Summary
Microarray technology is currently used in the development of carbohydrate drugs and diagnostic tests. Here we model an inexpensive alternative to microarrays using derivatized microbeads. In this model we examine the binding of mannose-rich yeast to microbeads derivatized with concanavalin A (Con A), a mannose-binding lectin, in the presence of 30 different sugars and 9 different pH conditions. We developed a listing of effective saccharide inhibitors of immobilized Con A based on 3901 replicates. We suggest that this is the most extensive saccharide inhibitor list ever developed for this lectin and it may be useful to use this listing to replace the less extensive lists that have been in the literature for decades. Information is also provided on pH effects on immobilized Con A binding based on 918 trials. Two assays to study binding, one which qualitatively scores more or less binding than control in thousands of replicate samples, and another that quantitatively evaluates binding by counting the number of cells bound to each bead, are also modeled here. We know of no previous studies that provide such as extensive information on saccharide inhibition and pH effects on the binding of immobilized Con A. We suggest that this microbead approach, using beads derivatized with lectins or sugars, and the two simple assays presented here, can in some cases, substitute for more expensive microarray technology in the development of carbohydrate drugs and diagnostic tests. If, for example, our model Saccharomyces cerevisiae was a pathogen, these studies show that it binds via cell surface mannose residues and drugs to prevent binding could be developed using the inhibitors of binding identified here. The beads could be also used in the development of diagnostic tests that identify the presence of the organism in blood samples, etc. in much the same way as microarray technology is being used today.
Keywords: Immobilized Con A, yeast, sugar inhibitors, pH, microarrays
Introduction
Lectins are among the most widely used compounds in biomedical research. They are carbohydrate-binding proteins that are widely used in thousands of studies involving cell surface histochemistry and purification of glycans. They are used in the purification of glycan-containing molecules and in histochemical studies that have identified important properties such as differences between cancer and non-cancer cell surfaces (Sharon and Lis, 1989; Sharon and Lis, 2004). Pathogenic organisms often attach to human cell surfaces via lectin-glycan interactions and drug companies are developing carbohydrate-based drugs that inhibit these adhesive interactions (Borman, 2005; Szymanski and Wren, 2005; Mulvey et al., 2001; Xin, et al. 2005; Dinglasan and Jacobs-Lorena, 2005; Rojo and Delgado, 2004).
Many scores of lectins are commercially available. Lectins bind best to naturally occurring ligands on cells that are often more complex than monosaccharides commonly quoted as their binding partners. It is important that lectin-binding partners that possess the greatest avidity for lectins be identified for use in countless types of experiments that require inhibitors of lectin binding. This study examines this issue by testing an unusually large number of sugars, including monosaccharides and oligosaccharides, for their ability to inhibit lectin binding in a model system. This approach can be used to expand the information on sugar binding avidity in many other lectin binding systems.
Material and Methods
A suspension of (Saccharomyces cerevsiae ) yeast were washed three times washed in distilled water then 50μl droplets at a concentration of 0.07 mg per ml-0.7 mg per ml were mixed on glass microscope slides with 0.7 mg-3.0 mg Con A derivatized agarose beads (Sigma Chemical Co., St. Louis, Mo) per ml solution. The specific yeast and bead concentrations for each study are given in the figure legends. Yeast and bead concentrations were varied in some studies to learn if these factors influenced the results. The droplets contained either pH adjusted distilled water (pH 2–10) or specific saccharides (Sigma), at 0.05 M concentration in distilled water. The 0.05 M concentration was chosen after studies were completed using higher concentrations that did not allow as clear a differentiation of the most effective sugars. The droplets were mixed for 240 seconds with wooden toothpicks and observed using light microscopes at 100x-400x magnification.
Two different assay procedures were used. For the sugar inhibition studies, each microscope slide included droplets containing the specific saccharide at 0.05M concentration or no sugar (controls). Binding of yeast and beads was recorded for each slide as less binding in the sugar drops than in the control drops, more binding in the sugar drops than in the control drops or approximately equal binding in the sugar drops and control drops. A total of 3901 samples were assayed with an average of 130 replicates for each sugar.
The second assay was used in the pH experiments. In these experiments the number of yeast cells bound per bead was counted and recorded in a total of 918 trials spanning a pH range of 2 through 10. Results were tabulated as the mean number of yeast bound to Con A beads ± standard deviation.
The first assay, used in the sugar studies is less quantitative than the second assay used in the pH experiments, but it is based on over 100 replicates for each sugar. Several independent investigators scored the binding results by comparison with control (with no sugar). Both assays are modeled here and their advantages and disadvantages are discussed.
Results
Table 1 shows the ranking of the most effective saccharide inhibitors of yeast-Con A bead binding at 0.05M sugar concentration, in order of decreasing inhibition effectiveness. D(+) melezitose, D(+) trehalose, maltotriose, maltose and D(−) fructose were the most effective inhibitors. D(+) glucose, D(+) galactosamine, methylα-D- mannopyranoside, D(+) mannose, L(−) fucose, D(+) glucosamine and methylα-D-glucopyranoside were somewhat less effective. D-mannoheptose, β-cyclodextrin, D(+) raffinose, methyl β-D-glucopyranoside,α-Lactose, β-lactose, D-lactose, L-sorbose, α-cyclodextrin, L(−) xylose, L (+) arabinose, D(−) arabinose, D(+) cellobiose, L-rhamnose, melibiose, D(+) xylose, D(+) galactose (Sigma 6404), and D(+) galactose (Sigma 0750) were least effective.
Table 1.
Saccharide inhibitors, at 0.05M concentration, of yeast binding to Con A beads in order of decreasing effectiveness. %s given are for % of replicates that showed inhibition, no change or promotion over controls with no sugar. Based on 3901 replicates for each sugar. Inhibitory means that yeast-Con A bead binding was less than control in absence of sugar. No Change means that yeast-Con A bead binding was the same as control in absence of sugar. Promotional means that there was more yeast-Con A bead binding in the sugar sample than in the control (absence of sugar). The differences in numbers of replicates for each sugar simply reflect numbers of experiments done over a year-long period by 24 investigators for each sugar.
| Saccharide | Inhibitory | no change | promotional | # replicates |
|---|---|---|---|---|
| D(+)Melezitose | 72% | 18% | 10% | 118 |
| D(+)Trehalose | 63% | 25% | 12% | 126 |
| Maltotriose | 60% | 25% | 14% | 111 |
| Maltose | 60% | 22% | 18% | 118 |
| D(-)Fructose | 59% | 21% | 20% | 153 |
| D(+)Glucose | 54% | 33% | 13% | 132 |
| D(+) Galactosamine | 53% | 26% | 21% | 131 |
| Methyl α-D-mannopyranoside | 53% | 37% | 10% | 127 |
| D(+)Mannose | 52% | 27% | 21% | 157 |
| L(−)Fucose | 50% | 31% | 19% | 123 |
| D(+)Glucosamine | 49% | 36% | 15% | 162 |
| Methyl -α D- Glucopyranoside | 48% | 30% | 22% | 166 |
| D-Mannoheptose | 46% | 28% | 26% | 112 |
| β-Cyclodextrin | 44% | 29% | 27% | 84 |
| D(+)Raffinose | 44% | 45% | 11% | 122 |
| Methyl β-D- Glucopyranoside β | 42% | 33% | 25% | 139 |
| α-Lactose | 39% | 30% | 31% | 140 |
| β-Lactose | 38% | 34% | 28% | 130 |
| D-Lactose | 37% | 41% | 22% | 131 |
| L-Sorbose | 36% | 43% | 21% | 132 |
| α-Cyclodextrin | 35% | 34% | 31% | 105 |
| L(−)Xylose | 35% | 41% | 24% | 118 |
| L(+)Arabinose | 32% | 30% | 38% | 114 |
| D(−)Arabinose | 31% | 38% | 31% | 129 |
| D(+)Cellobiose | 29% | 44% | 27% | 126 |
| L-Rhamnose | 28% | 48% | 24% | 124 |
| Melibiose | 28% | 42% | 30% | 131 |
| D(+)Xylose | 26% | 38% | 36% | 132 |
| D(+)Galactose Sigma (6404) | 25% | 39% | 36% | 154 |
| D(+)Galactose Sigma (0750) | 14% | 42% | 44% | 154 |
Figure 1 shows examples of yeast binding to Con A beads in the presence and absence of inhibitory sugar.
Figure 1.
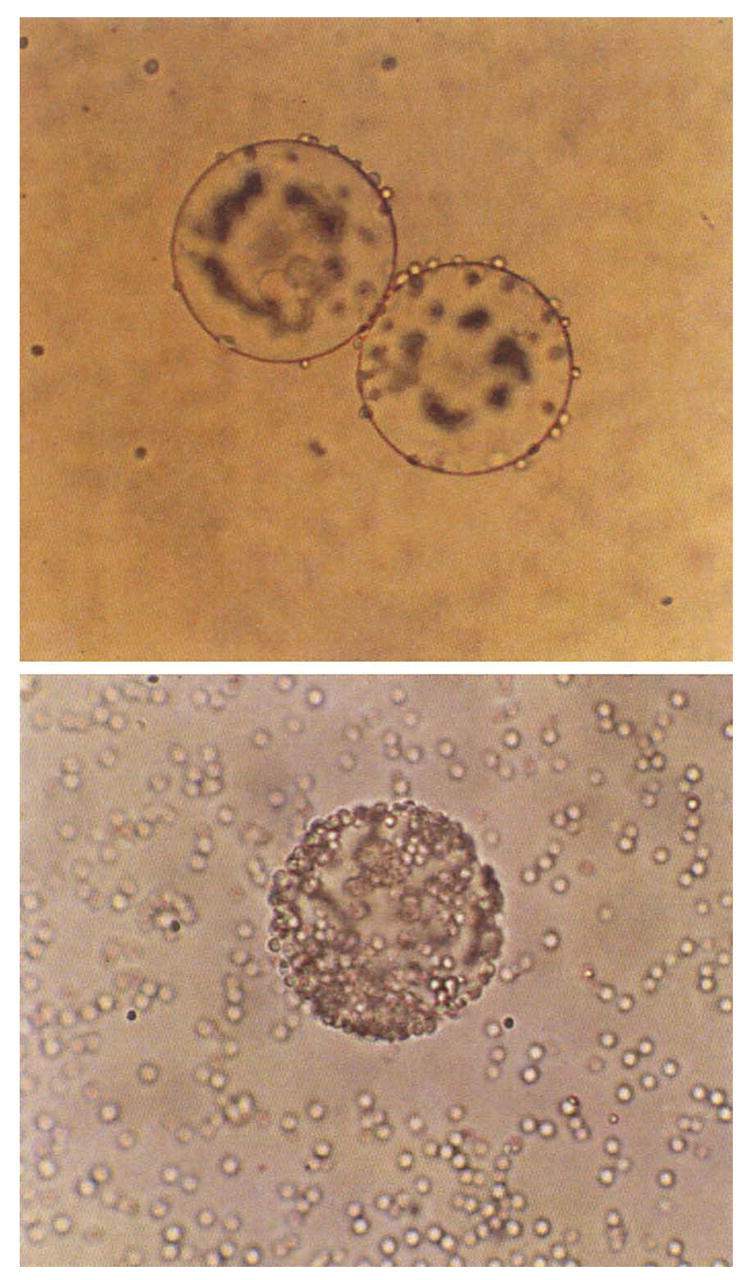
Top. An example of inhibition of yeast-Con A-bead binding with 0.05M D (+) melezitose.
Bottom. Control with no sugar. Large spheres are beads, tiny spheres are cells. Magnification 300x. Melezitose sample is focused to show small number of cells adhering to bead edges. Control sample shows cells coating entire bead.
Figures 2a-2d provide 4 different sets of pH experiments each using different bead and yeast concentrations. As can be seen, regardless of bead and yeast concentrations, the patterns of the results were very similar with most yeast-Con A bead binding occurring at pH 4–8. Very little binding occurred at pH 2 and pH 10, with slightly more binding at pH 3 and 9.
Figure 2.
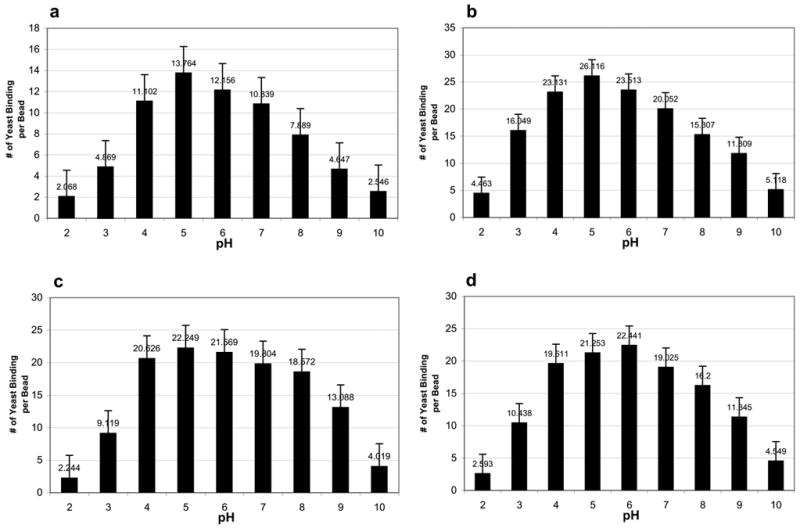
Effects of pH on Con A bead-yeast binding under varying bead and yeast concentrations. Numbers are means of number of yeast bound per bead. Error bars show Standard Deviation.
a. 3 mg beads, 0.7 mg yeast per ml distilled water, 238 trials. b. 1.5 mg beads, 0.07 mg yeast per ml distilled water, 118 trials. c. 1.5 mg beads, 0.15 mg yeast per ml distilled water, 227 trials. d. 0.7 mg beads, 0.15 mg yeast per ml distilled water, 335 trials.
Discussion
Glycobiology is rapidly becoming of major interest worldwide. Carbohydrate microarrays and lectin microarrays are being used to screen thousands of compounds, cell extracts, and cells to assess their carbohydrate binding properties (Borman, 2005; Disney and Seeberger, 2004; Kuno et al., 2005; Nimrichter et al., 2004). The method used here is an inexpensive alternative to microarrays. It can be used with hundreds of glycan derivatized beads or lectin derivatized beads (as in this study) to quickly assess glycan-lectin interaction and the factors that influence it.
Microarray technology, in which sugars or proteins, such as lectins, are bound to small spots on solid surfaces or microchips is becoming widely used to screen biological compounds, cell extracts, cells, and other samples to assess the binding of the samples to the compounds bound to the chips (Borman, 2005; Disney and Seeberger, 2004; Kuno et al., 2005; Nimrichter et al., 2004). This technology is being used to produce potential anti-HIV vaccines and anti-infection drugs, to detect bacteria in food and blood, and to develop blood tests for diseases such as Crohn’s disease (Borman, 2005). Microarray technology, however, can cost hundreds of thousands of dollars. The microbead technology used here can accomplish some of the same goals as microarrays at very low cost.
Over the past decade we have used microbeads derivatized with over 100 different compounds to study cell surface properties (Ngo et al., 2003; Khurrum et al., 2003; Navarro et al., 2002; Salbilla et al., 1999; Latham and Oppenheimer, 1999; Heinrich et al., 2005; Welty et al. 2005; Latham et al. 1995; 1995a). We are unaware of any studies that have defined the microbead system as we have done in the present paper. In this study we focus on one microbead, Con A immobilized on agarose. Concanavalin A has been used in thousands of studies and is probably the most widely used lectin (Sharon and Lis, 2004; Sharon and Lis, 1989).
In order for microbeads to be correctly and effectively used in binding studies, the parameters affecting binding and inhibition of binding must be rigorously defined. We are unaware of any past studies that have examined the parameters and inhibitors in a microbead system in as comprehensive a manner as we have done here. Past studies have examined many parameters affecting free Con A binding. One of the most thorough studies was done by Ambrosino, et al. (1987) using a calorimetric technique. They found that melezitose, a trisaccharide, showed higher affinity for Con A than other oligosaccharides and monosaccharides tested. That study was done with free Con A. The findings here using Con A beads, not free Con A, were almost identical to those of Ambrosino, et al. (1987). This validates our approach to study effective inhibitors of Con A binding, but what is different in the study reported here is not only the use of Con A beads but the numbers of sugars tested and numbers of replicates examined. Here we tested 30 sugars, not the 5 tested by Ambrosino, et al. (1987). In this study, we were able to generate a much more comprehensive list of sugar inhibitors of Con A binding than any previous study (Ambrosino et al., 1987; Al-Arhabi et al., 2002; Tawaki et al., 2005; Wong, 1980; Figlas, et al., 1997; Ooya et al., 2005; Wright and Cooper, 1979). In these previous studies, the findings also generally matched our results indicating that specific oligosaccharides are often better lectin inhibitors than monosaccharides.
Here we show that the most inhibitory sugars of yeast binding to immobilized Con A at 0.05M concentration were the oligosacharrdes D(+) melezitose, D(+) trehalose and maltotriose. The listing of sugars presented here in order of decreasing inhibition effectiveness is the most extensive listing of sugar inhibitors of immobilized Con A-cell binding ever developed. This listing was made possible by the ease and rapidity of the microbead assay. We suggest that this listing, based on 3901total replicates, replace the less extensive lists for sugar inhibitors of immobilized Con A/free Con A that have been in the literature for decades.
In this study, we model two assays for determining cell binding to beads. The assay used in the sugar analysis is based on thousands of replicates using the principle: at a given sugar concentration, does a sugar sample show more, less, or the same cell binding to beads than controls without sugar on the same slide? Although this approach is based on thousands of trials, it does not answer the question: in a single trial for example, how much less cell binding to beads occurred in the experimental versus control sample? The virtue of this assay, however, is its great rapidity, facilitating thousands of trials. Its validity is clear because our results, using this assay, match those of other less inclusive studies, examining a smaller number of sugars, for example Ambrosino, et al., (1987), quoted above.
For more precise quantification of binding of cells to beads, we developed the second assay, used here in the pH study. In this method, numbers of cells bound to beads are manually counted, tabulated and statistically assessed. Here again, we know of no past studies that have as extensively examined the effects of pH on cell binding to immobilized Con A, generating the quantitative results in this study. While other studies have shown that free Con A is most active in the pH range of 4–8 (Liener, 1976), we know of no studies that have examined cell binding to immobilized Con A over a range of pH in as many trials as performed here, and at varying cell and bead concentrations. The results indicate that pH 4–8 is most permissive for facilitating cell binding to immobilized Con A.
Interestingly, we also show that cell binding to immobilized Con A occurs at pH below 4 and above 8. Minimal binding occurred at pH 2 and 10. These results should be of interest to those who are using immobilized Con A in many different types of studies. In some cases , due to experimental constraints, use of a pH in the standard range may not be possible. Our results make it clear how immobilized Con A-cell binding is influenced by pH that is not in the standard range.
In summary, we present two assays to measure cell binding to immobilized Con A. We use these assays to model the effects of two important factors on immobilized Con A-cell binding: sugars and pH, using different approaches than those used in the past. Most past studies have been done using free Con A. Immobilized Con A has not been as thoroughly evaluated but is used extensively worldwide. And finally, we propose that using lectin derivatized and saccharide derivatized microbeads offer an inexpensive way of accomplishing many of the same experiments in the area of carbohydrate drug and diagnostic test development that are currently being performed using microarray technology that is comparatively much more costly (Borman, 2005). If, for example, the Saccharomyces cerevisiae model presented here was a pathogen that bound to human tissue via its cell surface mannose residues, the development of drugs that prevent binding, based on the inhibitors of binding identified in this study, could be facilitated. On the other hand, if it was not known what surface sugars or lectins were present on the Saccharomyces cerevisiae cell surface, it would be easy to run these cells through libraries of beads derivatized with different sugars or lectins to see to which beads cell binding occurs. This would help define the cell surface molecules that could be involved in attachment of the cells to human tissue. In addition to the development of drugs that could inhibit such attachment, the beads could be used in the development of diagnostic tests for identifying the presence of the organism in blood samples, etc. in much the same way as microarray technology is being used today (Borman, 2005).
Acknowledgments
This work was supported by grants from NIH NIGMS, SCORE, MARC, RISE, ITQ program and the Joseph Drown Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Arhabi M, Mrestani Y, Richter H, Neubert RHH. Characterization of interaction between protein and carbohydrate using CZE. J Pharmaceutical and Biomedical Analysis. 2002;29:555–560. doi: 10.1016/s0731-7085(02)00082-1. [DOI] [PubMed] [Google Scholar]
- Ambrosino R, Barone G, Castronuovo G, Ceccarini C, Cultrera O, Elia V. Protein-ligand interaction. A calorimetric study of the interaction of oligosaccharides and hen ovalbumin glucopeptides with concanavalin. A Biochemistry. 1987;26:3971–3975. doi: 10.1021/bi00387a034. [DOI] [PubMed] [Google Scholar]
- Borman S. Carbohydrate advances. Chemical and Engineering News. 2005;83:41–50. [Google Scholar]
- Dinglasan RR, Jacobs-Lorena M. Insight into a conserved lifestyle: protein-carbohydrate adhesion strategies of vector-borne pathogens. Infection and Immunity. 2005;73:7797–7807. doi: 10.1128/IAI.73.12.7797-7807.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disney MD, Seeberger PH. The use of carbohydrate microarrays to study carbohydrate- cell interactions and to detect pathogens. Chem Biol. 2004;11:1701–1707. doi: 10.1016/j.chembiol.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Figlas DN, Arias HR, Fernandez A, Alperin DM. Dramatic saccharide-mediated protection of chaotropic-induced deactivation of concanavalin A. Arch Biochem Biophys. 1997;340:154–158. doi: 10.1006/abbi.1997.9929. [DOI] [PubMed] [Google Scholar]
- Heinrich EL, Welty LY, Banner LR, Oppenheimer SB. Direct targeting of cancer cells: a multiparameter approach. Acta Histochemica. 2005;107:335–344. doi: 10.1016/j.acthis.2005.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurrum MR, Weerasinghe GR, Soriano ES, Riman R, Badali O, Gipson S, Medina J, Alfaro J, Navarro VM, Harieg CB, Ngo L, Sakhakorn T, Kirszenbaum L, Khatibi D, Abedi K, Barajas M, Zem G, Kirszenbaum A, Razi A, Oppenheimer SB. Analysis of surface properties of human cancer cells using derivatized beads . Acta Histochemica. 2002;104:217–23. doi: 10.1078/0065-1281-00656. [DOI] [PubMed] [Google Scholar]
- Kuno A, Uchiyama N, Koseki-Kuno S, Ebe Y, Takashima S, Yamada M, Hiraboyashi J. Evanescent-field fluorescence-assisted lectin microarray: a new strategy for glycan profiling. Nat Methods. 2005;2:851–856. doi: 10.1038/nmeth803. [DOI] [PubMed] [Google Scholar]
- Latham V, Ducut J, Rostamiani K, Chun H, Lopez M, Herrera S, Oppenheimer S. A rapid lectin receptor binding assay: comparative evaluation of sea urchin embryo cell surface lectin receptors. Acta Histochemica. 1995;97:89–87. doi: 10.1016/S0065-1281(11)80209-6. [DOI] [PubMed] [Google Scholar]
- Latham V, Herrera S, Rostamiani K, Chun H, Oppenheimer S. Rapid identification of lectin receptors and their possible function in sea urchin cell systems. Acta Histochemica. 1995a;97:373–382. doi: 10.1016/S0065-1281(11)80062-0. [DOI] [PubMed] [Google Scholar]
- Latham V, Oppenheimer S. A simple image analysis method for evaluating cell binding to derivatized beads. Acta Histochemica. 1999;101:263–270. doi: 10.1016/S0065-1281(99)80027-0. [DOI] [PubMed] [Google Scholar]
- Liener IE. Isolation and properties of concanavalin A. In: Bittiger H, Schnebli HP, editors. Concanavalin A as a Tool. Wiley; London: 1976. pp. 17–31. Chapter 2. [Google Scholar]
- Mulvey G, Kitov PI, Marcato P, Bundle DR, Armstrong GD. Glycan mimicry as a basis for novel anti-infective drugs. Biochimie. 2001;83:841–847. doi: 10.1016/s0300-9084(01)01291-3. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Walker SL, Badali O, Abundis M, Ngo LL, Weerasinghe G, Barajas M, Zem G, Oppenheimer SB. Analysis of surface properties of fixed and live cells using derivatized agarose beads. Acta Histochemica. 2002;104:99–106. doi: 10.1078/0065-1281-00617. [DOI] [PubMed] [Google Scholar]
- Ngo L, Barajas M, Weerasinghe G, Zem G, Oppenheimer SB. A new histochemical approach for studying sperm cell surfaces. Acta Histochemica. 2003;105:21–28. doi: 10.1078/0065-1281-00689. [DOI] [PubMed] [Google Scholar]
- Nimrichter L, Gargir A, Gortler M, Alstock RT, Shtevi A, Weisshaus O, Fire E, Dotan M, Schnaar RL. Intact cell adhesion to glycan microarrays. Glycobiology. 2004;14:197–203. doi: 10.1093/glycob/cwh022. [DOI] [PubMed] [Google Scholar]
- Ooya T, Utsunomiya H, Eguchi M, Yui N. Rapid binding of concanavalin A and maltose- polyrotaxane conjugates due to mobile motion of alpha-cyclodextrins threaded onto a poly (ethylene glycol) Bioconjug chem. 2005;16:62–69. doi: 10.1021/bc049809h. [DOI] [PubMed] [Google Scholar]
- Rojo J, Delgado R. Glycodendritic structures: promising new antiviral drugs. Journal of Antimicrobial Chemotherapy. 2004;54:579–581. doi: 10.1093/jac/dkh399. [DOI] [PubMed] [Google Scholar]
- Salbilla BA, Vaghefi H, Chhabra P, Hall G, Brown D, Sadoughi F, Francisco E, Attas L, Walker S, Nguyen BN, Oppenheimer SB. Analysis of cell surface properties using derivatized agarose beads. Acta Histochemica. 1999;101:271–9. doi: 10.1016/s0065-1281(99)80028-2. [DOI] [PubMed] [Google Scholar]
- Sharon N, Lis H. Lectins. Chapman and Hall; London UK: 1989. [Google Scholar]
- Sharon N, Lis H. History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology. 2004;14:53R–62R. doi: 10.1093/glycob/cwh122. [DOI] [PubMed] [Google Scholar]
- Szymanski CM, Wren BW. Protein glycosylation in bacterial mucosal pathogens. Nat Rev Microbiol. 2005;3:225–237. doi: 10.1038/nrmicro1100. [DOI] [PubMed] [Google Scholar]
- Tawaki S, Uchida Y, Maeda Y, Ikeda I. HRP-catalyzed polymerization of sugar-based phenols in aqueous organic solvents. Carbohydrate Polymers. 2005;59:71–74. [Google Scholar]
- Welty LAY, Heinrich EL, Garcia K, Banner LR, Summers ML, Baresi L, Metzenberg S, Coyle Thompson C, Oppenheimer SB. Analysis of unconventional approaches for the rapid detection of surface lectin-binding ligands on human cell lines. Acta Histochemica. 2005 doi: 10.1016/j.acthis.2005.10.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PP. Interactions between Rhizobia and lectins of lentil, pea, broad, and jackbean. Plant Physiol. 1980;65:1049–1052. doi: 10.1104/pp.65.6.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RK, Cooper EL. Specificity of carbohydrate inhibition on leopard frog (Rana pipiens) erythocyte agglutinating activity by concanavalin A and phytohemagglutinin. Comp Biochem Physiol B. 1979;63:13–18. [PubMed] [Google Scholar]
- Xin J, Olinger GG, Aris S, Chen Y, Gewurz H, Spear GT. Mannose-binding lectin binds to Ebola and Marburg envelope glycoproteins, resulting in blocking of virus interaction with DC-SIGN and complement-mediated virus neutralization. J Gen Virol. 2005;86:2535–2542. doi: 10.1099/vir.0.81199-0. [DOI] [PubMed] [Google Scholar]


