complete delineation of the impact of antibiotics on some microbes, especially in the stratified analysis, potentially leading to type 2 errors. On the other hand, the examination of the impact on multiple microbes raises the question of multiple comparisons and possible type 1 errors, as significant findings may occur purely by chance. In addition, the findings may not be applicable to antibiotics other than the ones used in this study given varying coverage profiles. Finally, we did not conduct an exhaustive evaluation of potential endometrial microorganisms. We are therefore unable to assess the impact on or interaction with organisms such as fungi, viruses or other microbes that can only be identified using molecular techniques.
Our findings do indicate that antibiotics can be useful in protecting the endometrium from microbial colonization and inflammation by preventing their acquisition and/or promoting resolution. Specifically, the impact on Gardnerella vaginalis is predictably due to metronidazole [12] while that on Gram negative rods (predominantly aerobic) is attributable to azithromycin [13–14]. It is paradoxical that these changes did not translate to improved birth outcomes [11]. It is also unclear why the impact on aerobes in general was restricted to resolution. An interaction between baseline floral organisms and at least one of these antibiotics is plausible. There is a paucity of similar endometrial studies for comparison, but one older study of women with recurrent abortion and infertility reported the eradication of mycoplasmas from the endometrium, as well as the cervix, after doxycycline therapy [15]. A Cochrane systematic review of multiple studies[10] including the large NICHD MFMU trial [16] evaluating the impact of antibiotics on bacterial vaginosis during pregnancy did not find any beneficial effect on pregnancy outcomes despite significant reductions in bacterial vaginosis. The antibiotics included single use or combinations of metronidazole (most frequent), clindamycin, erythromycin and amoxicillin. Similarly, metronidazole therapy induced a marked reduction in Trichomonas vaginalis relative to placebo but was paradoxically associated with increased preterm delivery [17]. Aerobic and anaerobic cervical flora have also been evaluated before and after administration of perioperative cesarean prophylactic cephalosporins vs. placebo [18]. There was a postpartum increase in cervical colonization with Escherichia coli and Bacteroides fragilis that was independent of treatment. However, there was a significant increase in enterococcal colonization of the cervix in the antibiotic arm. In our trial we did not find any such significant increase in endometrial enterococci, although aerobic Gram-positive cocci and related Streptococcus viridans provided the only instances in which the prevalence of acquisition of microbes was higher in the antibiotic arm than in the placebo arm. These differences are likely due primarily to differences in the spectrum of antibiotic coverage. Genital tract location (cervix vs. endometrium) and timing of assessment in relation to pregnancy could also contribute to these differences.
Regarding the timing of assessment it is important to note that genital cultures and histology were empirically obtained two weeks after initial treatment at a time remote from conception. The findings may therefore differ from the actual endometrial microbial flora and histology following additional cycles of antibiotics or at the time of conception and pregnancy.
Our findings add to an accumulating body of evidence indicating that antibiotics produce expected changes in micro-flora and reduce susceptible pathogens in the human genital tract. Unfortunately, the desirability of this antibiotic manipulation prior to or during pregnancy is questionable, as it generally has not resulted in improved pregnancy outcomes even when the pathogens or microbes are established risk factors for poor outcomes such as preterm delivery. This paradox, which is further compounded in some instances by an apparent increase in adverse outcomes, remains largely unexplained. Further studies are needed to clarify this issue. Such studies may examine the specific content of microbial flora in relation to pregnancy outcomes, as well as predisposing host factors.
Footnotes
To be presented at the 27th Annual Meeting of the Society for Maternal-Fetal Medicine on February 9th, 2007 in San Francisco, CA
No reprints will be available
Funded by a grant from the National Institute of Child Health and Human Development (HD33883). Searle Pharmarceuticals provided the metronidazole and placebos. Pfizer Pharmaceuticals provided the azithromycin and placebos
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Espinoza J, Erez O, Romero R. Preconceptional antibiotic treatment to prevent preterm birth in women with a previous preterm delivery. Am J Obstet Gynecol. 2006;194(3):630–7. doi: 10.1016/j.ajog.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 2.Andrews WW, Goldenberg RL, Hauth JC, Cliver SP, Conner M, Goepfert AR. Endometrial microbial colonization and plasma cell endometritis after spontaneous or indicated preterm versus term delivery. Am J Obstet Gynecol. 2005;193(3 Pt 1):739–45. doi: 10.1016/j.ajog.2005.02.128. [DOI] [PubMed] [Google Scholar]
- 3.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 4.Andrews WW, Hauth JC, Goldenberg RL. Infection and preterm birth. Am J Perinatol. 2000;17:357–365. doi: 10.1055/s-2000-13448. [DOI] [PubMed] [Google Scholar]
- 5.Hillier SL, Martius J, Krohn M, Kiviat N, Holmes KK, Eschenbach DA. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988;319:972–978. doi: 10.1056/NEJM198810133191503. [DOI] [PubMed] [Google Scholar]
- 6.Hillier SL, Krohn MA, Kiviat NB, Watts DH, Eschenback DA. Microbiologic causes and neonatal outcomes associated with chorioamnion infection. Am J Obstet Gynecol. 1991;165:955–961. doi: 10.1016/0002-9378(91)90447-y. [DOI] [PubMed] [Google Scholar]
- 7.Hauth JC, Andrews WW, Goldenberg RL. Infection-related risk factors predictive of spontaneous preterm birth. Prenat Neonat Med. 1998;3:86–90. [Google Scholar]
- 8.Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol. 1988;31:553–584. doi: 10.1097/00003081-198809000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Andrews WW, Hauth JC, Goldenberg RL, Gomez R, Romero R, Cassell GH. Amniotic fluid interleukin-6: correlation with genital tract microbial colonization and gestational age in women delivered after spontaneous labor versus indicated delivery. Am J Obstet Gynecol. 1995;173:606–12. doi: 10.1016/0002-9378(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 10.McDonald H, Brocklehurst P, Parsons J. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst Rev. 2005;(1):CD000262. doi: 10.1002/14651858.CD000262.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Andrews WW, Goldenberg RL, Hauth JC, Cliver SP, Copper R, Conner M. Interconceptional antibiotics to prevent spontaneous preterm birth: a randomized clinical trial. Am J Obstet Gynecol. 2006;194(3):617–23. doi: 10.1016/j.ajog.2005.11.049. [DOI] [PubMed] [Google Scholar]
- 12.Bannatyne RM, Jackowski J, Cheung R, et al. Susceptibility of Gardnerella vaginalis to metronidazole, its bioactive metabolites, and tinidazole. Am J Clin Pathol. 1986;87:640–1. doi: 10.1093/ajcp/87.5.640. [DOI] [PubMed] [Google Scholar]
- 13.Piscitelli SC, Danziger LH, Rodvold KA. Clarithromycin and azithromycin: New macrolide antibiotics. Clin Pharm. 1992;11:137–52. [PubMed] [Google Scholar]
- 14.Bahal N, Nahata MC. The new macrolide antibiotics: Azithromycin, clarithromycin, dirithromycin, and roxithromycin. Ann Pharmacother. 1992;26:46–55. doi: 10.1177/106002809202600112. [DOI] [PubMed] [Google Scholar]
- 15.Stray-Pedersen B, Eng J, Reikvam TM. Uterine T-mycoplasma colonization in reproductive failure. Am J Obstet Gynecol. 1978;130(3):307–11. [PubMed] [Google Scholar]
- 16.Carey JC, Klebanoff MA, Hauth JC, Hillier SL, Thom EA, Ernest JM, et al. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 2000;342(8):534–40. doi: 10.1056/NEJM200002243420802. [DOI] [PubMed] [Google Scholar]
- 17.Klebanoff MA, Carey JC, Hauth JC, Hillier SL, Nugent RP, Thom EA, et al. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med. 2001;345 (7):487–93. doi: 10.1056/NEJMoa003329. [DOI] [PubMed] [Google Scholar]
- 18.Stiver HG, Forward KR, Tyrrell DL, Krip G, Livingstone RA, Fugere P, et al. Comparative cervical microflora shifts after cefoxitin or cefazolin prophylaxis against infection following cesarean section. Am J Obstet Gynecol. 1984;149(7):718–21. doi: 10.1016/0002-9378(84)90109-1. [DOI] [PubMed] [Google Scholar]


