Summary
This laboratory has developed and used a novel histochemical assay using derivatized agarose beads for over a decade to examine the surface properties of various cell types. Most recently we have used this assay to examine lectin binding ligands on two human cell types, CCL-220, a colon cancer cell line, and CRL-1459, a non-cancer colon cell line. We found that CCL-220 cells bound specific lectins better than CRL-1459, and this information was used to test for possible differential toxicity of these lectins in culture, as a possible approach in the design of more specific anti-cancer drugs. Although we have examined the validity of the bead-binding assay in sea urchin cell systems, we have not validated this technique for mammalian cells. Here the binding results of the bead assay are compared with conventional fluorescence assays, using lectins from 3 species (Triticum vulgaris, Phaseolus vulgaris, and Lens culinaris) on the 2 colon cell lines. These lectins were chosen because they seemed to interact with the two cell lines differently.
Binding results obtained using both assays were compared for frozen, thawed and fixed; cultured and fixed; and live cells. Both qualitative and quantitative fluorescence results generally correlated with those using the bead assay. Similar results were also obtained with all of the 3 different cell preparation protocols. The fluorescence assay was able to detect lower lectin binding ligand levels than the bead assay, while the bead assay, because it can so rapidly detect cells with large numbers of lectin binding ligands, is ideal for initial screening studies that seek to identify cells that are rich in surface binders for specific molecules. The direct use of frozen, thawed, and fixed cells allows rapid mass screening for surface molecules, without the requirement for costly and time consuming cell culture.
Keywords: lectins, fluorescence, derivatized beads, human colon cancer cells, WGA, PHA, LCA
Introduction
Over the past decade, this laboratory has developed and used a novel histochemical assay to examine cell surface properties (Heinrich, et al. 2005; Khurrum, et al. 2002; Navarro et al. 2002; Salbilla et al. 1999; Latham, et al. 1995; 1995a; Latham and Oppenheimer, 1999). This technique involves mixing various cell types to be studied separately with agarose beads derivatized with about 100 different amino acids, sugars, lectins and other proteins, nucleotides and other molecules. Most histochemical assays use stains to identify the biochemical components of cells and tissues. The bead assay uses derivatized beads to do the same thing. If a cell adheres to a bead derivatized with a specific lectin and the adhesion is blocked by the lectin’s preferential binding sugar, such results would indicate that a ligand for that lectin is present on the cell surface.
Many past studies have used derivatized beads to examine cell surface properties (Goldstein et al. 1976; Oketa et al. 1979; Iweg et al. 1980; Takahashi and Tavassoli 1981; Nenci 1983; Pincus 1984; Johnson and Silver 1989; Matsuoka and Tavassoli 1989; Hayes et al. 1990; Kijimoto-Ochiai and Uede 1995), but we are unaware of any studies, other than ours, that use so many different beads to rapidly assay cell surface properties (Khurrum et al. 2002; Navarro et al. 2002; Salbilla et al. 1999; Roque et al. 1996; Latham and Oppenheimer, 1999; Latham et al. 1995; 1995a; Heinrich et al. 2005). Previous studies from our laboratory have involved many unconventional approaches. For example, previously frozen cells that were fixed with formaldehyde were used instead of freshly cultured cells (Khurrum, et al. 2002). Fixation did not alter authentic surface properties (Navarro et al. 2002). In some cases we used live cells in their physiological medium (Latham, et al. 1995; 1995a; Navarro et al. 2002).
Previously experiments were conducted to examine the validity of the bead binding assay using sea urchin embryos and fluorescent (FITC) labeled lectins (Latham, et al. 1995; 1995a). When lectin beads bound to the cells, the FITC lectins also bound. When the lectin beads did not bind, neither did the FITC labeled lectins bind (Latham et al. 1995, 1995a). In both cases, hapten sugars inhibited lectin binding to the cells. These results led to the conclusion that the bead assay was a valid approach to examining cell surface properties such as lectin binding ligands on the cell types tested.
Live sea urchin cells in sea water, however, do not present the same sort of challenge as other cell types under other conditions. Because of the usefulness and rapidity of the bead assay, it is important to examine its validity with other cell types under other conditions. In this study, we examine lectin binding ligands on two human colon cell lines: CCL-220, a colon cancer cell line and CRL-1459, a non-cancer colon cell line using both the bead and conventional fluorescence assays. We also examined binding using both assays and 3 cell preparation protocols: (1) frozen, thawed and fixed cells assayed in distilled water, (2) cultured and fixed cells assayed in distilled water, and (3) live cells assayed in saline.
The main purpose and novelty of this study, therefore, is to determine if the conventional fluorescence assay gives similar results as the unconventional bead assay, as a means of validating the use of the bead assay in mammalian cell systems. In addition, the study explores which cell preparation procedure is best for the most rapid detection of specific cell surface molecules.
Material and Methods
Cell lines
Two human cell lines, colon cancer cells tumorigenic in nude mice (CCL-220/Colo320DM) and non-cancerous colon cells (CRL-1459 /CCD-18Co) were obtained frozen from the American Type Culture Collection (ATCC, Manassas, VA). In some experiments the frozen ATCC cells were directly used. In those experiments, cells were thawed in a 37 °C water bath for 40–60 seconds, washed by centrifugation for 3 minutes at 1000 x g in 1.5 ml Dulbecco’s phosphate buffered saline with calcium and magnesium chloride (PBS; Sigma, St. Louis, Missouri, U.S.A.), and fixed in 1% formaldehyde in PBS for at least 30 minutes. Prior to use, the fixed cells were washed thrice by centrifugation at 1000 x g for 3 minutes in 1.5 ml of distilled water. Cell suspensions were then diluted by adding 8.5 ml of distilled water yielding 10 ml aliquots with a final cell concentration of approximately 1 million cells per ml (Khurrum et al., 2002).
Other cells were cultured in 75 cm2 closed Falcon Flasks (Fisher Scientific, Tustin, CA) using RPMI 1640 cell culture medium (Fisher Scientific, Hampton, NH) with L-glutamine, supplemented with 10% heat-inactivated fetal bovine serum and 2.5g/l of glucose at 37°C and 5% CO2 atmosphere (Heinrich et al., 2005). All cell lines were maintained without the use of antibiotics. All reagents were of tissue culture grade and were obtained from Sigma Chemical Co (St. Louis, MO) unless otherwise indicated.
Subculturing
CCL-220
CCL-220 cells grew in suspension with a small percentage of adherent cells, and were subcultured by the following method. Suspended cells and medium were removed from the flask and added to a 15 ml conical plastic centrifuge tube. Adherent cells were rinsed with 2 ml Dulbecco's phosphate buffered saline (PBS - without CaCl2 and MgCl2) prior to using 1 ml 0.25% trypsin-EDTA solution to detach adherent cells. Adherent cells were incubated at 37°C with trypsin-EDTA for 3 minutes, and the flask rinsed with 2 ml RPMI 1640 medium to remove cells. This rinse was added to the centrifuge tube. Cells were pelleted at 28 x g for 3 minutes and washed once in 3 ml PBS, resuspended in 1 ml trypsin-EDTA and incubated for 3 minutes to create a single cell suspension. 2 ml of RPMI 1640 medium were added to inhibit further activity of the trypsin-EDTA solution. Cells were pelleted by 3 minutes centrifugation at 78 x g, resuspended in 3 ml RPMI 1640 medium for counting and subculturing. Cells were counted using a hemacytometer (Hausser Scientific, Horsham, PA) and cell viability was determined using trypan blue exclusion. All cultures used exhibited viability of 90% or higher. New cultures were seeded at 5 X 105 cells/ml (Heinrich et al. 2005).
CRL-1459
CRL-1459 grew as adherent cells and were harvested from the flask after removing the medium. The monolayer was then rinsed with 2 ml PBS which was removed and discarded. 1 ml 0.25% trypsin-EDTA solution was added to the monolayer to detach cells. The cells were incubated at 37 °C with trypsin-EDTA for 3 minutes. The flask was rinsed with 2 ml RPMI 1640 medium to remove cells and inhibit further activity of the trypsin-EDTA solution. This rinse was added to a 15 ml conical plastic centrifuge tube. Cells were washed, resuspended, counted and transferred as above. All cultures used exhibited viability of 90% or higher. The use of trypsin in this and most studies may alter cell surface molecules. Here, however, it will be shown that very different cell preparation protocols yield similar results, suggesting that factors such as trypsinization do not significantly affect lectin binding ligands.
Bead Assay
Fixing Cultured Cells
CCL-220 and CRL-1459 were cultured and removed from flasks following the subculturing protocol. Upon removal, cells were centrifuged at 113 x g for 3 minutes to remove media and resuspended in 1 ml serum-free RPMI 1640 medium. The cells were then washed 3 times with PBS (without CaCl2 and MgCl2) and centrifuged for 3 minutes each time before being fixed in 1% formaldehyde in PBS for 45 minutes at room temperature. Fixed cells were washed 3 times in distilled water by centrifugation as described previously and resuspended in 1 ml distilled water. The cells were used within 3 days of fixation.
Lectin Beads
Lectins used in the histochemical bead assay were: wheat germ agglutinin – (WGA), Phaseolus vulgaris agglutinin – (PHA), and Lens culinaris agglutinin – (LCA). These lectins were chosen because they appeared to interact differently with the different cell lines under investigation. We found that the two isolectins of PHA, PHA-E and PHA-L, gave similar results (data not shown), so both of these lectins were used here interchangeably depending upon availability. The lectin bound agarose beads were obtained from Sigma and were washed 3 times by centrifugation at 1000 x g with distilled water suspended at a concentration of 3mg/ml and stored at 4 °C before use. All beads were used within 3 days of preparation.
Sugars
Haptenic sugars are those to which lectins preferentially bind. Non-haptenic sugars are those to which lectins do not preferentially bind. The haptenic sugars (Sigma, 0.2M in distilled water)included: N-acetyl-D-glucosamine for Triticum vulgaris agglutinin, D (+)-glucosamine and N-acetyl-D-glucosamine for Phaseolus vulgaris agglutinin and α-methyl mannose for Len culinaris agglutinin. L-fucose served as a non-haptenic sugar for the 3 lectins (Liener, et al., 1986). In the case of PHA, monosaccharides not usually considered effective happen inhibitors. In this system, however, inhibition of lectin-cell binding was observed using the monosaccharides cited.
Haptenic Inhibition
Slides were prepared in triplicate containing droplets of 40 μl of distilled water, distilled water with a haptenic sugar (0.2M) or distilled water with a non-haptenic sugar (0.2M). Approximately 4 μg of washed lectin beads were added to the droplets on the slide and gently swirled using a toothpick for 1 minute to give the lectin beads and sugar time to interact. This was followed by the addition of 4 μl of washed fixed cells suspended in distilled water. The cell-lectin bead suspension was also gently swirled for 1 minute and observed using a light microscope at 100 X and 200 X magnification to determine if binding was present between cells and beads. Positive or negative binding was recorded. Binding was confirmed by agitating the slides to ascertain if cells touching beads were or were not bound to the beads.
Fluorometry and Fluorescence Quantification Study
A Turner Designs model 700 laboratory fluorometer equipped with a blue mercury vapor source, 486/510–700nm excitation/emission filters and minicell adapter (Sunnyvale, CA) was used to measure fluorescence emission. Lectins from Lens culinaris agglutinin (LCA, Sigma L-9262), Phaseolus vulgaris agglutinin (PHA, EY Labs F-1802–5) and Triticum vulgaris agglutinin (WGA, Sigma L-4895), derivatized with fluorescein 5(6)-isothiocyanate (FITC) were used in fluorescence experiments.
The fluorometer was calibrated using a TD-700 FITC solid standard (Turner Designs, TD-7000–993) with distilled water as the blank. Fluorescence of samples was determined relative to the standard set as 80% of maximum sensitivity and expressed as raw fluorescence units relative to the standard (relative fluorescence in the graphs).
The background autofluorescence emission of each 230 μl sample of cells was measured prior to the addition of the each lectin conjugated to FITC using cuvettes pre-cleaned with 70% ethanol. FITC labeled lectin (0.5mg) was resuspended in 50 μl distilled water and mixed with the fixed cells for 1 minute. An initial fluorescence emission reading was taken and the cells and FITC labeled lectin were then covered in aluminum foil to prevent any photobleaching and incubated overnight at 37 °C. A fluorescence emission reading was taken following the overnight incubation and afterwards the cells were washed with 1 ml of distilled water by centrifugation for 3 minutes at 1000 x g. The supernatant was removed and cells were resuspended in 0.75 ml of distilled water for 1 minute prior to taking a fluorescence emission reading. This washing procedure was repeated 4 times. A fluorescence emission reading and a digital fluorescence photograph of the cells were taken after each wash. The binding study was repeated six times for each lectin with the CCL-220 cultured and fixed cells and the CCL-220 ATCC frozen thawed and fixed cells. This binding study was also repeated with each lectin for the cultured and fixed CRL-1459 line.
A control experiment was performed in order to account for any free FITC labeled lectin or free FITC in excess that may have been carried over in the solution or adhered to the glass cuvette that could artificially increase the fluorescence emission. This experiment was conducted in cell-free distilled water with only FITC labeled lectin added. The emission of free lectin was subtracted from the total emission. This control was carried out for all three lectins in exactly the same way as with samples containing cells.
Another control experiment was done in order to quantify the autofluorescence of the cells alone that could artificially increase the fluorescence emission. The autofluorescence control without FITC labeled lectin was carried out in exactly the same way as the experimental samples.
FITC Labeled Lectin and Haptenic Inhibition Study
The background autofluorescence emission of each 230 μl sample of cells in distilled water was measured prior to the addition of each lectin derivatized with FITC suspended in 50 μl distilled water and equal molar concentrations of either its specific haptenic sugar or a non-haptenic sugar, as indicated previously. Cells were treated and fluorescence readings taken with the appropriate controls as cited previously. Final concentration of sugars was 0.2M.
Qualitative fluorescence microscopy was also used to examine cell fluorescence at 1000 X magnification. Cells were digitally photographed under normal light and UV light. Samples were washed with 1 ml of distilled water and centrifuged for 3 minutes to obtain a pellet. After removing the supernatant, the pellet was resuspended in distilled water for 1 minute. A 20 μl sample was viewed on a slide and photographed at 1000 X magnification. This was repeated until 4 washes were completed.
Titration Study
Six different concentrations of FITC labeled lectin were used to find the minimum amount of FITC labeled lectin required to produce visually fluorescent cells at 1000 X magnification. Samples of FITC labeled LCA, PHA or WGA at 0.5, 1.0, 5.0, 10.0, 50.0, and 100.0 ng per milliliter were incubated with cells (90 μl) bringing the total volume to 100 μl.
Sodium Azide Live Cell Study
Live cells were incubated with a low concentration (0.05%) of sodium azide to reduce internalization of labeled lectins, degradation of labeled lectins or other processes that require cellular energy and may interfere with surface-labeling of live cells (Stackpole et al. 1974).
Cancer Cell Line CCL-220
Live colon cancer cells CCL-220 were grown in the tissue culture flask and split using the standard trypsinization procedure. The RPMI 1640 medium was removed and the live cells were washed with 0.05% sodium azide in PBS. The cells were incubated at 37 °C with 0.05% sodium azide in PBS and 50.0 ng FITC labeled LCA, PHA or WGA in four trials for 1 hour in separate centrifuge tubes. After incubation, cells were washed by centrifugation once with PBS. 20 μl of cells were placed on a slide with a coverslip and viewed at 1000 X magnification. This study was repeated four times with each lectin.
Normal Colon Cell Line CRL-1459
Cells were trypsinized and removed from the tissue culture flask, and plated on a slide with removable chambers in serum free media. The slides were incubated for 24 hours to allow the cells to adhere. Cells were then washed with 0.05% sodium azide in PBS and incubated at 37 °C in 0.05% sodium azide and PBS and 50.0 ng FITC labeled lectin (WGA, LCA, and PHA) for 1 hour, each in separate chambers. The chambers were removed and cells were washed by dipping the slide in a solution of PBS. The plated cells were viewed with a coverslip at 1000 X magnification. This study was repeated four times with each labeled lectin.
Photography
Cells in the bead binding and fluorescence study were photographed digitally at 400 X or 1000 X magnification using a Zeiss (Oberkochen, Germany) Axiolab fluorescence microscope. In the fluorescence experiments photographs were taken in bright field and UV light. An optical filter XF-100 (Omega Optical, Brattleboro, VT) was used for the FITC fluorescence studies.
Results
Figure 1A shows that cultured and fixed CCL-220 cells adhered to beads derivatized with LCA. Figure 1B shows that CRL-1459 cells did not bind to LCA beads. Figure 2A shows that cultured and fixed CCL-220 cells adhered to beads derivatized with PHA. Figure 2B shows that the CRL-1459 cell did not bind to the PHA beads. Figure 2C shows that cultured and fixed CCL-220 cells did not bind to PHA beads in the presence of 0.2M D-glucosamine, while they did bind to PHA beads in the presence of 0.2M L-fucose (Fig. 2D). Cultured and fixed CCL-220 cells adhered to WGA beads. Cultured and fixed CRL-1459 did not bind to WGA beads (data not shown). In all of these experiments, similar results were obtained in 6 repetitions of each test.
Figure 1.
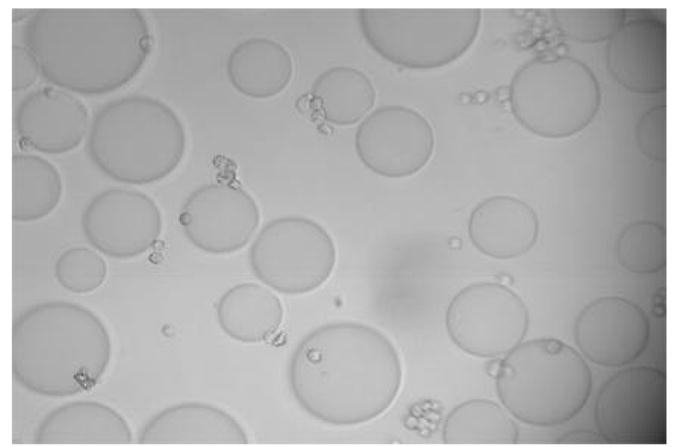
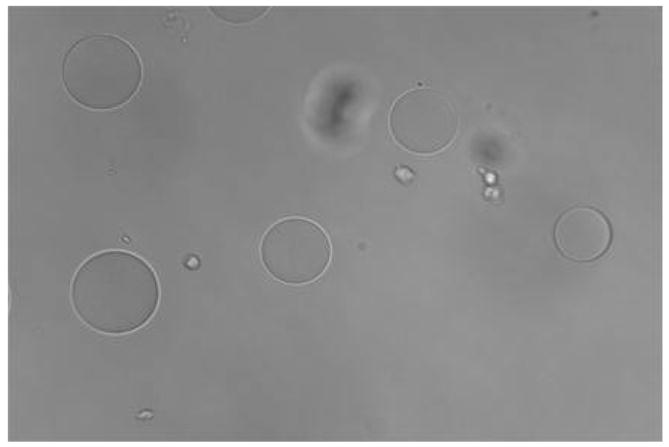
Figure 1a. Positive binding of cultured and fixed colon cancer cells (CCL-220) to agarose beads derivatized with Lens culinaris agglutinin (LCA) in distilled water. Larger spheres are the beads, smaller spheres are the cancer cells. Viewed at magnification 200X.
Figure 1b. Negative binding of cultured and fixed colon non-cancer cells (CRL-1459) to agarose beads derivatized with Lens culinaris agglutinin (LCA) in distilled water. Larger spheres are the beads, smaller spheres are the cancer cells. Viewed at magnification 200X.
Figure 2.
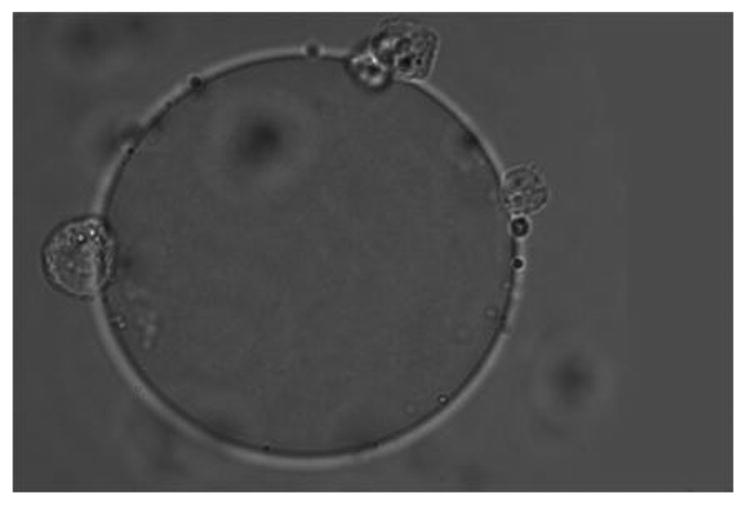
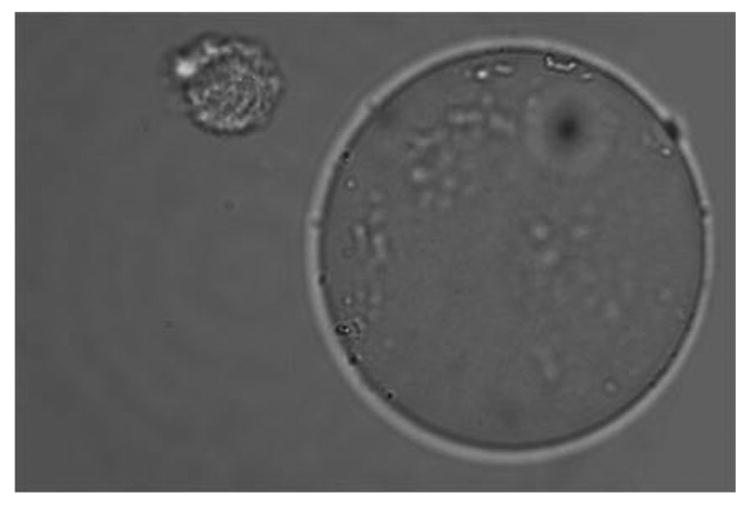
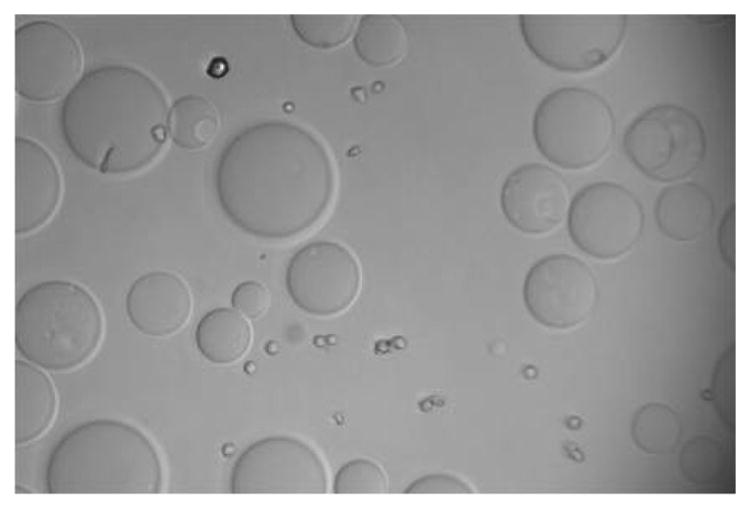
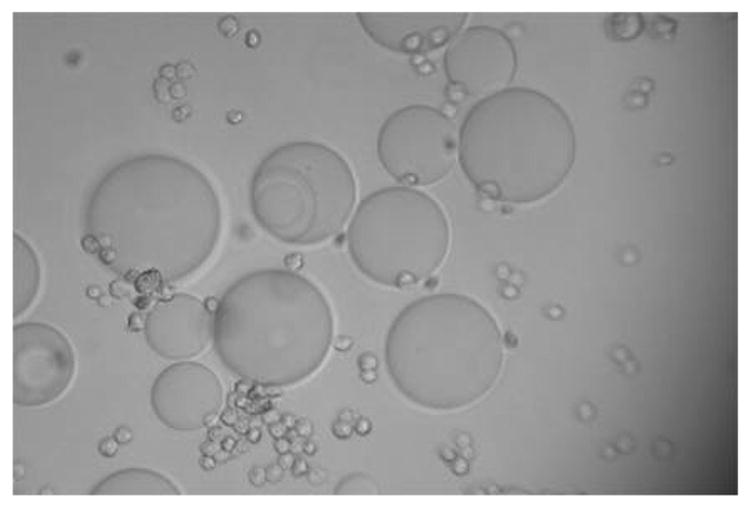
Figure 2a. Positive binding of cultured and fixed colon cancer cell (CCL-220) to an agarose bead derivatized with Phaseolus vulgaris agglutinin (PHA) in distilled water. Larger sphere is the bead, smaller spheres are the cancer cells. Viewed at magnification 1000X.
Figure 2b. Negative binding of cultured and fixed colon non-cancer cell (CRL-1459) to an agarose bead derivatized with Phaseolus vulgaris agglutinin (PHA) in distilled water. Larger sphere is the bead, smaller sphere is the cancer cell. Viewed at magnification 1000X.
Figure 2c. Negative binding of cultured and fixed colon cancer cells (CCL-220) to agarose beads derivatized with Phaseolus vulgaris agglutinin (PHA) and 0.2M D-glucosamine in distilled water. Larger spheres are the beads, smaller spheres are the cancer cells. Viewed at magnification 200X.
Figure 2d. Positive binding of cultured and fixed colon cancer cells (CCL-220) to agarose beads derivatized with Phaseolus vulgaris agglutinin (PHA) and 0.2M L-fucose in distilled water. Larger spheres are the beads, smaller spheres are the cancer cells. Viewed at magnification 200X.
In all cases where bead binding occurred, the haptenic sugar inhibited binding, while the non-haptenic sugar (L-fucose) did not. Cultured and fixed CCL-220 cells always bound to the 3 types of lectin beads while cultured and fixed CRL-1459 cells did not (as found for frozen, thawed and fixed cells in Khurrum et al. 2002).
Figures 3 and 4 illustrate sample fluorescence micrographs of some of the results that are summarized in Table 1. The purpose of this study is not to examine each and every possible combination of lectin and preparation method using both assays, but to test a sufficient number of such combinations to obtain reliable information on the assays and cell preparation procedures. Bright field images were always examined to confirm that cells were present in all fluorescence experiments. Where the term ‘data not shown’ is used, it was felt that just showing countless near identical fluorescence images was inappropriate.
Figure 3.
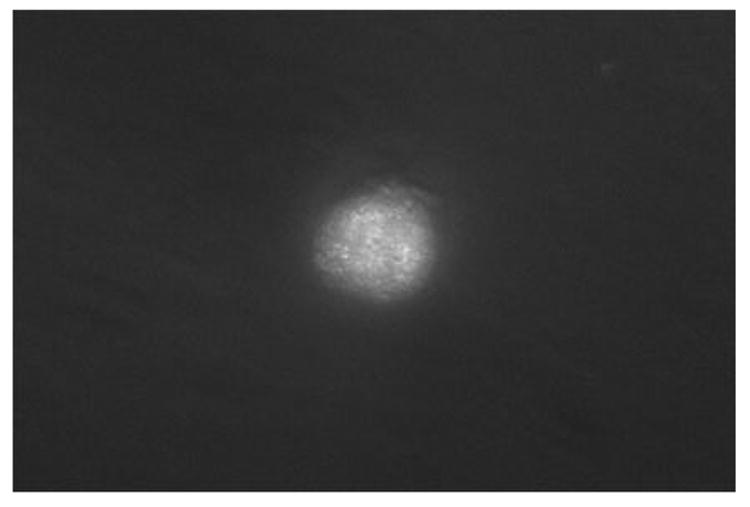
Fluorescence image of cultured and fixed colon cancer cell (CCL-220) stained with FITC labeled Phaseolus vulgaris agglutinin (PHA). Viewed at magnification 1000X.
Figure 4.

Fluorescence image of ATCC frozen, thawed and fixed colon cancer cell (CCL-220) stained with FITC labeled Lens culinaris agglutinin (LCA). Viewed at magnification 1000X.
Table 1.
Percent Fluorescing CCL-220 and CRL-1459 cells following various treatments using 3 FITC labeled lectins. A total of 380 cells were examined. Frozen CRL-1459 cells were unavailable.
| % Fluorescing CCL-220 Cells | % Fluorescing CRL-1459 Cells | ||||
|---|---|---|---|---|---|
| Cultured | Frozen | Live | Cultured | Live | |
| FITC Lectin | |||||
| LCA | 96 | 90 | 60 | 36 | 41 |
| PHA | 90 | 99 | 80 | 47 | 44 |
| WGA | 91 | 96 | 100 | 41 | NA |
CRL-1459 fluorescence was much less intense than CCL-220 fluorescence. Cultured= cultured, fixed. Frozen=frozen, thawed, fixed. Live=unfixed. NA=Not done, cells unavailable. Details in Material and Methods. % Fluorescing cells= (number of fluorescing cells/total number of cells) x 100.
Figure 3 is a fluorescence image showing that FITC labeled PHA bound to a cultured and fixed CCL-220 cell. Similar results were obtained in 6 repeated experiments. Figure 4 is a fluorescence image showing that FITC labeled LCA bound to a frozen, thawed and fixed CCL-220 cell. Similar results were obtained in 6 repeated experiments. FITC conjugated WGA adhered to the cultured, fixed CCL-220 line and the haptenic sugar (0.2M N-acetyl-D-glucosamine) inhibited this binding, while 0.2M L-fucose did not (data not shown). Similar results were obtained in 6 repeated experiments. FITC conjugated WGA bound to live CCL-220 cells (data not shown). Similar results were obtained in 6 repeated experiments.
Figure 5 is a titration curve that shows substantial increases in fluorescence emission occurring for the FITC labeled lectins between 50ng and 100ng of FITC conjugated lectin per ml, with the most dramatic increases occurring with WGA and LCA, with cultured and fixed CCL-220 cells. Preliminary experiments indicated that 2 washes were optimal for removing unattached fluorescent lectins and maintaining high cell-specific fluorescence (data not shown). Figure 6 shows that under the conditions of the assay, for cultured and fixed CCL-220 cells, the fluorescence emission of FITC labeled WGA was about twice as great as that for FITC labeled PHA, while FITC labeled PHA was greater than FITC labeled LCA, but the error bars were overlapping.
Figure 5.
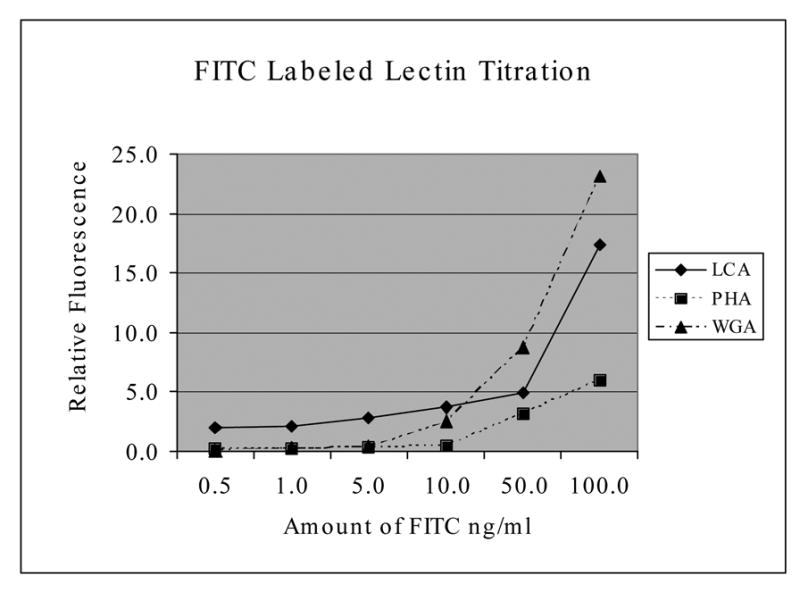
FITC labeled lectin titration. Shows relative fluorescence emission of cultured and fixed CCL-220 cells with increasing concentrations of FITC labeled lectins after 2 washes. Results based on 3 trials. Fluorescence on the Y axis is in relative units
Figure 6.
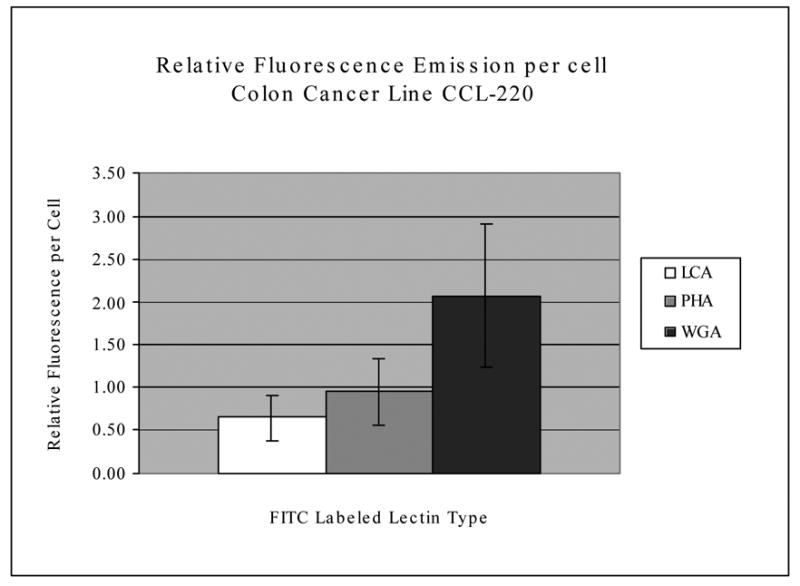
Relative fluorescence emission per CCL-220 cell incubated with FITC labeled lectins. Raw fluorescence was divided by the number of cells per sample to get the relative fluorescence per cell. Average readings based upon 6 separate samples. Bars indicate standard error of the mean. Fluorescence on the Y axis is in relative units.
Table 1 shows that cultured and fixed, frozen-thawed and fixed and live CCL-220 cells displayed similar percentages of fluorescing cells with the 3 FITC labeled lectins, while cultured and fixed and live CRL-1459 both displayed reduced percentages of fluorescing cells with the lectins tested. The CCL-220 cells, no matter what the conditions of the preparation, showed similar percent fluorescence. The same was true for the CRL-1459 cells. The results in Table 1 were based on 6 repeated experiments for each of the cultured and frozen-thawed cell experiments, and 3 repeated experiments for each of the live cell experiments. When CRL-1459 cells were found to fluoresce, fluorescence was markedly less intense with all lectins tested than found for the CCL-220 cells. CRL-1459 fluorescence was barely visible under the microscope and did not register well on any photographs. The results, however, suggest that the fluorescence assay can detect low quantities of cell surface lectin receptors.
Discussion
Numerous studies have examined the effects of lectins on cancer cells (Minko, 2003; Gabor, et al. 1998; 2002; 2004; Wirth, et al. 1998;2002; Kiss, et al. 1997; Valentiner, et al. 2002; 2003, Bangchonglikitbul, 2002; Lorea, et al., 1997; Lityńska, et al. 2001; Schwarz, et al. 1999) and some studies report use of lectins in supplemental cancer therapy in humans (Fritz, et al., 2004; Thies, et al., 2005). Few studies have screened cancer cells and their normal counterparts for binding compounds such as lectins in order to determine if compounds can be identified that differentially bind to cancer cells and minimally to their normal counterparts (Bakalova and Ohba, 2003; Heinrich, et al., 2005). This is important especially in light of the findings that lectins need to bind to cells so that they can be internalized by the cells to exert their toxic effects (Gorelick, et al., 2001; Wirth, et al., 2002; Gabor, et al., 1998; 2002; 2004; Schwarz, et al. 1999; Kim, et al., 1993).
Past studies have shown that human colon cancer cells bind better to specific lectins than do their normal counterparts, using the bead binding assay (Khurrum, et al., 2002; Heinrich, et al., 2005) and that lectins that bind better to the cancer cells are more toxic to these cells under certain culture conditions and incubation times (Heinrich, et al. 2005). Those studies, however, used only lectin bead binding and cultured cancer cells. Here, we expand the study to ascertain if conventional fluorescent-lectin binding techniques, which are more time consuming than the bead assay, validate the bead-binding results. We show that the quickest of all approaches to screen cells for binding affinity, namely the use of frozen, thawed and fixed cells provides similar results to using cultured and fixed cells (Khurrum, et al., 2002) with both the bead and fluorescence assays. This is important because frozen, thawed and fixed cells can be used in hundreds of binding affinity assays in minutes and do not depend upon continuous cell culture. This is of benefit for research purposes since thousands of compounds, therefore, can be rapidly screened for differential binding to cancer and non-cancer cells for possible development of new anticancer drugs or drug delivery agents based on binding affinities. This result also supports the possibility that this technique can be used in the future for diagnostic purposes using frozen thawed cell and tissue samples.
This study therefore shows that cultured and fixed cells; frozen, thawed and fixed cells; and live cells all display similar lectin binding characteristics, and also helps explain and validate some important past findings. All previous studies using only the bead assay suggested that only CCL-220 cells bound the lectins studied, while CRL-1459 did not. Assuming that lectins need to bind to cells, to be internalized, and exert their toxic effects (Gorelick, et al., 2001; Wirth, et al., 2002; Gabor, et al., 1998; 2002; Schwarz, et al., 1999; Kim, et al., 1993), bead binding studies could not explain why CRL-1459 cells were also killed in culture (to a lesser extent at some incubation times) by the lectins studied (Heinrich, et al. 2005). This work illustrates that the bead assay is useful for identifying cells with large numbers of lectin binding ligands, but may not pick up cells with low ligand numbers. The fluorescence assay is more sensitive than the bead assay and appears able to pick up cells with fewer ligands, suggesting that lectin binding ligands on CRL-1459 are reduced not totally absent.
The results of this and past studies combine to provide a detailed picture of the nature of the bead assay. Many years ago it was found that beads derivatized with the same compound sometimes behaved very differently (Salbilla, et al. 1999), and depend upon the nature of the spacers that separate the derivatized compound from the surface of the bead. The number of spacers can vary widely, leading to different distances that the derivatized compounds are from the bead surface. This influences the ability of a bead to bind to a cell as would the density of derivatized compounds on the bead surface (Salbilla, et al., 1999). We have studied the spacer problem allowing the prediction of possible problems with certain types of beads that can be excluded from experiments (Salbilla, et al., 1999).
Optimization of experimental parameters is not confined to the bead assay. We, for example, showed here that the fluorescence assay also must be carefully designed so that there is enough of the FITC derivatized lectin to provide detectible fluorescence emission, as illustrated by the titration study. In addition, the fluorescence assay requires numerous controls as in the experiments described here. The validity of both the bead and the fluorescence assays can be confirmed by hapten inhibition experiments.
It is clear, therefore, that each assay has distinct advantages and disadvantages. These have been thoroughly described in this study, improving our understanding of the nuances associated with these assays and the nuances that must be considered to effectively use them in various experimental systems. It is this comparative in depth examination of the nuances of both assays that make this study novel and useful. In addition, this study provides evidence that the use of frozen, thawed and fixed cells along with the bead assays is the most rapid mass screening approach to identify various cell surface properties.
Acknowledgments
This work was supported by grants from NIH NIGMS SCORE (GM-48680), NIH NIGMS RISE (GM-63787), MARC (GM-08395), and the Joseph Drown Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aketa K, Yoshida M, Miyasaki S, Ohta T. Sperm binding to an egg model composed of agarose beads. Experimental Cell Research. 1979;123:281–284. doi: 10.1016/0014-4827(79)90469-5. [DOI] [PubMed] [Google Scholar]
- Bakalova R, Ohba H. Interaction of soybean agglutinin with leukemic T-cells and its use for their in vitro separation from normal lymphocytes by lectin-affinity chromatography. Biomedical Chromatography. 2003;17:239–49. doi: 10.1002/bmc.218. [DOI] [PubMed] [Google Scholar]
- Banchonglikitkul C, Smart JD, Gibbs RV, Donovan SJ, Cook DJ. An in vitro evaluation of lectin cytotoxicity using cell lines derived from the ocular surface. Journal of Drug Targeting. 2002;10:601–6. doi: 10.1080/1061186021000060747. [DOI] [PubMed] [Google Scholar]
- Fritz P, Dippon J, Kierschke T, Siegle I, Mohring A, Moisa A, Murdter TE. Impact of mistletoe lectin binding in breast cancer. Anticancer Research. 2004;24:1187–92. [PubMed] [Google Scholar]
- Gabor F, Bogner E, Weissenboeck A, Wirth M. The lectin-cell interaction and its implications to intestinal lectin-mediated drug delivery. Advanced Drug Delivery Reviews. 2004;56:459–80. doi: 10.1016/j.addr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Gabor F, Klausegger U, Wirth M. The interaction between wheat germ agglutinin and other plant lectins with prostate cancer cells Du-145. International Journal of Pharmaceutics. 2001;221:35–47. doi: 10.1016/s0378-5173(01)00650-0. [DOI] [PubMed] [Google Scholar]
- Gabor F, Schwarzbauer A, Wirth M. Lectin-mediated drug delivery: binding and uptake of BSA-WGA conjugates using the Caco-2 model. International Journal of Pharmaceutics. 2002;237:227–39. doi: 10.1016/s0378-5173(02)00049-2. [DOI] [PubMed] [Google Scholar]
- Gabor F, Stangl M, Wirth M. Lectin-mediated bioadhesion: Binding characteristics of plant lectins on the enterocyte-like cell lines Caco-2, HT-29 and HCT-8. Journal of Controlled Release. 1998;55:131–42. doi: 10.1016/s0168-3659(98)00043-1. [DOI] [PubMed] [Google Scholar]
- Gorelik E, Galili U, Raz A. On the role of cell surface carbohydrates and their binding proteins (lectins) in tumor metastasis. Cancer and Metastasis Reviews. 2001;20:245–77. doi: 10.1023/a:1015535427597. [DOI] [PubMed] [Google Scholar]
- Hayes DF, Silberstein DS, Rodrique SW, Kufe DW. DF3 antigen, a human epithelial cell mucin, inhibits adhesion of eosinophils to antibody-coated targes. Journal of Immunology. 1990;145:962–970. [PubMed] [Google Scholar]
- Heinrich EL, Welty LY, Banner LR, Oppenheimer SB. Direct targeting of cancer cells: a multiparameter approach. Acta Histochemica. 2005 doi: 10.1016/j.acthis.2005.06.013. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwig M, Lasch J, Glaesser D. Growth regulation of lens epithelial cells. Chemcially modified sepharose as a suitable substratum for studying cell-substratum interactions. Cell Diff. 1980;9:1–12. doi: 10.1016/0045-6039(80)90002-0. [DOI] [PubMed] [Google Scholar]
- Johnson KE, Silver MH. Cells from Rana pipiens gastrulate and arrested hybrid gastrulae show differences in adhesion to fibronectin-sepharose beads. Journal of Experimental Zoology. 1989;251:155–166. doi: 10.1002/jez.1402510205. [DOI] [PubMed] [Google Scholar]
- Khurrum MR, Weerasinghe GR, Soriano ES, Riman R, Badali O, Gipson S, Medina J, Alfaro J, Navarro VM, Harieg CB, Ngo L, Sakhakorn T, Kirszenbaum L, Khatibi D, Abedi K, Barajas M, Zem G, Kirszenbaum A, Razi A, Oppenheimer SB. Analysis of surface properties of human cancer cells using derivatized beads. Acta Histochemica. 2002;104:217–23. doi: 10.1078/0065-1281-00656. [DOI] [PubMed] [Google Scholar]
- Kijimoto-Ochiai S, Uede T. CD23 molecule acts as a galactose-binding lectin in the cell aggregation of EBV-transformed human B-cell lines. Glycobiology. 1995;5:443–448. doi: 10.1093/glycob/5.4.443. [DOI] [PubMed] [Google Scholar]
- Kim M, Rao MV, Tweardy DJ, Prakash M, Galili U, Gorelik E. Lectin-induced apoptosis of tumor cells. Glycobiology. 1993;3:447–53. doi: 10.1093/glycob/3.5.447. [DOI] [PubMed] [Google Scholar]
- Kiss R, Camby I, Duckworth C, De Decker R, Salmon I, Pasteels J-L, Danguy A, Yeaton P. In vitro influence of Phaseolus vulgaris, Griffonia simplicifolia, Concanavalin A, wheat germ, and peanut agglutinins on HCT-15, LoVo, and SW837 human colorectal cancer cell growth. Gut. 1997;40:253–61. doi: 10.1136/gut.40.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham V, Ducut J, Rostamiani K, Chun H, Lopez M, Herrera S, Oppenheimer S. A rapid lectin receptor binding assay: comparative evaluation of sea urchin embryo cell surface lectin receptors. Acta Histochemica. 1995;97:89–97. doi: 10.1016/S0065-1281(11)80209-6. [DOI] [PubMed] [Google Scholar]
- Latham V, Herrera S, Rostamiani K, Chun H, Oppenheimer S. Rapid identification of lectin receptors and their possible function in sea urchin cell systems. Acta Histochemica. 1995a;97:373–382. doi: 10.1016/S0065-1281(11)80062-0. [DOI] [PubMed] [Google Scholar]
- Latham V, Oppenheimer S. A simple image analysis method for evaluating cell binding to derivatized beads. Acta Histochemica. 1999;101:263–270. doi: 10.1016/S0065-1281(99)80027-0. [DOI] [PubMed] [Google Scholar]
- Liener IE, Sharon N, Goldstein IJ, editors. The Lectins. Academic Press; Orlando: 1986. pp. 33–247. [Google Scholar]
- Lityńska A, Przybylo M, Pochec E, Hoja-Lukowicz D, Cloczyk D, Laidler P, Gill D. Comparison of the lectin-binding pattern in different human melanoma cell lines. Melanoma Research. 2001;11:205–12. doi: 10.1097/00008390-200106000-00001. [DOI] [PubMed] [Google Scholar]
- Lorea P, Goldschmidt D, Darro F, Salmon I, Bovin N, Gabius HJ, Kiss R, Danguy A. In vitro characterization of lectin-induced alterations of the proliferative activity of three human melanoma cell lines. Melanoma Research. 1997;7:353–63. doi: 10.1097/00008390-199710000-00001. [DOI] [PubMed] [Google Scholar]
- Matsuoka T, Tavassoli M. A minibead method for detection of membrane lectins. Journal of Histochem Cytochem. 1989;37:91–96. doi: 10.1177/37.1.2908884. [DOI] [PubMed] [Google Scholar]
- Matsuoka T, Tavassoli M. Electron microscopic identification of hemopoietic progenitor cells by exploiting their sugar-recognizing receptors using a newly developed mini-bead technique. Experimental Hematology. 1989a;17:326–329. [PubMed] [Google Scholar]
- Minko T. Drug targeting to the colon with lectins and neoglycoconjugates. Advanced Drug Delivery Reviews. 2003;56:491–509. doi: 10.1016/j.addr.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Walker SL, Badali O, Abundis M, Ngo LL, Weerasinghe G, Barajas M, Zem G, Oppenheimer SB. Analysis of surface properties of fixed and live cells using derivatized agarose beads. Acta Histochemica. 2002;104:99–106. doi: 10.1078/0065-1281-00617. [DOI] [PubMed] [Google Scholar]
- Nenci I. Specific cell adhesion to estradiol-derivatized agarose beads. Journal of Steroid Biochemistry. 1983;19:109–111. [PubMed] [Google Scholar]
- Pincus SH. Eosinophil-particle interactions: a model system for study of cellular adherence and activation. Cell Immunology. 1984;86:460–471. doi: 10.1016/0008-8749(84)90401-5. [DOI] [PubMed] [Google Scholar]
- Salbilla BA, Vaghefi H, Chhabra P, Hall G, Brown D, Sadoughi F, Francisco E, Attas L, Walker S, Nguyen BN, Oppenheimer SB. Analysis of cell surface properties using derivatized agarose beads. Acta Histochemica. 1999;101:271–9. doi: 10.1016/s0065-1281(99)80028-2. [DOI] [PubMed] [Google Scholar]
- Schwarz RE, Wojciechowicz DC, Picon AI, Schwarz MA, Paty PB. Wheat germ agglutinin-mediated toxicity in pancreatic cancer cells. British Journal of Cancer. 1999;80:1754–62. doi: 10.1038/sj.bjc.6690593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackpole CW, Jacobson JP, Lardis MP. Two distinct types of capping of surface receptors on mouse lymphoid cells. Nature. 1974;248:232–234. doi: 10.1038/248232a0. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tavassoli M. A minibead method to study cell surface receptors for various proteins coupled to latex particles. Journal of Ultrastruct Res. 1981;75:205–217. doi: 10.1016/s0022-5320(81)80136-0. [DOI] [PubMed] [Google Scholar]
- Thies A, Nugel D, Pfuller U, Moll I, Schumacher U. Influence of mistletoe lectin and cytokines induced by them on cell proliferation of human melanoma cells in vitro. Toxicology. 2005;207:105–16. doi: 10.1016/j.tox.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Valentiner U, Fabian S, Schumacher U, Leathem A. The influence of dietary lectins on the cell proliferation of human breast cancer cell lines in vitro. Anticancer Research. 2003;23:1197–206. [PubMed] [Google Scholar]
- Valentiner U, Pfuller U, Baum C, Schumacher U. The cytotoxic effect of mistletoe lectins I, II and III on sensitive and multidrug resistant human colon cancer cell lines in vitro. Toxicology. 2002;171:187–99. doi: 10.1016/s0300-483x(01)00581-9. [DOI] [PubMed] [Google Scholar]
- Wirth M, Fuchs A, Wolf M, Ertl B, Gabor F. Lectin-mediated drug targeting: Preparation, binding characteristics, and antiproliferative activity of wheat germ agglutinin conjugated doxorubicin on Caco-2 cells. Pharmaceutical Research. 1998;15:1031–7. doi: 10.1023/a:1011926026653. [DOI] [PubMed] [Google Scholar]
- Wirth M, Kneuer C, Lehr CM, Gabor F. Lectin-mediated drug delivery: Discrimination between cytoadhesion and cytoinvasion and evidence for lysosomal accumulation of wheat germ agglutinin in the Caco-2 model. Journal of Drug Targeting. 2002;10:439–48. doi: 10.1080/1061186021000038300. [DOI] [PubMed] [Google Scholar]


