SUMMARY
Lectins have been widely used in cell surface studies and in the development of potential anti-cancer drugs. Many past studies that have examined lectin toxicity have only evaluated the effects on cancer cells, not their non-cancer counterparts. In addition, few past studies have evaluated the relationship between lectin-cell binding and lectin toxicity on both cell types. Here we examine these parameters in one study: lectin-cell binding and lectin toxicity with both cancer cells and their normal counterparts. We found that the human colon cancer cell line CCL-220/Colo320DM bound to agarose beads derivatized with Phaseolus vulgaris agglutinin (PHA-L) and wheat germ agglutinin (WGA), while the non-cancer human colon cell line CRL-1459/CCD-18Co did not. When these lectins were tested for their effects on cell viability in culture, both cell lines were affected by the lectins but at 6, 48 and 72 hours incubation times, PHA-L was most toxic to the cancer cell line in a concentration dependent manner. At 48 hours incubation, WGA was more toxic to the cancer cell line. The results suggest that it may be possible to develop lectin protocols that selectively target cancer cells for death. In any case, examination of both malignant cells and their non-malignant counterparts, analysis of their binding characteristics to immobilized lectins, and examination of the toxicity of free lectins in culture, provides a multiparameter model for obtaining more comprehensive information than from more limited approaches.
Keywords: Immobilized lectin binding, lectin toxicity in culture, human colon cancer and non-cancer colon cells, PHA-L, WGA
Introduction
Many studies over several decades have focused on the development of anticancer drugs that are designed to specifically target cancer cells. The approaches used in these studies have varied considerably. There is a great deal of evidence that suggests that in many cases the drugs that bind to cancer cells better than to normal cells, are internalized by the cancer cells, leading to rapid death. This is not a new concept, but many studies have not focused on the cell binding aspects of drug action.
It is well known that lectins often interact better with cancer cells than with normal cells because of greater numbers of cell surface lectin receptors or because of altered distributions of these receptors on the surfaces of the cancer cells (Sharon and Lis, 1989). As a result, lectins have been used in the design of anticancer drugs, by their direct toxicity or as carriers of other toxic agents (Banchonglikitkul et al., 2002; Gabor et al., 1998; 2002; 2004; Wirth et al., 2002; Minko, 2003).
Most studies using lectins as potential anticancer agents have not fully explored the interaction of the lectins with cells and have not carefully examined their effects on not only the cancer cells but on non-cancer cells from the same tissue. Studies in this area would yield more information if all of these aspects were examined.
One approach to screening a large number of compounds, such as lectins, for potential use in the development of anticancer drugs, is to first determine which compounds bind better to cancer cells than to their non-cancer counterparts and if these agents are differentially toxic to the cancer cells.
Our laboratory has developed a rapid assay that allows screening of limitless numbers of compounds for binding to cells. It utilizes agarose beads derivatized with many different compounds such as amino acids, sugars, lectins and other proteins (Khurrum et al., 2002; Navarro et al., 2002; Salbilla et al., 1999; Latham and Oppenheimer, 1999; Latham et al., 1995; 1995a). In fact, almost any compound can be immobilized on agarose beads for such studies.
This assay was shown to provide the same results as the more commonly used fluorescence assay, but it takes only a fraction of the time and requires no expensive equipment (Latham et al., 1995; 1995a). It can be used to screen a hundred compounds for cell binding in the time it takes to screen one compound using fluorescent label.
Using this assay, we found that the lectins Triticum vulgaris agglutinin (wheat germ agglutinin, WGA) and Phaseolus vulgaris agglutinin (also known as phytohemagglutinin-L, PHA-L) bound better to human colon cancer cells than they did to human non-cancer colon cells (Khurrum et al., 2002). In the past studies, the cells were thawed and fixed directly from the vials purchased from the vendor and not grown in culture.
In the present study, we examine these lectins for their binding and toxicity to the two cell types in culture. We find differential binding and toxicity of WGA and PHA-L to cancerous and non-cancerous colon cells. More important than the specific results obtained in this study, is the multiparameter model developed that approaches this type of work by examining: (1) both the cancer and non-cancer cells of the same tissue, (2) binding of both cell types grown in culture to the immobilized lectins, and (3) lectin toxicity to both cell types in culture. This approach provides a more comprehensive overview than do the more limited approaches used in the past.
Material and Methods
Cell lines
Two cell lines, human colon cancer cells tumorigenic in nude mice (CCL-220/Colo320DM) and non-cancerous human colon cells (CRL-1459 /CCD-18Co) were obtained from American Type Culture Collection (ATCC, Manassas, VA). Cells were cultured in 75 cm2 closed Falcon Flasks (Fisher Scientific, Tustin, CA) using RPMI 1640 cell culture medium with L-glutamine, supplemented with 10% heat-inactivated fetal bovine serum and 2.5g/l of glucose at 37°C and 5% CO2. All cell lines were maintained without the use of antibiotics. All reagents were of tissue culture grade and were obtained from Sigma Chemical Co (St. Louis, MO) unless otherwise indicated.
Subculturing
CCL-220
CCL-220 cells grow in suspension with a small percentage of adherent cells. They were subcultured by the following method. Suspension cells and medium were removed from the flask and added to a 15 ml conical plastic centrifuge tube. Adherent cells were rinsed with 2 ml Dulbecco's Phosphate Buffered Saline (PBS - without CaCl2 and MgCl2) prior to using 1 ml 0.25% trypsin-EDTA solution to detach adherent cells. Cells were incubated at 37°C with trypsin-EDTA for 3 minutes. The flask was rinsed with 2 ml RPMI 1640 medium to remove cells. This rinse was added to the centrifuge tube. Cells were spun at 28 x g for 3 minutes and the supernatant discarded. The cells were washed in 3 ml PBS, spun at 28 x g for 3 minutes and the supernatant discarded. They were then resuspended in 1 ml trypsin-EDTA and incubated for 3 minutes to create a single cell suspension. 2 ml of RPMI 1640 medium were added to inhibit further activity of the trypsin-EDTA solution. Cells were spun for 3 minutes at 78 x g and the supernatant discarded. Cells were resuspended in 3 ml RPMI 1640 medium for counting and subculturing. Cells were counted using a hemacytometer (Hausser Scientific, Horsham, PA) and cell viability was determined using trypan blue exclusion. All cultures used exhibited viability of 90% or higher. New cultures were seeded at 5 X 105 cells/ml.
CRL-1459
CRL-1459 is an adherent cell line. Cells were subcultured by removing and discarding the medium in the flask. The monolayer was then rinsed with 2 ml PBS which was removed and discarded. 1 ml 0.25% trypsin-EDTA solution was added to the monolayer to detach cells. The cells were incubated at 37°C with trypsin-EDTA for 3 minutes. The flask was rinsed with 2 ml RPMI 1640 medium to remove cells and inhibit further activity of the trypsin-EDTA solution. This rinse was added to a 15 ml conical plastic centrifuge tube. Cells were spun for 3 minutes at 78 x g and the supernatant was discarded. The cells were resuspended in 3 ml RPMI 1640 medium for counting and subculturing. Cells were counted using a hemacytometer (Hausser Scientific) and cell viability was determined using trypan blue exclusion. All cultures used exhibited viability of 90% or higher. New cultures were seeded at 5 X 105 cells/ml.
Histochemical Assay
Fixing Cells
CCL-220 and CRL-1459 were cultured and removed from flasks following the subculturing protocol. Upon removal, cells were spun at 113 x g for 3 minutes to remove media and resuspended in 1 ml serum-free RPMI 1640 medium. The cells were then washed 3 times with PBS (without CaCl2 and MgCl2) and spun for 3 minutes each time before being fixed in 1% formaldehyde in PBS for 45 minutes at room temperature. Fixed cells were washed by centrifugation 3 times in distilled water and resuspended in 1 ml distilled water. The cells were used within 3 days of fixation.
Lectin Beads
Lectins used in the histochemical assay, wheat germ agglutinin - WGA and Phaseolus vulgaris agglutinin – PHA-L, were bound to agarose beads. Poly L-lysine derivatized beads were also used as a positive control for the non-cancer line. All were obtained from Sigma. The lectin-beads were washed 3 times by centrifugation with distilled water and stored at 4°C before use. All lectins were used within 3 days of preparation.
Sugars
The haptenic sugars (0.2M) used were: N-acetyl-D-glucosamine for Triticum vulgaris agglutinin and D (+)-glucosamine and N-acetyl-D-glucosamine for Phaseolus vulgaris agglutinin. L-(-)-fucose served as the non-haptenic sugar for both lectins.
Haptenic Inhibition
Slides were prepared in triplicate containing 40 μl of distilled water, haptenic sugar or non-haptenic sugar. Haptenic sugars are those to which lectins preferentially bind. Non-haptenic sugars are those to which lectins do not preferentially bind. Approximately 4 μg of lectin beads were added and gently swirled with a toothpick for 1 minute to give the lectin beads and sugar time to interact. This was followed by the addition of 4 μl of fixed cells suspended in distilled water. The cell-lectin bead suspension was also gently swirled for 1 minute and observed using a light microscope at 100x and 200x magnification to determine if binding was present between cells and beads. Positive or negative binding was recorded. A positive control, poly L-lysine beads, was also tested under the distilled water condition with the non-cancer cells. Binding was confirmed by agitating the slides to ascertain if cells touching beads were or were not bound to the beads.
Serum contains many substances which may interfere with lectin binding to cells. To circumvent this issue, low serum medium was used in all lectin culture experiments (below) and in the bead experiments cells were washed multiple times with PBS to remove any interfering substances prior to fixation and assay in distilled water.
Photography
Cell-bead binding was digitally photographed using a Zeiss (Oberkochen, Germany) Axiolab microscope at 100x and 200x.
Cell Viability Assay
Lectins
Free lectins (Sigma) were reconstituted in sterile PBS (without CaCl2 and MgCl2). Stock solutions at 1 mg/ml were made using serum-free RPMI 1640 medium and stored at -20°C. Dilutions were also carried out using serum-free RPMI 1640.
MTT Assay
Tetrazolium derivative reduction (MTT) assay (Sigma) was used to determine the number of live cells present under each experimental condition. MTT was reconstituted in sterile PBS to a concentration of 5 mg/ml. Any MTT solution not used immediately was stored at −20°C. MTT is light sensitive so all vials were wrapped in foil to keep out light. 10 μl of MTT were added to each cell sample. Living cells reduce yellow MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-biphenyl tetrazolium bromide) to purple formazan crystals. After a 3 hour incubation at 37°C, 5% CO2, the crystals were dissolved in 10% Triton X-100 plus 0.1 N HCl in anhydrous isopropanol. Following a second 3 hour incubation at 37°C, 5% CO2, absorbance was measured at 570 nm using a spectrophotometer (Spectramax 190, Molecular Devices, Sunnyvale, CA) with background absorbance measured at 690 nm. The intensity of the colored product formed, as quantified by the spectrophotometer, is directly proportional to the number of live cells present in a sample. Each concentration of both lectins was tested with each cell line at least 6 times giving a minimum of 12 data points for each condition. Due to the variation of absorbances between plates, results of the MTT assay are expressed as percentage of the absorbance of the control wells. Average absorbance of the control cells was considered 100% survival. Absorbances of treated cells were expressed as percent of control survival. Standard error of the mean (SEM) was used as an indicator of variation between trials. Other studies have examined the correspondence between MTT results and results obtained through direct counting (Kiss et al., 1997; Remmelink et al., 1999) and confirmed that the MTT assay is a valid indicator of the number of viable cells present.
In this study it was deemed important to culture the cell lines without the use of antibiotics since routine use of antibiotics can mask problems with aseptic technique. This led to initial difficulty in cell culturing as well as the loss of many cells. When contamination became apparent, the affected flasks were disposed of and a new culture was begun using cells purchased from ATCC or frozen stock cells previously cultured and stored. At the time MTT assays were run, all cells were determined to be free of contamination. If a culture is contaminated with bacteria or mycoplasma, these cleave the tetrazolium ring of the MTT reagent just as live cells would. Because of the high numbers of bacteria or mycoplasma which are generally present in a contaminated culture, the reagents in contaminated wells turn deep purple within minutes of introducing MTT. When this happened those cultures were disposed of and the assay stopped.
Optimal Cell Number Determination for MTT Assay
Before beginning the toxicity study, it was necessary to determine the optimal number of cells to use with the MTT based in vitro toxicology assay. CCL-220 cells, which were available in large numbers and therefore used here, were removed by trypsinization, counted and serially diluted to concentrations of 2.5x104, 5x104, 7.5x104, 1.0x105, 2.5x105, 5x105, 7.5x105 and 1.0x106 cells/ml. 130 μl of cells were added to 6 wells for each cell concentration in a multi-well plate. The MTT assay was run 24 hours later according to the aforementioned procedure. This gave information on the optimal number of cells to use in order to get absorbance readings in an appropriate range to be read by the spectrophotometer. It also produced a standard curve which showed the approximate number of cells present at a given absorbance.
Cell Viability Assessment
Sixty-five μl of cells at a concentration of 1.0x106 cells/ml were plated in multi-well plates for 24 hours before the addition of 65 μl of lectin solution. Lectin solutions, in serum-free RPMI 1640 medium, were added to a final concentration of 0.5, 1.0, 10.0, 50.0, and 100.0 μg/ml for the 24, 48 and 72 hour incubations and 0.05, 0.25, 0.5, 5.0, 25.0, and 50.0 μg/ml for the 6 and 12 hour incubations. Controls had 65 μl of serum-free RPMI 1640 medium added in place of the lectin solution. The cells, when added to the wells, were in the same RPMI 1640 medium in which they were cultured. This contained 10% FBS. Because serum-free medium was added to each well, both for the control and the lectin samples, these experiments were effectively carried out in 5% fetal bovine serum. MTT based in vitro toxicology assay kit was used to measure relative cell number after 6, 12, 24, 48 or 72 hours incubation with the lectins.
Statistical Analysis
The results of the MTT assay are stated as means +/− SEM. Statistical comparisons were performed by Fisher F-test (one-way analysis of variance) to compare differences between cell lines under each condition. Values of p<0.05 were considered significant. Statistical analyses were done using Excel (Microsoft Office 2003).
RESULTS
Histochemical Assay
The fixed, tumorigenic colon cancer cells (CCL-220) showed binding in most trials to immobilized PHA-L and WGA under the distilled water and non-haptenic sugar conditions (Table 1). Binding was inhibited under the haptenic sugar conditions for PHA-L and WGA in all trials.
Table 1. Results for histochemical assay with CCL-220.
Percent positive binding trials of lectin beads and cultured, fixed tumorigenic colon cells (CCL-220) in distilled water, haptenic sugar solution and non-haptenic sugar solution. The 78% positive binding trials is probably not significantly different from 100% and could be due to experimental error in 2 trials showing negative binding.
| Lectin Bead | Sigma # | Conditions | # positive trials | % positive binding trials |
|---|---|---|---|---|
| Phaseolus vulgaris agglutinin | L3007 | Distilled water | 7/9 | 78% |
| 0.2M D-glucosamine | 0/9 | 0% | ||
| 0.2 M N- acetyl-D-glucosamine | 0/6 | 0% | ||
| 0.2M L (-)-fucose | 9/9 | 100% | ||
| Triticum vulgaris agglutinin | L6257 | Distilled water | 7/9 | 78% |
| 0.2 M N- acetyl-D-glucosamine | 0/3 | 0% | ||
| 0.2 M L (-)-fucose | 6/6 | 100% |
The fixed, non-cancer colon line (CRL-1459) did not bind to the lectins tested under the distilled water condition (Table 2). To determine if these cells could bind to any immobilized compounds, poly L-lysine, known to bind readily to cells and substrates (Roque et al., 1996) was used as a positive control. In this case, binding was seen in 6 out of 6 trials.
Table 2. Results for histochemical assay with CL-1459.
Percent positive binding trials of lectin beads to non-cancerous colon cells (CCL-1459) in distilled water.
| Lectin Bead | Sigma # | Conditions | # positive trials | % positive binding trials |
|---|---|---|---|---|
| Phaseolus vulgaris agglutinin | L3007 | Distilled water | 0/3 | 0% |
| Triticum vulgaris agglutinin | L6257 | Distilled water | 0/3 | 0% |
| Poly L-Lysine | P6893 | Distilled water | 6/6 | 100% |
Photographs showing representative examples of results seen in this study are in Figures 1a–d.
Figure 1.
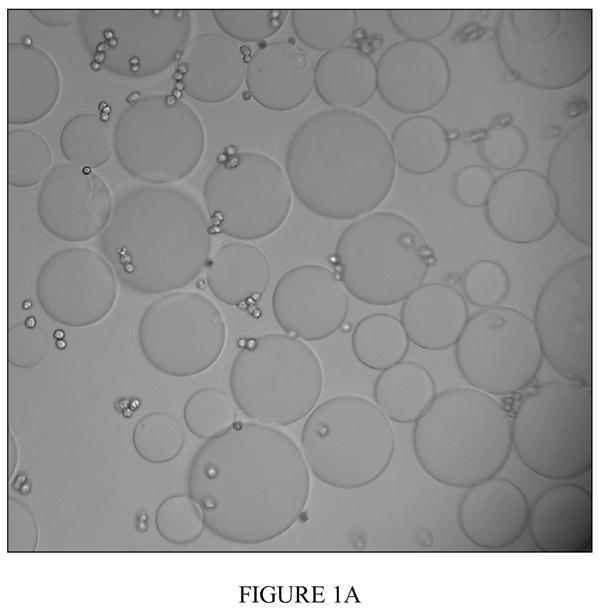

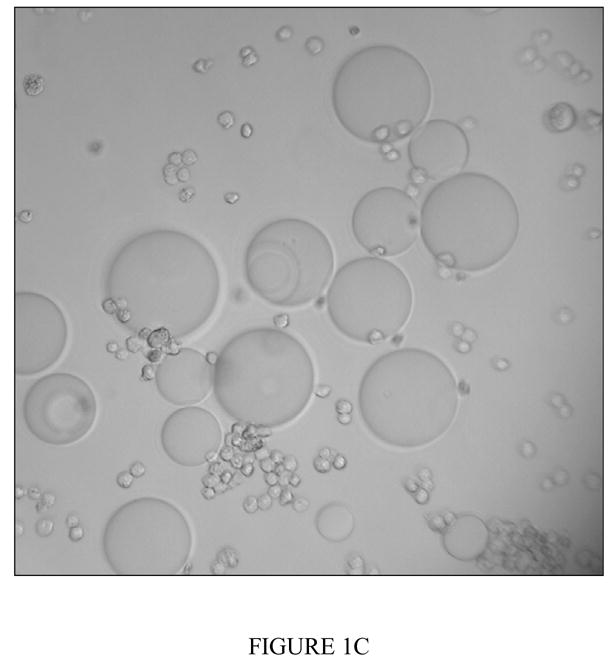

Figure 1a Binding of PHA-L derivatized agarose beads and CCL-220 cells in distilled water
Positive binding result shown. Fixed CCL-220 cells are small spheres, PHA-L derivatized beads are large spheres. Tapping the slide did not separate bound cells and beads. Magnification 200x.
Figure 1b Binding of PHA-L derivatized agarose beads and CCL-220 in 0.2M D (+)-glucosamine
Negative binding result shown. Fixed CCL-220 cells are small spheres, PHA-L derivatized beads are large spheres. Tapping the slide did not separate bound cells and beads. Magnification 200x.
Figure 1c Binding result of PHA-L derivatized agarose beads and CCL-220 in 0.2M L (−)-fucose, a non-inhibiting sugar
Positive binding result shown. Fixed CCL-220 cells are small spheres, PHA-L derivatized beads are large spheres. Magnification 200x.
Figure 1d Binding result of PHA-L derivatized agarose beads and CRL-1459 in distilled water
Negative binding result shown. Fixed CRL-1459 cells are small spheres, PHA-L derivatized beads are large spheres. Magnification 200x.
Optimal Cell Number Determination
Preliminary MTT studies were done to determine what cell concentration would give a strong absorbance reading. This produced a standard curve showing the absorbance at each cell concentration (Figure 2). 106 cells/ml was selected as the concentration of cells to use for initial plating. This concentration was diluted to 5x105 cells/ml after the addition of lectin solution. 5x105 cells/ml was preferable because it gave a mid-range absorbance reading which would enable differences in cell concentration in either direction to be easily detected.
Figure 2. Standard curve for absorbance and cell concentration.
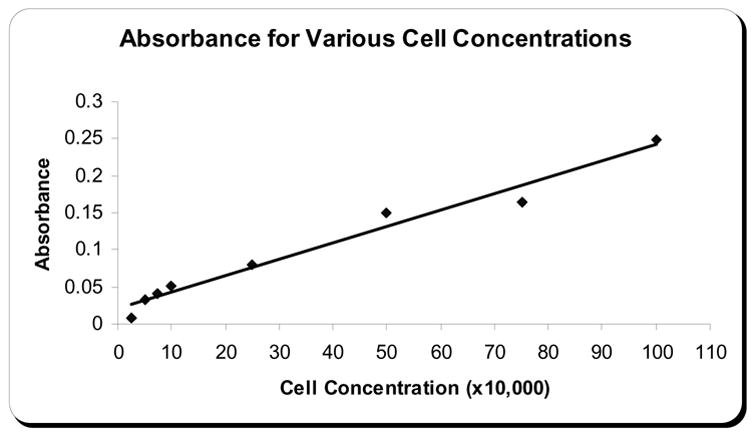
Shows the absorbance plotted against CCL-220 cell concentrations after MTT assay.
MTT Assay
Due to the variation in absorbances between plates, results of the MTT assay are expressed as percentage of the absorbance of the control wells. The absorbances for the control wells were averaged for each plate. All other absorbance readings for that plate were divided by this average control absorbance and multiplied by 100 to get the percent of control viability. Standard error of the mean was used as an indicator of variation among trials.
Phaseolus vulgaris agglutinin
Incubation of CRL-1459 with low concentrations of PHA-L (0.05 to 5.0 μg/ml) for 6 hours resulted in an increase in cell proliferation. This stimulatory effect was highest for the lowest concentration (0.05 μg/ml) and decreased with increasing lectin concentration. At the two highest concentrations there was an inhibitory effect by the lectin with 76.6% viable cells at 25.0 μg/ml and 50% at 50.0 μg/ml. The CCL-220 line generally showed a decrease in percent viable cells as the lectin concentration increased (except at the 0.5 μg/ml concentration) with 100% viable cells at 0.05 μg/ml, 89.5% at 0.25 μg/ml, 64.9% at 5.0 μg/ml, 60% at 25 μg/ml and 50.3% at 50.0 μg/ml. The CRL-1459 line showed a significantly higher (p>0.001) proportion of viable cells than the CCL-220 line at all concentrations except 0.5 μg/ml and 50.0 μg/ml (Fig. 3a). Twelve and twenty four hour incubations showed minimal differences between CCL-220 and CRL-1459 (data not shown).
Figure 3.
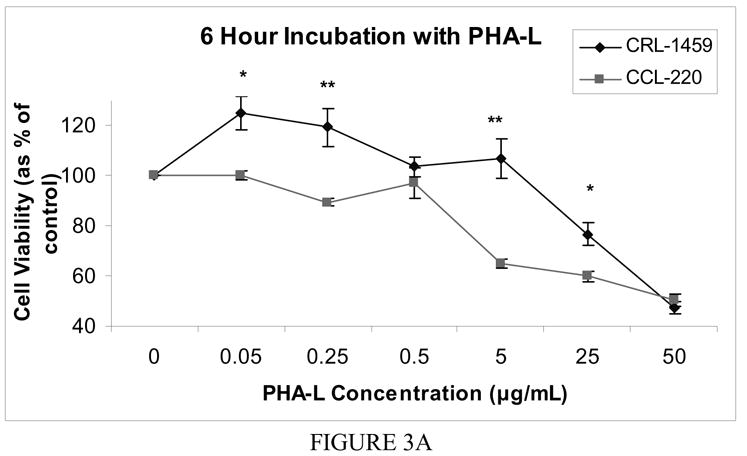
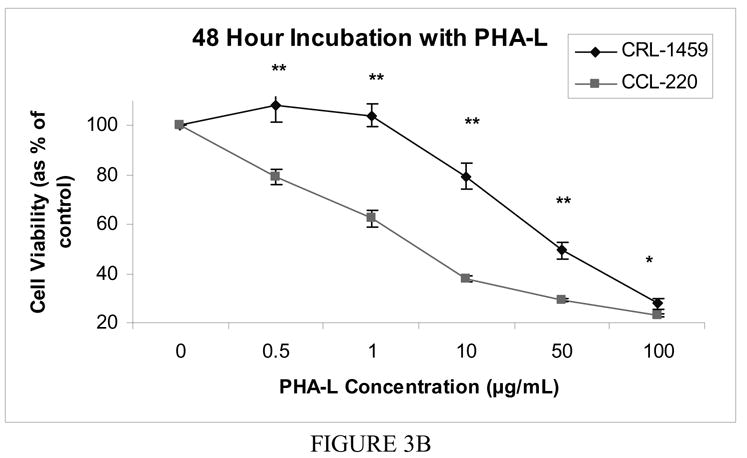
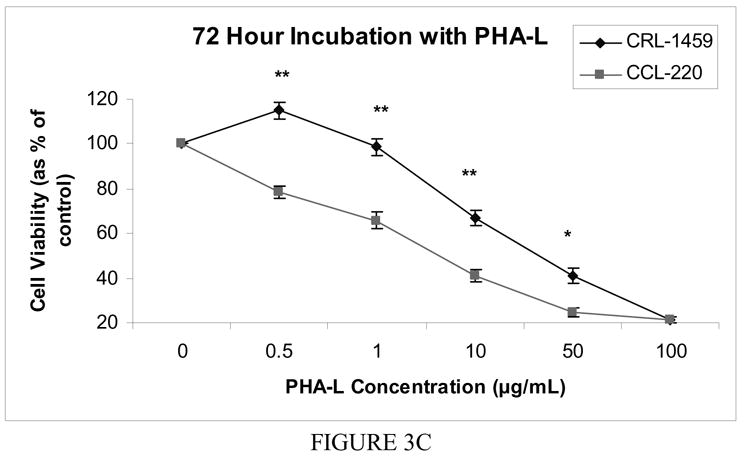
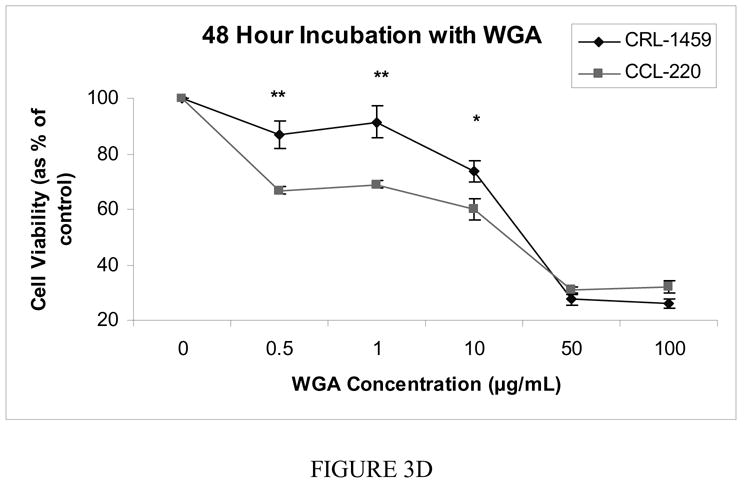
Figure 3a Cell viability after 6 hour incubation with free PHA-L
Cell viability was determined by MTT colorimetric assay. The mean value of the optical density measured in the control condition (no lectin added) was defined as 100% survival with other conditions reported as a percent of the control value. Error bars show standard error of the mean. * indicates p < 0.05, ** indicates p<0.0001.
Figure 3b Cell viability after 48 hour incubation with free PHA-L
Cell viability was determined by MTT colorimetric assay. The mean value of the optical density measured in the control condition (no lectin added) was defined as 100% survival with other conditions reported as a percent of the control value. Error bars show standard error of the mean. * indicates p < 0.05, ** indicates p<0.0001.
Figure 3c Cell viability after 72 hour incubation with free PHA-L
Cell viability was determined by MTT colorimetric assay. The mean value of the optical density measured in the control condition (no lectin added) was defined as 100% survival with other conditions reported as a percent of the control value. Error bars show standard error of the mean. * indicates p < 0.05, ** indicates p<0.0001.
Figure 3d Cell viability after 48 hour incubation with free WGA
Cell viability was determined by MTT colorimetric assay. The mean value of the optical density measured in the control condition (no lectin added) was defined as 100% survival with other conditions reported as a percent of the control value. Error bars show standard error of the mean. * indicates p < 0.05, ** indicates p<0.0001.
Following incubation for 48 hours, CRL-1459 showed a slight stimulatory effect at low concentrations of PHA-L (0.5 and 1 μg/ml), followed by a decrease in percent viable cells. CCL-220 showed a steady decrease in percent viable cells with increasing lectin concentration. Differences at all concentrations were significant with the CRL-1459 line having a higher percentage of viable cells than the CCL-220 line (p<0.0001, except at 100 μg/ml where p<0.04) (Fig. 3b).
For the 72 hour incubation, the trend seen in the 48 hour incubation continued. The CRL-1459 line had a significantly higher proportion of viable cells than the CCL-220 line at all concentrations (p<0.0001) except 100.0 μg/ml where both lines experienced substantial cell death with cell number at only approximately 20% of the controls (Fig. 3c).
Wheat germ agglutinin
The cell lines showed no significant differences in viability after 6, 12 and 24 hour incubations with WGA. At 48 hours, however, significant differences were found between the cell lines at 0.5 μg/ml, 1.0 μg/ml and 10.0 μg/ml with the CRL-1459 line showing a greater proportion of viable cells (p<0.02). At the two highest concentrations of WGA there was a drastic decrease in viable cells for both cell lines with live cell number at approximately 30% of controls (Fig. 3d).
Discussion
Recent studies have used lectins to directly target and kill cancerous cells by exploiting the changes made to the carbohydrates present on cell surfaces as a cell becomes transformed. There are two ways in which this has been done – using a non-toxic lectin conjugated to a drug which serves as the toxic entity (Banchonglikitkul et al., 2002; Gabor et al., 1998; 2002; 2004; Wirth et al., 2002) or using a toxic lectin to serve as both the targeting compound and the toxic entity (Minko, 2003). In either case, the lectin binding to the carbohydrates on the cell surface is the first step. Cells not containing the carbohydrate residues to which the lectins bind would presumably not be affected by the lectin (Gorelik et al., 2001).
As mentioned previously, several lectins examined by Khurrum et al. 2002 showed significantly different binding to thawed, fixed tumorigenic colon cancer cells as compared to non-tumorigenic colon cells, with the cancer cells exhibiting more binding than the non-cancer cells. Some of these lectins have demonstrated toxicity, to varying degrees, against some cancer cell lines in vitro, including: colon (Wirth et al., 1998; Kiss et al., 1997), breast (Valentiner et al., 2003), melanoma (Lorea et al., 1997; Litynska et al., 2001) and pancreatic cancers (Schwarz et al., 1999). These lectins have the potential to be used to target cancer cells for death.
Results obtained by Khurrum et al. 2002, however, using fixed cells will not necessarily be replicated with live cells as the fixation process can change the properties/ structure of the carbohydrates on the cell surface. Thies et al. 2005 examined binding differences of three mistletoe lectins when six different melanoma cell lines were either unfixed, fixed in methanol or fixed in paraformaldehyde. Some cell lines showed a difference in binding intensity which was dependent upon whether the cells were fixed or not and which fixative was used. Navarro et al. (2002) found that the assay used here with fixed cells and derivatized beads in distilled water gave nearly identical results to those using live cells. Our binding assay data confirm the results of Navarro et al. 2002 and show that, for the lectins tested, binding is preserved in both fixed and growing colon cells.
Studies by Gabor et al. 2001, 2000 have shown that free WGA is internalized by cells after binding in vitro. Schwarz et al. 1999 found that WGA must be internalized to exhibit a cytotoxic effect in their study of pancreatic cancer cells. Free WGA inhibited cell proliferation while WGA immobilized on a plastic tissue culture plate did not.
Kim et al. 1993 examined the cytotoxic activity of WGA and Griffonia simplcifolia 1-B4 both as free lectins and bead-bound lectins against nine different murine tumor cell lines and found that cells that did not have a binding site for the lectin were not affected. Further, only soluble lectin exhibited a cytotoxic effect. This indicates that binding and internalization are necessary for lectin-mediated toxicity, for these particular lectins. Also, if the cells were pre-incubated with the lectin-specific sugar, the lectin effects could be blocked. But if the cells were incubated with the lectin for 1–4 hours before the haptenic sugar was added there was no blocking. The lectin was already internalized.
Camby et al. 1997 found a protective effect of confluence against the cytotoxic effects of the lectins. Incubation times in the present study were much shorter and the cells were not yet confluent. Regardless of whether cells are in a suspension or monolayer, Wirth et al. 2002 found that 80% of WGA which bound to a cell was internalized within 4 hours. The initial rate of uptake was faster for cells in a single cell suspension, but by four hours internalization rates were comparable. The shortest incubation time used in this present study was 6 hours.
Several past studies have examined the effects of lectins on cancer cell lines without examining the effects of those lectins on non-cancerous cells of the same origin. If the non-cancerous cells are affected in a major way by the addition of the lectin, use of these compounds for targeting might be ill advised. In order to suggest that these lectins are safe for use with non-cancerous cells, studies should be conducted which also examine the effects of free lectins on cancer and non-cancer cells of the same tissue. Ohba and Bakalova (2003) found that WGA had substantial interaction with both normal and leukemic lymphocytes, although interactions were decreased with the normal lymphocytes. This indicates that the cell surface receptors which WGA bind are not specific to the cancer cells used in their study.
WGA’s effect has been described in Gastman et al. 2004 as apoptotic, triggered through a mitochondrial pathway. Apoptosis is dependent upon binding of lectin to surface carbohydrates and uptake into the cell. After just 30 minutes of incubation with WGA, Jurkat cells showed signs of apoptosis including disruption of the inner mitochondrial membrane and release of cytochrome c. The speed with which apoptosis was initiated is thought to be due to speed of binding and uptake. This is further reason to examine earlier time points. Schwarz et al. 1999 also describe WGA’s effects as indicating apoptosis is the mechanism of action. WGA incubation with pancreatic cells led to nuclear fragmentation, chromatin condensation and DNA release. Kim et al. 1993 found that the mechanism of action for many lectins is apoptosis with DNA fragmentation.
The binding of lectins in vitro to unfixed cells does not necessarily correspond to binding in vivo. It is important to consider whether any in vitro effect due to a binding relationship will have any effect in vivo. Clark (1995) found a high level of concordance between results from studies of the lectin Ulex europaeus binding to fixed mouse Peyer’s patch M-cells, found in the intestine, and binding in in vivo studies using a mouse Peyer’s patch gut loop model. Jordinson (1998) found that incubation of colorectal cancer cells with Vicia faba agglutinin (VFA) for 21 days led to increased differentiation of the cells in vitro. When grown in the same conditions without VFA or with a different lectin, no change was seen.
Mistletoe extract is currently one of the most often used alternative or complementary therapies for breast cancer patients in Germany and other parts of Europe (Fritz et al., 2004). It is also used with melanoma patients, although less frequently (Thies et al., 2005). The active ingredient in mistletoe extract is mistletoe lectin (Thies et al., 2005). It has been shown to have a cytotoxic effect on certain breast cancer cell lines in vitro (Fritz et al., 2004; Valentiner et al., 2002).
The two cell lines used in this study grow in different manners. The colon cancer line, CCL-220, grows mainly in suspension, with approximately 10% of the cells adherent. The adherent portion of the cells did not come close to confluence at any point. In contrast, the non-cancer colon line, CRL-1459, grows as a monolayer and was subcultured just prior to reaching confluence. This is not believed to influence the differential toxicity seen.
In the current study, PHA-L acted as a mitogen at low concentrations, particularly with non-cancerous cells after 6 hour incubations, at concentrations from 0.05 μg/ml up to 5 μg/ml. This effect was also found by Kiss et al. 1997 with 3 different human colorectal cancer cell lines and by Lorea et al. 1997 for PNA with melanoma cells. Camby et al. 1997 found a weak stimulatory effect of PHA-L with astrocytic tumor cell lines during 48–96 hour incubations. Beyond these time points, PHA-L had an inhibitory effect on cell proliferation that was concentration and time dependent.
Even though there were statistically significant differences in the viability of the two cell lines under some of the conditions, it is not the case that overall the non-tumorigenic colon cells were unaffected by the addition of the lectin. At 12 and 24 hours both cell lines were affected by PHA-L in a similar way, suggesting that incubation time is an important factor in differential lectin effects. For incubations with PHA-L however, at the 48 and 72 hour time points, the 1 μg/ml concentration resulted in the non-cancer line having almost the same cell proliferation as the control (104% and 98.6% respectively) while the cancer cell line had a significant decrease in proliferation (62.3% of control at 48 hours, p<0.00001 and 65.8% at 72 hours, p<0.00001). This suggests that it may be possible, at the right combination of lectin concentration and incubation time, to selectively target cancer cells for death.
The results presented here are less important than the multiparameter approach modeled in this study. Examination of both cancer cells and their non-cancerous counterparts, analysis of their binding characteristics to immobilized lectins and examination of the toxicity of free lectins in culture to both cell types, provide a model for obtaining more comprehensive information than by the more limited approaches used in the past.
Acknowledgments
The work was supported by grants from NIH NIGMS SCORE, NIH NIGMS RISE and the Joseph Drown Foundation. We thank Paul Wilson for expert assistance with the statistical analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bakalova R, Ohba H. Interaction of soybean agglutinin with leukemic T-cells and its use for their in vitro separation from normal lymphocytes by lectin-affinity chromatography. Biomedical Chromatography. 2003;17:239–49. doi: 10.1002/bmc.218. [DOI] [PubMed] [Google Scholar]
- Banchonglikitkul C, Smart JD, Gibbs RV, Donovan SJ, Cook DJ. An in vitro evaluation of lectin cytotoxicity using cell lines derived from the ocular surface. Journal of Drug Targeting. 2002;10:601–6. doi: 10.1080/1061186021000060747. [DOI] [PubMed] [Google Scholar]
- Camby I, Salmon I, De Decker R, Pasteels J-L, Brotchi J, Danguy A, Kiss R. Lectin histochemistry of astrocytic tumors and in vivo characterization of lectin-induced modifications on the proliferations of the SW1088, U373 and U87 human astrocytic cell lines. Journal of Neuro-Oncology. 1997;34:111–22. doi: 10.1023/a:1005783321916. [DOI] [PubMed] [Google Scholar]
- Clark MA, Jepson MA, Simmons NL, Hirst BH. Selective binding and transcytosis of Ulex europaeus 1 lectin by mouse Peyer’s patch M-cells in vivo. Cell & Tissue Research. 1995;282:455–61. doi: 10.1007/BF00318877. [DOI] [PubMed] [Google Scholar]
- Fritz P, Dippon J, Kierschke T, Siegle I, Mohring A, Moisa A, Murdter TE. Impact of mistletoe lectin binding in breast cancer. Anticancer Research. 2004;24:1187–92. [PubMed] [Google Scholar]
- Gabor F, Bogner E, Weissenboeck A, Wirth M. The lectin-cell interaction and its implications to intestinal lectin-mediated drug delivery. Advanced Drug Delivery Reviews. 2004;56:459–80. doi: 10.1016/j.addr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Gabor F, Schwarzbauer A, Wirth M. Lectin-mediated drug delivery: binding and uptake of BSA-WGA conjugates using the Caco-2 model. International Journal of Pharmaceutics. 2002;237:227–39. doi: 10.1016/s0378-5173(02)00049-2. [DOI] [PubMed] [Google Scholar]
- Gabor F, Klausegger U, Wirth M. The interaction between wheat germ agglutinin and other plant lectins with prostate cancer cells Du-145. International Journal of Pharmaceutics. 2001;221:35–47. doi: 10.1016/s0378-5173(01)00650-0. [DOI] [PubMed] [Google Scholar]
- Gabor F, Stangl M, Wirth M. Lectin-mediated bioadhesion: Binding characteristics of plant lectins on the enterocyte-like cell lines Caco-2, HT-29 and HCT-8. Journal of Controlled Release. 1998;55:131–42. doi: 10.1016/s0168-3659(98)00043-1. [DOI] [PubMed] [Google Scholar]
- Gastman B, Wang K, Han J, Zhu Z, Huang X, Wang G, Rabinowich H, Gorelik E. A novel apoptotic pathway as defined by lectin cellular interaction. Biochemical and Biophysical Research Communications. 2004;316:263–71. doi: 10.1016/j.bbrc.2004.02.043. [DOI] [PubMed] [Google Scholar]
- Gorelik E, Galili U, Raz A. On the role of cell surface carbohydrates and their binding proteins (lectins) in tumor metastasis. Cancer and Metastasis Reviews. 2001;20:245–77. doi: 10.1023/a:1015535427597. [DOI] [PubMed] [Google Scholar]
- Jordinson M, El-Hariry I, Calnan D, Pignatelli M. Vicia faba agglutinin, the lectin present in broad beans, stimulates differentiation of undifferentiated colon cancer cells. Gut. 1999;44:709–14. doi: 10.1136/gut.44.5.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurrum MR, Weerasinghe GR, Soriano ES, Riman R, Badali O, Gipson S, Medina J, Alfaro J, Navarro VM, Harieg CB, Ngo L, Sakhakorn T, Kirszenbaum L, Khatibi D, Abedi K, Barajas M, Zem G, Kirszenbaum A, Razi A, Oppenheimer SB. Analysis of surface properties of human cancer cells using derivatized beads. Acta Histochemica. 2002;104:217–23. doi: 10.1078/0065-1281-00656. [DOI] [PubMed] [Google Scholar]
- Kim M, Rao MV, Tweardy DJ, Prakash M, Galili U, Gorelik E. Lectin-induced apoptosis of tumor cells. Glycobiology. 1993;3:447–53. doi: 10.1093/glycob/3.5.447. [DOI] [PubMed] [Google Scholar]
- Kiss R, Camby I, Duckworth C, De Decker R, Salmon I, Pasteels J-L, Danguy A, Yeaton P. In vitro influence of Phaseolus vulgaris, Griffonia simplicifolia, Concanavalin A, wheat germ, and peanut agglutinins on HCT-15, LoVo, and SW837 human colorectal caner cell growth. Gut. 1997;40:253–61. doi: 10.1136/gut.40.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham V, Ducut J, Rostamiani K, Chun H, Lopez M, Herrra S, Oppenheimer S. A rapid lectin receptor binding assay: comparative evaluation of sea urchin embryo cell surface lectin receptors. Acta Histochemica. 1995;97:89–97. doi: 10.1016/S0065-1281(11)80209-6. [DOI] [PubMed] [Google Scholar]
- Latham V, Herrera S, Rostamiani K, Chun H, Oppeheimer S. Rapid identification of lectin receptors and their possible function in sea urchin cell systems. Acta Histochemica. 1995a;97:373–382. doi: 10.1016/S0065-1281(11)80062-0. [DOI] [PubMed] [Google Scholar]
- Latham V, Oppenheimer S. A simple image analysis method for evaluating cell binding to derivatized beads. Acta Histochemica. 1999;101:263–270. doi: 10.1016/S0065-1281(99)80027-0. [DOI] [PubMed] [Google Scholar]
- Litynska A, Przybylo M, Pochec E, Hoja-Lukowicz D, Cloczyk D, Laidler P, Gill D. Comparison of the lectin-binding pattern in different human melanoma cell lines. Melanoma Research. 2001;11:205–12. doi: 10.1097/00008390-200106000-00001. [DOI] [PubMed] [Google Scholar]
- Lorea P, Goldschmidt D, Darro F, Salmon I, Bovin N, Gabius HJ, Kiss R, Danguy A. In vitro characterization of lectin-induced alterations of the proliferative activity of three human melanoma cell lines. Melanoma Research. 1997;7:353–63. doi: 10.1097/00008390-199710000-00001. [DOI] [PubMed] [Google Scholar]
- Minko T. Drug targeting to the colon with lectins and neoglycoconjugates. Advanced Drug Delivery Reviews. 2003;56:491–509. doi: 10.1016/j.addr.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Walker SL, Badali O, Abundis M, Ngo LL, Weerasinghe G, Barajas M, Zem G, Oppenheimer SB. Analysis of surface properties of fixed and live cells using derivatized agarose beads. Acta Histochemica. 2002;104:99–106. doi: 10.1078/0065-1281-00617. [DOI] [PubMed] [Google Scholar]
- Remmelink M, Darro F, Decaestecker C, DeDecker R, Bovin NV, Gebhart M, Kaltner H, Gabius HJ, Kiss R, Salmon I, Danguy A. In vitro influence of lectins and neoglycoconjugates on the growth of three human sarcoma cell lines. Journal of Cancer Research and Clinical Oncology. 1999;125:275–85. doi: 10.1007/s004320050274. [DOI] [PubMed] [Google Scholar]
- Roque RL, Herrera S, Yeh TYJ, Borisavljevic TL, Brunick L, Miles A, Haritunians T, Addy C, Bada RA, Vaghefi H, Matsumoto SS, Piccionelli GA, Rodriguez L, Oppenheimer SB. Cell adhesion mechanisms: Modeling using derivatized beads and sea urchin cell systems. Acta Histochemica. 1996;98:441–51. doi: 10.1016/s0065-1281(96)80011-0. [DOI] [PubMed] [Google Scholar]
- Salbilla BA, Vaghefi H, Chhabra P, Hall G, Brown D, Sadoughi F, Francisco E, Attas L, Walker S, Nguyen BN, Oppenheimer SB. Analysis of cell surface properties using derivatized agarose beads. Acta Histochemica. 1999;101:271–9. doi: 10.1016/s0065-1281(99)80028-2. [DOI] [PubMed] [Google Scholar]
- Schwarz RE, Wojciechowicz DC, Picon AI, Schwarz MA, Paty PB. Wheat germ agglutinin-mediated toxicity in pancreatic cancer cells. British Journal of Cancer. 1999;80:1754–62. doi: 10.1038/sj.bjc.6690593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon N, Lis H. Lectins. Chapman and Hall; London, UK: 1989. [Google Scholar]
- Thies A, Nugel D, Pfuller U, Moll I, Schumacher U. Influence of mistletoe lectin and cytokines induced by them on cell proliferation of human melanoma cells in vitro. Toxicology. 2005;207:105–16. doi: 10.1016/j.tox.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Valentiner U, Fabian S, Schumacher U, Leathem A. The influence of dietary lectins on the cell proliferation of human breast cancer cell lines in vitro. Anticancer Research. 2003;23:1197–206. [PubMed] [Google Scholar]
- Valentiner U, Pfuller U, Baum C, Schumacher U. The cytotoxic effect of mistletoe lectins I, II and III on sensitive and multidrug resistant human colon cancer cell lines in vitro. Toxicology. 2002;171:187–99. doi: 10.1016/s0300-483x(01)00581-9. [DOI] [PubMed] [Google Scholar]
- Wirth M, Kneuer C, Lehr CM, Gabor F. Lectin-mediated drug delivery: Discrimination between cytoadhesion and cytoinvasion and evidence for lysosomal accumulation of wheat germ agglutinin in the Caco-2 model. Journal of Drug Targeting. 2002;10:439–48. doi: 10.1080/1061186021000038300. [DOI] [PubMed] [Google Scholar]
- Wirth M, Fuchs A, Wolf M, Ertl B, Gabor F. Lectin-mediated drug targeting: Preparation, binding characteristics, and antiproliferative activity of wheat germ agglutinin conjugated doxorubicin on Caco-2 cells. Pharmaceutical Research. 1998;15:1031–7. doi: 10.1023/a:1011926026653. [DOI] [PubMed] [Google Scholar]


